Abstract
Helicobacter pylori strains associated with severe tissue damage and inflammation possess a unique genetic locus, cag, containing 31 genes originating from a distant event of horizontal transfer and retained as a pathogenicity island. The cag system is an Helicobacter-specific type IV secretion engine involved in cellular responses like induction of pedestals, secretion of IL-8, and phosphorylation of proteic targets. It has previously been reported that cocultivation of epithelial cells with Helicobacter pylori triggers signal transduction and tyrosine phosphorylation of a 145-kDa putative host cell protein. Herein, we demonstrate that this protein is not derived from the host but rather is the bacterial immunodominant antigen CagA, a virulence factor commonly expressed in peptic ulcer disease and thought to be an orphan of a specific biological function. Thus, CagA is delivered into the epithelial cells by the cag type IV secretion system where it is phosphorylated on tyrosine residues by an as yet unidentified host cell kinase and wired to eukaryotic signal transduction pathways and cytoskeletal plasticity.
Keywords: pathogenicity island, type IV secretion system, virulence
The microaerophilic bacterium Helicobacter pylori (Hp) has been under intense study for its causative role in several gastric pathologies, including peptic ulcer disease, chronic active gastritis, mucosal-associated lymphoid tissue lymphoma, and gastric adenocarcinoma of the antrum (1). Factors involved in the gastric survival, including motility, urease production, and nickel incorporation, have been described. A molecule possessing toxic activity, VacA, was isolated, image reconstructed, and linked to late endosome traffic and vacuolar ATPase activity (2, 3). Despite two different genomic efforts, few virulence factors were further identified (4, 5). A pathogenicity island encoding a secretion apparatus responsible for several virulent traits observed in in vitro experimental conditions was associated with the increased virulence of strains isolated from human gastric mucosal specimens with severe injuries. Helicobacter strains were adapted to colonize the stomachs of mice (6), dogs (7), Mongolian gerbils (8), and monkeys (9), and stomach lesions were obtained after prolonged incubation. This subset (type I) of Hp isolates contains a 40-kilobase fragment of DNA known as cag (cag PAI) inserted at the end of the glutamate racemase gene after being horizontally transferred from an unknown source a million years ago into the Hp chromosome (10). When incubated with tissue culture cells, cag-positive strains activate host cell signal transduction, including induction of the transcription factor NF-κB with consequent secretion of the proinflammatory cytokine IL-8 (11). In addition, these virulent strains induce reorganization of the cytoskeleton with pedestal formation and tyrosine phosphorylation of an unidentified 145-kDa putative host cell protein (12). The 145-kDa molecule is localized at the contact site between Hp and host cells, at the base of the pedestals that cups the bacterium. Strains lacking cag or isogenic mutants deleted for cag genes are impaired in the ability to induce these signaling events (13). The cag secretion system is thought to be an organelle used to secrete macromolecules serving as a conduit for protein(s) keyed to interact with cellular structures and delivered during contact (10). Various pathogens use a variant of the basic type IV secretion machinery for conjugational DNA transfer (Escherichia coli), for transfer of virulence plasmids into plant cells (Agrobacterium tumefaciens), or for toxin secretion (Bordetella pertussis). Recently, type IV secretion systems were also described in Rickettsia prowazekii, Legionella pneumoniae, and Brucella suis (1). Also encoded by cag is the immunodominant antigen CagA, the size of which can vary from 128 to 146 kDa in different strains and which is widely used as a marker of Hp virulence (14). Despite the correlation between CagA expression and virulence, thus far, no progress has been made in understanding the function of this protein.
Materials and Methods
Bacterial Strains.
Helicobacter strains G27, 87A300, and 342 and mutants G27ΔcagA, G27ΔcagM, G27ΔcagE, and G27ΔcagI have been described (10, 12, 13). The mutant strains G27ΔHP528 (virB9), G27ΔHP527 (virB10), G27ΔHP525 (virB11), G27ΔHP524 (virD4), and 342ΔcagA were produced in our lab (M. Marchetti, R.R., and A.C., unpublished work). All mutants except G27ΔHP528 were generated with the same principle: briefly, short regions of the 5′ end and 3′ end coding sequence were amplified with appropriate restriction sites by PCR and cloned into the pBluescript SK(+) vector. A kanamycin-resistance cassette was then cloned between the two fragments. After transformation, the G27 mutants were selected for kanamycin resistance, and the corresponding gene region was sequenced after PCR amplification. G27ΔHP528 was generated by insertion of a stop codon signal within the coding sequence by using the SacB cassette system and negatively selected for sucrose resistance (15).
