Abstract
In Aplysia, three distinct phases of memory for sensitization can be dissociated based on their temporal and molecular features. A single training trial induces short-term memory (STM, lasting <30 min), whereas five trials delivered at 15-min intervals induces both intermediate-term memory (ITM, lasting >90 min) and long-term memory (LTM, lasting >24 h). Here, we explore the interaction of amount and pattern of training in establishing ITM and LTM by examining memory for sensitization after different numbers of trials (each trial = one tail shock) and different patterns of training (massed vs. spaced). Under spaced training patterns, two trials produced STM exclusively, whereas four or five trials each produced both ITM and LTM. Three spaced trials failed to induce LTM but did produce an early decaying form of ITM (E-ITM) that was significantly shorter and weaker in magnitude than the late-decaying ITM (L-ITM) observed after four to five trials. In addition, E-ITM was induced after three trials with both massed and spaced patterns of training. However, L-ITM and LTM after four to five trials require spaced training: Four or five massed trials failed to induce LTM and produced only E-ITM. Collectively, our results indicate that in addition to three identified phases of memory for sensitization—STM, ITM, and LTM—a unique temporal profile of memory, E-ITM, is revealed by varying either the amount or pattern of training.
The search for the cellular and molecular basis of memory has been significantly enhanced by the elucidation of general principles of memory formation across diverse species. For example, memory retention is highly sensitive not only to the total amount of training but also to the pattern of trials used during training. In particular, in a variety of tasks across species ranging from invertebrates to humans, training trials distributed over time (spaced training) typically lead to superior retention compared with training in which trials are presented with little or no rest interval (massed training) (Ebbinghaus 1885; Carew et al. 1972; Salafia et al. 1973; Lefebvre and Sabourin 1977; Fanselow and Tighe 1988; Tully et al. 1994; Hermitte et al. 1999; Muzzio et al. 1999; Menzel et al. 2001). Perhaps the most interesting feature of the superiority of spaced over massed training is that it is not a general effect for all temporal domains of memory but rather appears to become more pronounced with longer-lasting memories (e.g., Carew et al. 1972; Tully et al. 1994; Menzel 2001). Thus, an understanding of the behavioral, cellular, and molecular factors contributing to the massed versus spaced effect could yield considerable insight into the overall organization of multiple phases of memory.
Despite the widespread conservation of the massed versus spaced effect in behavioral studies of memory, some of the key cellular and molecular features that contribute to this feature of memory formation are only now beginning to be understood. For example, induction of a transcriptional activator isoform of cAMP response element binding protein (CREB) in Drosophila enhances long-term memory (LTM) for olfactory conditioning after massed patterns of training, which normally do not induce LTM; conversely, induction of a dominant-negative transcriptional repressor CREB isoform impairs LTM after spaced training (Yin et al. 1994, 1995). These findings led to the hypothesis that massed versus spaced training effects may depend on differential decay kinetics of transcriptionally activating versus transcriptionally repressing isoforms of CREB, allowing for the presumably slower decaying CREB activator to build up across spaced (but not massed) training trials (Yin et al. 1995; see also, Smolen et al. 1998). In addition to the possible role of transcription factors such as CREB, other evidence has indicated that differential activation of signaling molecules upstream from CREB may also contribute to the massed versus spaced effect. For example, Wu et al. (2001) have shown that four spaced depolarizations (3 min each) of cultured hippocampal neurons lead to a persistent phosphorylation (and, presumably, activation) of MAP kinase, whereas continuous (massed) depolarization for 12 min does not. Thus, the temporal dynamics of MAP kinase activation in the hippocampus seem to be highly dependent on the pattern of stimulation. In addition, using an analog of classical conditioning in Hermissenda, Muzzio et al. (1999) provide evidence that the accumulation of intracellular calcium and preferential activation of protein phosphatases by massed relative to spaced training may also contribute to massed versus spaced effects, indicating that massed training may, in some cases, generate processes that compete with memory formation. These studies highlight the number of potential molecular substrates that may contribute to superior memory retention after spaced relative to massed training. Moreover, they indicate that the relative superiority of spaced training may reflect an increased ability of spaced training to engage constructive memory processes or an increased propensity of massed training to engage processes that interfere with normal memory formation.
One model system that is well suited for studying the effects of patterning on memory formation is the marine mollusk Aplysia. Aplysia has proven particularly valuable for the cellular and molecular analysis of behavioral sensitization, an elementary form of nonassociative learning in which behavioral responses to a weak stimulus increase in magnitude and duration after the presentation of a noxious stimulus. In Aplysia, sensitization is most often assessed by the degree to which defensive reflexes, such as tail-elicited siphon withdrawal (T-SW), become enhanced after noxious stimuli such as tail shock. Considerable evidence indicates that facilitation of sensory neuron to motor neuron (SN-MN) synapses by serotonin (5HT), a neuromodulator released in the CNS after tail shock (Marinesco and Carew, 2002; see also Levenson et al. 1999), is an important cellular mechanism contributing to behavioral sensitization. 5HT can induce three temporally and mechanistically distinct phases of SN-MN synaptic facilitation. A single 5HT pulse induces short-term facilitation (STF) lasting <30 min, whereas five spaced pulses of 5HT induce both intermediate-term facilitation (ITF) lasting 1–3 h and long-term facilitation (LTF) lasting >24 h. Each of these phases also has unique macromolecular synthesis requirements for their induction: STF requires neither protein nor RNA synthesis, ITF requires protein but not RNA synthesis, and LTF requires both (Montarolo et al. 1986; Mercer et al. 1991; Emptage and Carew, 1993; Ghirardi et al. 1995; Mauelshagen et al. 1996, 1998; Sutton and Carew 2000). Moreover, at tail SN-MN synapses, ITF declines completely to baseline by 3 h and LTF first emerges 10–15 h after 5HT, demonstrating that these phases of synaptic facilitation in the CNS are temporally discontinuous (Mauelshagen et al. 1996).
These features of distinct phases of 5HT-induced synaptic facilitation are also reflected in distinct phases of memory for sensitization induced by tail shock. Whereas a single tail shock induces short-term memory (STM) for sensitization lasting minutes, repeated spaced shocks produce LTM for sensitization lasting days to weeks (Frost et al. 1985; Scholz and Byrne 1987; Castellucci et al. 1989; Goldsmith and Byrne 1993; Cleary et al. 1998; Levenson et al. 2000; Sutton et al. 2001a; see also, Carew et al. 1971; Pinsker et al. 1973). Recently, we distinguished a third phase of memory for sensitization, intermediate-term memory (ITM), that is induced by repeated spaced shocks and lasts 1–3 h after training (Sutton et al. 2001a). These three phases of memory for sensitization in Aplysia can be mechanistically distinguished in a similar fashion as their synaptic counterparts: STM requires neither protein nor RNA synthesis, ITM requires protein but not RNA synthesis, and LTM requires both (Castellucci et al. 1989; Levenson et al. 2000; Sutton et al. 2001a). ITM and LTM can also be distinguished in the same animals based on the temporal dynamics of memory for sensitization after training: five spaced tail shocks induces ITM that decays completely to baseline by about 3 h, several hours before the emergence of LTM (Sutton et al. 2001a). The lack of temporal overlap in these mechanistically distinct phases of memory is experimentally advantageous, allowing for an unambiguous means for studying ITM and LTM independently.
In this study, we undertook a detailed analysis of the training parameters required for the induction of ITM and LTM. We found that LTM (20–24 h after training) requires multiple, spaced (temporally distributed) trials for induction. We also found that ITM has two distinct components: an early decaying (E-ITM) phase and a late-decaying (L-ITM) phase. L-ITM (lasting >90 min), like LTM, requires multiple spaced trials for induction. E-ITM (lasting <75 min) also requires multiple training trials but can be induced by either massed or spaced patterns of training. Moreover, amounts of training that are sufficient for the induction of L-ITM and LTM when given in a spaced pattern produce only E-ITM when delivered as a massed pattern. Collectively, our results indicate that in addition to three identified phases of memory for sensitization—STM, ITM, and LTM—a unique temporal profile of memory, E-ITM, is revealed by varying either the amount or pattern of training.