AGS Cells.
AGS cells (European Collection of Cell Cultures 89090402) were grown at 37°C in 5% CO2/95% air in DMEM supplemented with 10% (vol/vol) FBS.
Infection of AGS Cells with Metabolically Labeled Bacteria.
Hp strain 87A300 was grown overnight in 20 ml of RPMI-based minimal medium 1640 (Met− and Cys−) supplemented with 0.4% cyclodextrin at 37°C in 5% CO2. After 16 h, 200 μCi of 35S-trans-label (ICN) was added, and the bacteria were incubated for an additional 4 h. AGS cells were seeded in 10-cm tissue culture dishes, and protein biosynthesis of the cells was blocked with cycloheximide (100 μg/ml) for 40 min before infection. AGS cells were infected for 3 h with the labeled Helicobacter at a multiplicity of 100 to 1.
Cell Lysates.
Infected cells were washed three times with ice-cold PBS containing 100 mM sodium vanadate. The cells were scraped on ice, transferred into a microfuge tube, and pelleted at 5,000 × g for 1 min. The cell pellet recovered from a single 10-cm tissue culture dish was resuspended in 1 ml of saponin lysis buffer [100 mM saponin/20 mM Tris⋅Cl, pH 7.4/5 mg/ml protease inhibitor mixture (Complete, Roche Molecular Biochemicals)/200 mM sodium vanadate] and incubated on ice for 5 min. Then, the cells were pelleted at 20,000 × g for 5 min and resuspended in 100 μl of RIPA buffer (0.15 mM NaCl/0.05 mM Tris⋅HCl, pH 7.2/1% Triton X-100/1% sodium deoxycholate/0.1% SDS) containing 200 mM vanadate and 5 mg/ml protease inhibitors. The cells were incubated on ice for 10 min and centrifuged. The supernatant was collected, and 20 μl of 5× sample buffer solution was added. Samples were heated at 90°C for 5 min and kept at −20°C.
Immunoprecipitation.
Unspecific rabbit serum (5 μl) was added to 100 μl of RIPA-buffer-soluble fractions of infected AGS cells. The lysate was diluted with 500 μl of immunoprecipitation buffer [1% NP-40/1 mM EDTA/5 mM MgCl2/100 mM NaCl/10% (vol/vol) glycerol/200 mM vanadate/5 mg/ml protease inhibitors], and the tube was slowly rotated at 4°C for 30 min. A suspension of protein A-coated Sepharose beads (50 μl) was added, kept in continued rotation for 1 h, and then washed three times in immunoprecipitation buffer. Beads were spun down for 5 min at 4°C, and the precleared supernatant was transferred to a fresh tube. The antibody was added to the supernatant (3 μl monoclonal or 5 μl polyclonal serum), and the mixture was rotated for 30 min more at 4°C. Before the rotation was continued for between 1 h to overnight at 4°C, 75 μl of protein A/Sepharose beads was added. When monoclonal antibodies were used, the beads were first coupled to rabbit anti-mouse antibodies (beads were washed in lysis buffer, mixed with rabbit-anti-mouse antibody, and rotated for 1 h). After the incubation time, the beads were pelleted for 5 min at 20,000 × g, and the supernatant was discarded. The beads were subsequently washed in 3× immunoprecipitation buffer and then in 20 mM Tris⋅Cl (pH 7.4). The antigen was dissolved from the protein A/Sepharose beads by boiling in 50 μl of sample solution (2×). The beads were removed by centrifugation (5 min at 20,000 × g), and the supernatant was stored at −20°C for PAGE.
Two-Dimensional Gel Electrophoresis.
Two-dimensional gel electrophoresis was done according to the protocol of Bini et al. (16) with minor modifications. Bacterial samples were washed in cold PBS before resuspension in loading buffer.
Cell Fractionation.