Some of the results in this paper have been previously presented in abstract form (Carew et al. 2001).
RESULTS
Intermediate- and Long-Term Memory for Sensitization Require Multiple Training Trials
Different amounts of training can induce three distinct phases of memory for sensitization: a single-tail shock produces STM for sensitization (lasting <30 min), whereas five spaced tail shocks (delivered at 15-min intervals) induces both ITM (>90 min) and LTM (>24 h) for sensitization (Sutton et al. 2001a). Whereas the temporal dynamics of memory for sensitization after each of these training regimens (one shock vs. five spaced shocks) were determined empirically, the specific features of training (e.g., trial number vs. trial pattern) that are critical for the induction of ITM or LTM are not clear. The goal of the experiments in the present study was to determine the training parameters that are critical for the induction of these distinct phases of memory for sensitization.
We know that five spaced training trials produce both ITM and LTM for sensitization (Sutton et al. 2001a). In the first experiment, we asked whether ITM and LTM depend strictly on the integration of five training trials or if time alone between the first and last of two training trials (which bracket the five-shock pattern) is sufficient for their induction. To explore this question, we compared the duration of memory for sensitization after training with either five spaced tail shocks (15 min ITI) or the first and last shocks alone (60 min ITI). In this and all subsequent experiments, sensitization is indicated by a significant post-training increase (relative to baseline) in the duration of siphon withdrawal elicited by tactile stimulation of the tail.
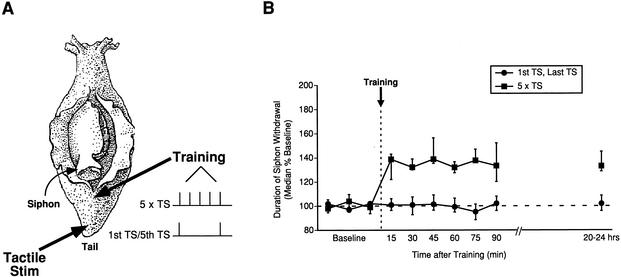
Consistent with our previous findings (Sutton et al. 2001a), animals receiving five spaced shocks (n = 8) showed both ITM for sensitization of T-SW lasting >90 min (Wilcoxon tests, p < 0.05 at all time points), as well as LTM for sensitization the following day (p < 0.05; Fig. 1B). In animals receiving the first and fifth shock only (n = 8), the duration of T-SW did not significantly differ from baseline at any of the post-training time-points (Friedman test, NS). Thus, this group showed neither ITM nor LTM for sensitization. Surprisingly, this group also did not show STM for sensitization (at 15 min), which would be expected following a single shock (Sutton et al. 2001a). Thus, the induction of STM by a single shock can apparently be inhibited by a previous shock delivered 60 min earlier. However, this inhibitory interaction between two trials is pattern-specific, as two shocks delivered 15 min apart produce significant STM for sensitization (see below). This intriguing effect awaits further study. Nonetheless, these results show that the induction of ITM and LTM for sensitization requires the integration of multiple training trials over time rather than the additive effect of two trials that bracket the five-trial training pattern.
Figure 1.
Intermediate- and long-term memory (ITM and LTM) for sensitization require multiple training trials. (A) Diagram of an Aplysia depicting the sites on the tail used for training and testing. Independent groups of animals received either five tail shocks delivered at 15-min intervals (5 × TS; n = 8) or the first and last shocks alone (1st TS/5th TS; n = 8). (B) Median (±interquartile range) duration of T-SW (normalized to baseline) before and at various times after training. In this and all subsequent figures, the horizontal dashed line indicates baseline and the vertical dashed line indicates training.