After infection, AGS cells were washed three times with 10 ml of ice-cold PBS containing 2 mM vanadate. All steps were done at 4°C on ice. The cells were scraped in 2 ml of PBS/vanadate. Cells from four dishes were combined, transferred into a 15-ml Falcon-tube, and pelleted for 5 min at 200 × g (Beckman–Spinco swing-out centrifuge; CS-6R). The supernatant was discarded, and the cells were carefully resuspended in 8 ml of homogenization buffer (250 mM sucrose/3 mM imidazole, pH 7.4/0.5 mM EDTA). The cells were pelleted for 10 min at 1,500 × g and resuspended in 600 μl of homogenization buffer. For mechanical lysis, the cells were aspirated slowly into a 1-ml syringe carrying a 0.22 gauge needle and expelled aggressively. This procedure was repeated four times. The cells were pelleted for 10 min at 1,500 × g, and the supernatant was transferred into centrifuge tubes. The pellet containing the bacteria, unlysed cells, and the cytoskeletal fraction was resuspended in 600 μl of 1× sample buffer. The supernatant was centrifuged for 20 min at 41,000 × g. The supernatant containing the host cell cytosol (200 μl) was mixed with 50 μl of 5× sample solution. The pellet, which contained the host cell membrane fraction, was resuspended in 200 μl of 1× sample solution. The samples were boiled for 5 min.
Computer Sequence Analyses.
DNA and protein sequences were analyzed by using the gcg software package (version 10; GCG) running on a Sun multiprocessor Enterprise 450 server under solaris 7 (Sun Microsystems, Palo Alto, CA). Internet-based searches were performed with netscape communicator (version 4.6; Netscape, Mountain View, CA) at the National Center for Biotechnology Information, Sanger Centre, Astra, and European Molecular Biology Laboratory sites with a Macintosh G3 (Apple) as the front-end computer. Accelerated Smith–Waterman searches were performed on a fast data finder (Paracel, Pasadena, CA) with the bioview toolkit.
Results
Tyrosine-Phosphorylated Target (PTYR) Is a Bacterial Protein.
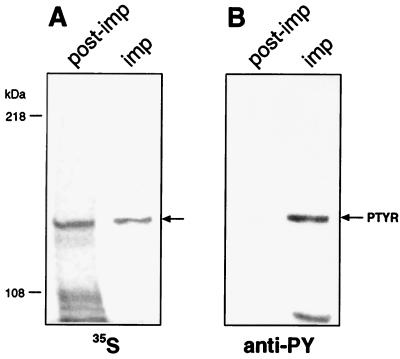
Trying to identify the PTYR, we infected AGS tissue culture cells with Hp wild-type strain 87A300, which was metabolically labeled with 35S before infection. Of the two phosphoproteins in the molecular range above 100 kDa that were immunoprecipitated from cell lysates, only one protein was radiolabeled (Fig. 1) and comigrated exactly with PTYR. When AGS cells were incubated with 35S in the absence of bacteria, no host protein of a similar molecular mass was radioactively labeled (data not shown), suggesting that PTYR could have a bacterial origin.
Figure 1.
Radioactive profile of tyrosine-phosphorylated proteins after infection of AGS cells with 35S-labeled Hp. AGS cells were infected with the 35S-labeled Hp strain 87A300. Tyrosine-phosphorylated proteins were immunoprecipitated from RIPA-buffer-soluble fractions by using monoclonal anti-phosphotyrosine antibody (anti-PY; PY20, Transduction Laboratories, Lexington, KY). Immunoprecipitates (imp) and proteins of the RIPA-soluble cell fraction after the immunoprecipitation with anti-PY (post-imp) were separated by SDS/6% PAGE and transferred onto nitrocellulose membranes in duplicate. One membrane was exposed to Kodak x-ray film (A); the second nitrocellulose membrane was probed with anti-phosphotyrosine antibody (anti-PY). Blots were developed with peroxidase-coupled secondary antibodies [GIBCO/BRL]. The arrow in A indicates the 35S-labeled protein immunoprecipitated with anti-PY and comigrating with PTYR (shown in B).
PTYR Is Recognized by Anti-CagA Antibodies.
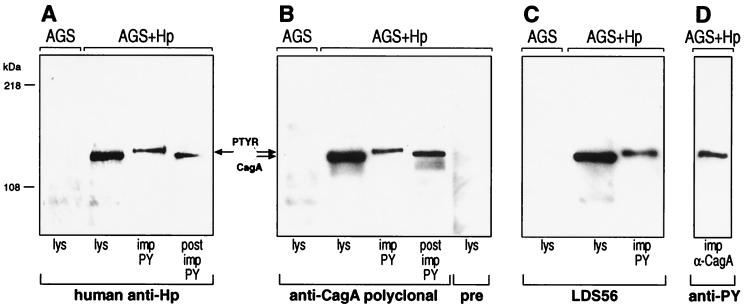
To verify the bacterial nature of PTYR cell lysates and proteins immunoprecipitated with anti-phosphotyrosine antibody, they were analyzed by Western blotting by using serum from a patient infected with Hp. The serum did not recognize any protein in lysates of uninfected AGS cells. However, it did recognize immunoprecipitated PTYR and a band of similar size in the complete cell lysate and postimmunoprecipitate of AGS cells incubated with Hp (Fig. 2A). This finding provided further evidence that PTYR could be a bacterial protein. A computer search of the Hp genome for proteins with a size similar to PTYR revealed that molecules in the range of 145 kDa are rare and that CagA is one of the few.
Figure 2.
Anti-CagA antibodies recognize PTYR. AGS cells were infected with the Hp strain 87A300. Lysis (lys), fractionation, and immunoprecipitation (imp) of infected (AGS+Hp) and uninfected (AGS) tissue culture cells were performed as described for Fig. 1. The fractions were analyzed with different antibodies: polyclonal human anti-Hp (A), polyclonal anti-CagA serum and preserum (pre; B), and monoclonal anti-CagA antibody (LDS 56; C). All three antibodies recognized the same bands including immunoprecipitated PTYR. (post imp, postimmunoprecipitation.) (D) CagA immunoprecipitated from infected cells with polyclonal anti-CagA serum (imp α-CagA) was recognized with anti-PY.
Therefore, we probed identical samples as shown in Fig. 2A with polyclonal and monoclonal (LDS 56) anti-CagA antibodies. Both antibodies stained the same bands as did the human anti-Hp serum shown in Fig. 2A, indicating that CagA and PTYR may be the same protein (Fig. 2 B and C, respectively). In agreement with these data, immunoprecipitated CagA proteins were detected with anti-phosphotyrosine antibody (Fig. 2D). Based on these results, our preliminary conclusion was that PTYR is identical to phosphorylated CagA (CagAtyr). Notably, only a minor amount of CagA was phosphorylated on tyrosine residues and showed a slight shift in the mobility on SDS/PAGE (Fig. 2).
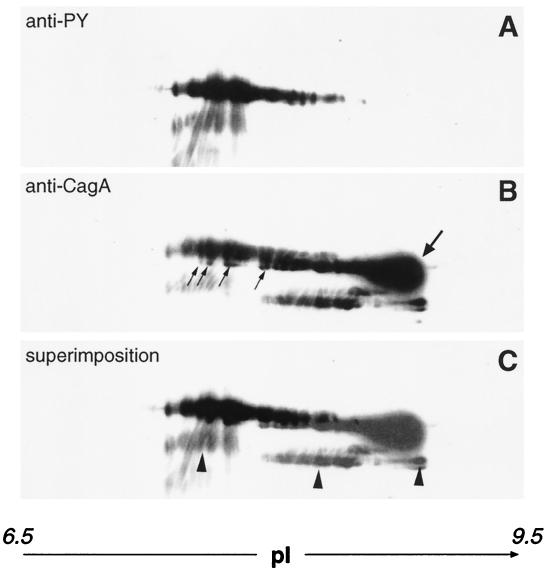
CagA and PTYR Have Overlapping Spots in Two-Dimensional Electrophoresis.
To provide further evidence that phosphorylated CagA is identical to PTYR, we resolved lysates of infected AGS cells by two-dimensional gel electrophoresis (16). The proteins were transferred onto poly(vinylidene difluoride) membranes and probed with anti-phosphotyrosine antibody or mouse anti-CagA serum. Superimposition of blots in Fig. 3 A and B showed that the anti-phosphotyrosine antibody recognized a subset of the spots also detected by anti-CagA serum (Fig. 3C). This result suggests that some isoforms of CagA are tyrosine phosphorylated. In particular, the anti-CagA antiserum recognized spots with higher pI values and also spots with slightly lower molecular masses, which likely indicate the nonphosphorylated form of CagA. An approximately 100-kDa minor breakdown product was recognized by both antibodies, indicating that PTYR had the same degradation pattern reported previously for CagA in Hp (14).
Figure 3.