Memory for Sensitization Varies with the Amount of Training
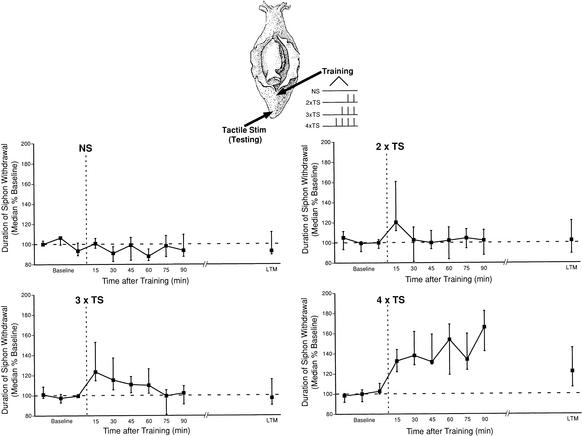
Given that both ITM and LTM require multiple training trials, we next determined the amount of training required for the induction of each phase. To examine this issue, we trained animals with either no shocks (controls) or two, three, or four shocks all with the same pattern (15 min ITI between shocks) and examined the time course of memory for sensitization in the short-term (15 min), intermediate-term (30–90 min), and long-term (20–24 h) time domains. The training of all shocked groups was coordinated so that the last trial was temporally aligned for all groups. As shown in Figure 2, the duration of T-SW in control animals (no shock; n = 7) was stable across the extent of the experiment (Friedman test, NS). Unlike two shocks delivered with a 60-min ITI, two shocks delivered 15 min apart (n = 8) produced STM for sensitization (15-min post-test, Wilcoxon test, p < 0.05); that decayed completely by 30 min and remained at baseline for all post-tests in the intermediate-term and long-term ranges (Wilcoxon tests, NS). This temporal profile is identical to that observed after a single shock, which also produces STM for sensitization that decays completely by 30 min (Sutton et al. 2001a). In contrast, four shocks (n = 8) produced robust ITM (15–90 min, Wilcoxon tests, all p < 0.05), as well as LTM (p < 0.05) 20–24 h after training, a time course of memory for sensitization indistinguishable from that produced by five shocks (see Fig. 1).
Figure 2.
Memory for sensitization varies with the amount of training. Top, diagram of an Aplysia illustrating the training and testing procedures. Independent groups of animals received no shocks (controls; n = 7), two tail shocks (2 × TS; n = 8), three tail shocks (3 × TS; n = 10), or four tail shocks (4 × TS; n = 8), each with an ITI of 15 min. Data are expressed as in Figure 1.
The duration of memory for sensitization after three shocks (n = 10) was somewhat intermediate between that of one to two shocks and four to five shocks. Memory for sensitization after three shocks extended beyond STM, lasting 60 min (15–60 min, Wilcoxon tests, p < 0.05). However, unlike the late-decaying ITM (L-ITM, lasting >90 min) observed after four or five spaced shocks, memory for sensitization after three shocks was weaker in magnitude from 30–60 min after training and decayed to baseline by 75 min (75–90 min, NS). Moreover, three shocks also failed to produce significant LTM 20–24 h following training. Thus, three spaced shocks are insufficient to induce LTM and appear to produce an early decaying form of ITM (E-ITM) lasting <75 min.
Intermediate- and Long-Term Memory for Sensitization Depend on the Pattern of Training
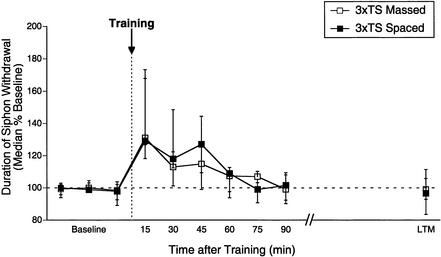
Having determined the relationship between trial number and duration of memory for sensitization with a given pattern of training, we next examined how the pattern of a given amount of training influenced memory for sensitization. Specifically, a fixed number of training trials were delivered in one of two temporal patterns: a massed pattern, with a 1-sec rest interval between shocks or a spaced pattern, with a 15-min rest interval between shocks. We began by examining whether the pattern of training influenced E-ITM observed after three spaced shocks (Fig. 3). As before, three spaced shocks (n = 13) produced a persistent memory for sensitization (15–45 min, Wilcoxon tests, p < 0.05) that decayed to baseline by 60–75 min (60–90 min, NS). The magnitude and time course of sensitization after three massed trials (n = 13) was virtually identical to that observed after three spaced trials (30–45 min, NS, Mann Whitney U), with significant memory for sensitization persisting for at least 45 min following training (15–45 min, Wilcoxon tests, p < 0.05) but not beyond 60–75 min (60–90 min, NS). Moreover, three shocks failed to induce LTM (20–24 h, NS) under either spaced or massed patterns of training. Thus, the duration of memory for sensitization after three shocks is insensitive to the pattern of training.
Figure 3.
Memory for sensitization after three trials is insensitive to the pattern of training. Independent groups received three tail shocks with either a 1-sec (massed) or 15-min (spaced) rest interval between shocks. Data are expressed as in Figure 1.
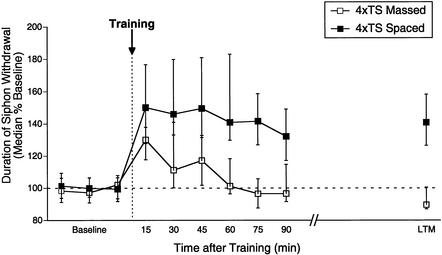
We next explored whether the induction of either L-ITM or LTM observed after four to five spaced shocks was influenced by the pattern of training. As shown in Figure 4, the duration of memory for sensitization after four shocks was strongly dependent on the pattern of training. Four spaced shocks (n = 12), as before, produced robust ITM lasting >90 min (15–90 min, p < 0.05). In contrast, memory for sensitization after four massed trials (n = 12) was significantly shorter than for spaced trials, persisting for <60 min (15–45 min, p < 0.05; 60–90 min, NS), and was significantly weaker in magnitude from 30–45 min relative to spaced training (Mann Whitney U, p < 0.05). Moreover, whereas spaced training with four shocks produced significant LTM for sensitization (20–24 h, p < 0.05), massed training did not (20–24 h, NS). These results show that four spaced trials induce both L-ITM and LTM, whereas four massed trials produce only E-ITM.
Figure 4.
Memory for sensitization after four trials is diminished by massed patterns of training. Independent groups received four tail shocks with either a 1-sec (massed) or 15-min (spaced) rest interval between shocks. Data are expressed as in Figure 1.
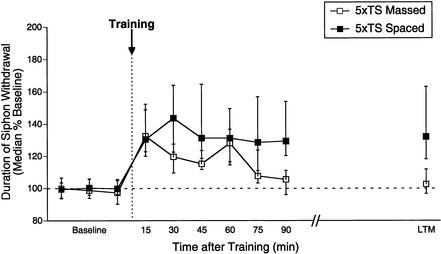
We found very similar results when we examined massed versus spaced training with five shocks (Fig. 5). Spaced training (n = 13) produced L-ITM for sensitization lasting >90 min (15–90 min, p < 0.05), as well as significant LTM for sensitization the following day (20–24 h, p < 0.05). In contrast, memory for sensitization after massed training (n = 15) was significantly shorter, persisting for <75 min (15–60 min, p < 0.05; 75–90 min, NS) and no LTM was observed (20–24 h, NS). Moreover, the magnitude of sensitization from 30–60 min after training was significantly weaker in massed trained animals (Mann Whitney U, p < 0.05), again revealing a significant attenuation of ITM for massed training. These results indicate that, unlike E- ITM observed after three shocks, which is insensitive to pattern (Fig. 3), both L-ITM and LTM for sensitization observed after four or five shocks are highly sensitive to the pattern of training.
Figure 5.
Memory for sensitization after five trials is diminished by massed patterns of training. Independent groups received five tail shocks with either a 1-sec (massed) or 15-min (spaced) rest interval between shocks. Data are expressed as in Figure 1.