Two-dimensional gel electrophoresis showing the similar migration pattern of PTYR and CagA. Infected AGS cells were denatured in electrophoresis buffer. After analysis by two-dimensional gel electrophoresis (16), the proteins were transferred onto poly(vinylidene difluoride) membrane. The membrane was probed with anti-PY (A) and then stripped and reprobed with polyclonal anti-CagA antibody (B). (C) A superimposition of A and B. Arrows in B point to the unphosphorylated form of CagA, and the major proteic spot at higher pI is indicated by a bold arrow. The CagA spots with slightly lower molecular masses are indicated with regular arrows. Arrowheads in C indicate a degradation product.
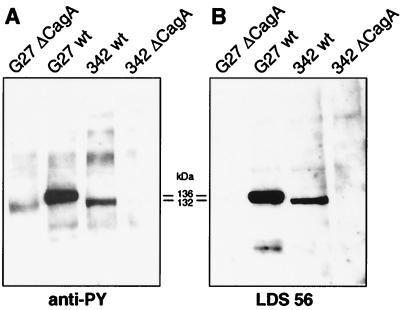
The Size Variability of CagA Correlates with the Size of PTYR.
To obtain further evidence of the identity between PTYR and CagA that would be independent from the specificity of antibodies, we took advantage of the interstrain size variability of cagA, which is caused by the number of 102-bp repeats present within the central region of cagA (14). AGS cells were incubated with Hp strains G27 and 342, whose cagA genes contain two and one 102-bp repeats and have molecular masses of 136 and 132 kDa, respectively. Fig. 4 shows that, in Hp strains G27 and 342, which produce CagA proteins of 136 and 132 kDa, respectively, the mobility of PTYR correlated precisely with the different CagA isoforms. The variation of the molecular mass of PTYR according to the variation of CagA provides a final confirmation that PTYR and CagA are the same protein. In agreement with this conclusion, cagA knockout strains did not have any PTYR (Fig. 4).
Figure 4.
The molecular mass of PTYR changes according to the molecular mass of the CagA antigen. Wild-type (wt) strains G27 and 342 and their isogenic cagA knockout mutants were incubated with AGS cells (12). Proteins were analyzed by SDS/6% PAGE and probed with anti-PY or with monoclonal anti-CagA antibody (LDS 56) by Western blotting. The mobility of CagA was estimated by SDS/PAGE and was also inferred by DNA sequence analysis of the 102-bp repeat region. The presence of two repeats in strain G27 and one repeat in strain 342 allowed precise calculation of the size of CagA and explains the kDa difference.
CagA Translocation Requires a Functional Type IV Secretion System.
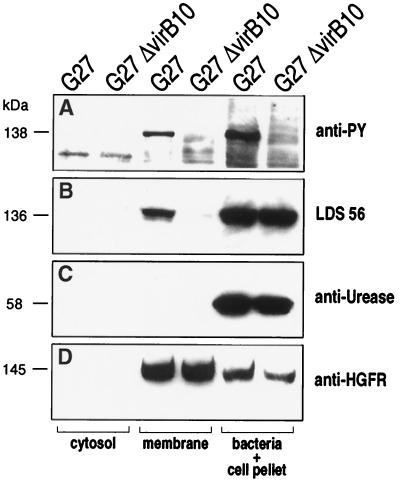
We tested knockout mutants of genes encoding the type IV secretion system (including virB4, virB9, virB10, virB11, and virD4 homologues; ref. 1) for their ability to induce CagAtyr. As shown in Table 1, all mutants were negative, suggesting that an intact secretion system is required. To provide evidence that CagA is translocated into the host cell via the type IV secretion system, we tested cytosol and membrane fractions of infected AGS cells for the presence of CagA. To show that the bacteria were not lysed during the fractionation procedure, we used urease as a negative control. Urease is an abundant protein of Helicobacter and is found in the bacterial cytosol and is associated with the outer bacterial membrane. Urease could not be detected in the host cell cytosol or membrane fraction (Fig. 5C). In contrast, CagA was enriched in the host cell membrane of cells infected with the wild-type strain with either monoclonal CagA or anti-phosphotyrosine antibody (Fig. 5 A and B) but not in membrane preparations of cells infected with the isogenic G27ΔHP527 (a virB10 homologue) mutant (Fig. 5B). Analysis of the fraction containing the bacterial proteins showed that CagA and urease antigens were expressed in both strains in approximately equal amounts. This finding indicates that type IV secretion mutants are unable to translocate CagA and that the CagA antigen does not have the ability to associate with the host membrane per se but must be injected by using an active mechanism. Translocation of CagA was recently confirmed by Segal et al. (17) by using deconvolution microscopy. HGFR was used as a marker protein to test the efficiency of extraction of the host membrane proteins (Fig. 5D). As discussed, only a minor amount of the total CagA became phosphorylated during the infection process, whereas most of the CagA protein detected in the host cell membrane seemed to be phosphorylated. Because no CagA phosphorylation can be detected in bacteria recovered from the supernatant of infected cells or in cells incubated with lysates (data not shown), our results suggest that CagA is phosphorylated only after translocation into the host cell membrane (Fig. 6).
Table 1.
cag-dependent tyrosine phosphorylation of CagA
| Helicobacter strains | CagA tyrosine phosphorylation |
|---|---|
| G27 (wild type) | + |
| G27ΔHP528 (virB9) | − |
| G27ΔHP527 (virB10) | − |
| G27ΔHP525 (virB11) | − |
| G27ΔHP524 (virD4) | − |
| G27ΔcagM | − |
| G27ΔcagE | − |
| G27ΔcagI | − |
| G27ΔcagA | − |
Figure 5.
PTYR fractionation. AGS cells were infected with the Hp wild-type strain G27 or its isogenic mutant Δ527 as described (12). Host cell cytosol and membrane proteins were separated from the cellular fraction containing the bacteria, unlysed cells, and the cytoskeleton proteins. The fractions were analyzed by SDS/8% PAGE and transferred onto four separate nitrocellulose membranes. Each membrane was probed with a different antibody [anti-PY (A), LDS 56 (B), anti-urease (rabbit polyclonal antiserum against recombinant UreA; C), or anti-HGFR (C28, Santa Cruz Biotechnology; D)]. Estimated mobility is provided for the phosphorylated (A; 138 kDa) and unphosphorylated CagA (B; 136 kDa), urease A (C; 58 kDa), and hepatocyte growth factor receptor (HGFR) major subunit (D; 145 kDa).
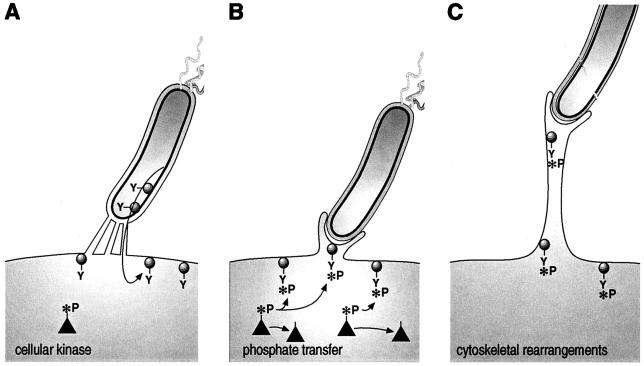
Figure 6.
Model for Helicobacter-mediated signaling events. (A) CagA (schematically drawn as a circle with a tyrosine residue, Y) is translocated via the type IV secretion system, represented as channels. (B) Black triangles represent cellular kinases that phosphorylate CagA on the tyrosine residue (the phosphate group is indicated by *P). CagA triggers signals that induce pedestal formation. In C, the pedestal elongates.
Discussion
Injection of virulence proteins via type III secretion machineries into the host cell is well established (18); however, whether this happens also in type IV secretion systems was unclear. Herein, we have demonstrated that CagA is actively translocated through the type IV secretion system into eukaryotic cells. Type III secretion systems are dedicated engines for intracellular delivery of bacterial proteins. They originated by duplication of the flagellar gene cluster and specialized into an injection organelle. Similarly, the bacterial type IV secretion systems share relatedness with the conjugative apparatus specializing in nucleoproteins transkingdom injection and in the export of pertussis toxin. In this work, we have described CagA as the first bacterial virulence protein translocated by a type IV secretion system. Thus, type III and type IV secretion systems can be used for translocation of an effector of virulence, suggesting a process of functional convergence of secretion engines in the evolution of pathogens.
Tyrosine or serine-threonine phosphorylation is a basic mechanism of signal transduction in eukaryotic cells and is rare in bacteria (19). In one case, a bacterial protein was shown to be tyrosine-phosphorylated by host kinases on transfer into the host cell membrane: the translocated intimin receptor of enteropathogenic E. coli (20). The translocated IncA of the obligate intracellular pathogen Chlamydia trachomatis is also phosphorylated but on serine-threonine residues (21). Herein, we add CagA of Helicobacter to this small family and show that translocation to eukaryotic cells and tyrosine phosphorylation of bacterial proteins may be more common than anticipated and is not restricted to type III secretion systems. This finding suggests that CagA, thus far considered an immunodominant antigen without apparent function, represents a module inserted by bacteria into the eukaryotic signal transduction pathways, which is likely to play a major role in Hp–host cell interactions and pathogenesis.
Because the cagA knockout and the wild type induce similar levels of IL-8 and NF-κB in tissue culture cells (22), these signaling events are independent and separate from the tyrosine phosphorylation of CagA.
CagA translocation and phosphorylation is followed by appearance of cell-surface protrusions (12), which, in turn, depend on actin polymerization at the cell cortex (23). Actin filament nucleation depends on activated Arp 2/3 complexes that interact with Rho family proteins (e.g., Cdc 42) via WASP-like family proteins (23). The bacterial pathogens Listeria monocytogenes and Shigella flexneri trigger actin polymerization either by a WASP-like bacterial product (Listeria ActA) or by stimulating the N-WASP recruitment (Shigella IcsA; refs. 24 and 25). Recent results on enteropathogenic E. coli-induced pedestals have shown activation of the Chp small GTPase and WASP recruitment (26). The data available for three different bacterial species suggest that (i) CagA may mimic WASP, (ii) CagA may recruit WASP through a specific small GTPase, (iii) CagA may activate the system inducing conformational changes in the Arp 2/3 complex after binding the N-WASP-like protein CA region, and (iv) there may be an alternative mechanism, not yet known, leading to the same effect.
Therefore, exploration of the molecular events involved in CagA-mediated signaling is likely to unravel hidden features of bacterial–host interactions.
Acknowledgments
We gratefully acknowledge B. Finlay (University of British Columbia), S. Lory (University of Washington), and C. Montecucco (University of Padova) for helpful discussions; R. DeVinney (University of British Columbia) for providing protocols and detailed comments; and S. Falkow, N. Salama, and T. McDaniel (Stanford University) for comments at various stages of the work. We are indebted to L. Bini and V. Pallini (University of Siena) for the precious help with two-dimensional gel electrophoresis. We would like to thank R. James (University of Leicester) and N. Figura (University of Siena) for supplying the monoclonal antibody LDS 56 and the human anti-serum. We gratefully thank M. Marchetti for the gift of unpublished cag isogenic mutants and the other members of our laboratory, S. Censini, S. Guidotti, F. Bagnoli, and A. Muzzi, for technical help and for discussions. R. Beltrami supported us with the computer system. Finally, we are deeply grateful to N. Valiante for critical reading of the manuscript, C. Mallia for editorial assistance, and G. Corsi for the artwork. M.S. is supported by a Marie Curie Fellowship from the European Commission.
Abbreviations
- Hp
Helicobacter pylori
- PTYR
tyrosine-phosphorylated target
- HGFR
hepatocyte growth factor receptor
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Covacci A, Telford J L, Del Giudice G, Parsonnet J, Rappuoli R. Science. 1999;284:1328–1333. doi: 10.1126/science.284.5418.1328. [DOI] [PubMed] [Google Scholar]
- 2.Papini E, Satin B, Bucci C, de Bernard M, Telford J L, Manetti R, Rappuoli R, Zerial M, Montecucco C. EMBO J. 1997;16:15–24. doi: 10.1093/emboj/16.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papini E, Gottardi E, Satin B, de Bernard M, Massari P, Telford J, Rappuoli R, Sato S B, Montecucco C. J Med Microbiol. 1996;45:84–89. doi: 10.1099/00222615-45-2-84. [DOI] [PubMed] [Google Scholar]
- 4.Alm R A, Ling L S, Moir D T, King B L, Brown E D, Doig P C, Smith D R, Noonan B, Guild B C, deJonge B L, et al. Nature (London) 1999;397:176–180. doi: 10.1038/16495. [DOI] [PubMed] [Google Scholar]
- 5.Tomb J F, White O, Kerlavage A R, Clayton R A, Sutton G G, Fleischmann R D, Ketchum K A, Klenk H P, Gill S, Dougherty B A, et al. Nature (London) 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 6.Marchetti M, Arico B, Burroni D, Figura N, Rappuoli R, Ghiara P. Science. 1995;267:1655–1658. doi: 10.1126/science.7886456. [DOI] [PubMed] [Google Scholar]
- 7.Rossi G, Rossi M, Vitali C G, Fortuna D, Burroni D, Pancotto L, Capecchi S, Sozzi S, Renzoni G, Braca G, et al. Infect Immun. 1999;67:3112–3120. doi: 10.1128/iai.67.6.3112-3120.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yokota K, Kurebayashi Y, Takayama Y, Hayashi S, Isogai H, Isogai E, Imai K, Yabana T, Yachi A, Oguma K. Microbiol Immunol. 1991;35:475–480. doi: 10.1111/j.1348-0421.1991.tb01577.x. [DOI] [PubMed] [Google Scholar]
- 9.Euler A R, Zurenko G E, Moe J B, Ulrich R G, Yagi Y. J Clin Microbiol. 1990;28:2285–2290. doi: 10.1128/jcm.28.10.2285-2290.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Censini S, Lange C, Xiang Z, Crabtree J E, Ghiara P, Borodovsky M, Rappuoli R, Covacci A. Proc Natl Acad Sci USA. 1996;93:14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma S A, Tummuru M K, Blaser M J, Kerr L D. J Immunol. 1998;160:2401–2407. [PubMed] [Google Scholar]
- 12.Segal E D, Falkow S, Tompkins L S. Proc Natl Acad Sci USA. 1996;93:1259–1264. doi: 10.1073/pnas.93.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Segal E D, Lange C, Covacci A, Tompkins L S, Falkow S. Proc Natl Acad Sci USA. 1997;94:7595–7599. doi: 10.1073/pnas.94.14.7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Covacci A, Censini S, Bugnoli M, Petracca R, Burroni D, Macchia G, Massone A, Papini E, Xiang Z, Figura N, et al. Proc Natl Acad Sci USA. 1993;90:5791–5795. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Copass M, Grandi G, Rappuoli R. Infect Immun. 1997;65:1949–1952. doi: 10.1128/iai.65.5.1949-1952.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bini L, Magi B, Marzocchi B, Cellesi C, Berti B, Raggiaschi R, Rossolini A, Pallini V. Electrophoresis. 1996;17:612–616. doi: 10.1002/elps.1150170333. [DOI] [PubMed] [Google Scholar]
- 17.Segal E D, Cha J, Lo J, Falkow S, Tompkins L S. Proc Natl Acad Sci USA. 1999;96:14559–14564. doi: 10.1073/pnas.96.25.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hueck C J. Microbiol Mol Biol Rev. 1998;62:379–433. doi: 10.1128/mmbr.62.2.379-433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ilan O, Bloch Y, Frankel G, Ullrich H, Geider K, Rosenshine I. EMBO J. 1999;18:3241–3248. doi: 10.1093/emboj/18.12.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kenny B, DeVinney R, Stein M, Reinscheid D J, Frey E A, Finlay B B. Cell. 1997;91:511–520. doi: 10.1016/s0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- 21.Bannantine J P, Stamm W E, Suchland R J, Rockey D D. Infect Immun. 1998;66:6017–6021. doi: 10.1128/iai.66.12.6017-6021.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crabtree J E, Covacci A, Farmery S M, Xiang Z, Tompkins D S, Perry S, Lindley I J, Rappuoli R. J Clin Pathol. 1995;48:41–45. doi: 10.1136/jcp.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Welch M D. Trends Cell Biol. 1999;9:423–427. doi: 10.1016/s0962-8924(99)01651-7. [DOI] [PubMed] [Google Scholar]
- 24.Loisel T P, Boujemaa R, Pantaloni D, Carlier M F. Nature (London) 1999;401:613–616. doi: 10.1038/44183. [DOI] [PubMed] [Google Scholar]
- 25.Egile C, Loisel T P, Laurent V, Li R, Pantaloni D, Sansonetti P J, Carlier M F. J Cell Biol. 1999;146:1319–1332. doi: 10.1083/jcb.146.6.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalman D, Weiner O D, Goosney D L, Sedat J W, Finlay B B, Abe A, Bishop J M. Nat Cell Biol. 1999;1:389–391. doi: 10.1038/14087. [DOI] [PMC free article] [PubMed] [Google Scholar]