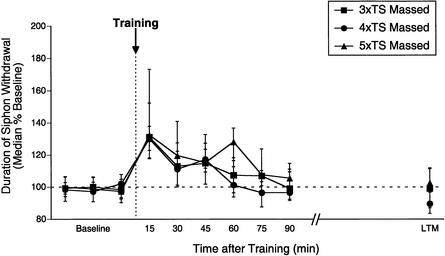
Our data indicate that the effect of training pattern on memory duration is also dependent on the amount of training. Thus, the magnitude and duration of memory for sensitization after three shocks were virtually identical regardless of training pattern (massed vs. spaced), whereas memory duration following four or five shocks was significantly diminished after massed relative to spaced training. From another perspective, our data also indicate that under conditions of massed training, additional training trials are ineffective in enhancing memory for sensitization in the intermediate-term or long-term domains. In Figure 6, we overlay memory for sensitization after massed training with either three, four, or five shocks. The magnitude and duration of memory for sensitization after massed training are very similar regardless of training amount, demonstrating that additional training trials are ineffective in prolonging memory for sensitization after massed training. Moreover, the profile of sensitization after massed training is also very similar to the magnitude and duration of memory for sensitization after three spaced shocks (Fig. 3), indicating that these temporal dynamics may reflect a phase of memory distinct from both STM (<30 min) and L-ITM (>90 min; see Fig. 7).
Figure 6.
Increasing amounts of training fail to prolong memory for sensitization after massed training. Median (±interquartile range) duration of T-SW (normalized to baseline) of massed trained groups receiving either three, four, or five tail shocks overlapped for comparison.
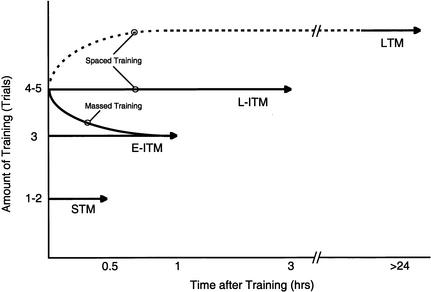
Figure 7.
Four distinct temporal domains of memory for sensitization can be distinguished by the amount and/or pattern of training. Schematic summary of our results illustrating the temporal limit (arrowheads) of memory for sensitization after different amounts of training. One to two trials produce short-term memory (STM), lasting <30 min; three spaced trials or any amount of massed training produce only an early decaying phase of intermediate-term memory (E-ITM), lasting ∼60 min; four to five spaced trials produce both a late-decaying phase of intermediate-term memory (L-ITM), lasting >90 min (but <3 h), and long-term memory (LTM), lasting >24 h. Dashed line indicates that LTM expression is delayed for >6 h after training and is temporally discontinuous with L-ITM expression (see Sutton et al. 2001a).
DISCUSSION
In this paper, we have examined the interaction of two training parameters—amount and pattern of training—in the induction of specific phases of memory for sensitization. Our results (summarized in Fig. 7) contribute to a large literature indicating that memory can be strongly affected by both the amount and pattern of training. In this study, however, we have exploited the added advantage that the parameters of amount and pattern of training could be examined in the context of temporally and mechanistically defined memory phases. Our results indicate that the maximal expression of ITM, as well as the induction of LTM, is similarly dependent on both the amount and pattern of training trials. Moreover, we observed a differential influence of training pattern on memory depending on the amount of trials used for training. These features of ITM and LTM at the behavioral level allow us to define constraints on the possible cellular and/or molecular mechanisms of each of these phases of memory.
Amount and Pattern of Training in Long-Term Memory
The most definitive effects of training amount and training pattern on memory that we observed were in the long-term domain 20–24 h after training. We found that the induction of LTM for sensitization requires multiple training trials (e.g., five), rather than two trials with a prolonged spacing interval that bracketed the five-shock pattern (Fig. 1). Under our training conditions (e.g., shock duration and intensity) with a 15-min ITI, we found that four trials was the minimum amount of training sufficient for LTM induction (Fig. 2). Thus, our results indicate that the induction of LTM is favored by the integration of multiple cellular and molecular events (each presumably driven at least in part by 5HT) occurring within a limited temporal domain (15-min ITI), rather than an interaction of two such events occurring over a prolonged temporal domain (60-min ITI). When the amount of training was held constant and the pattern was varied, we found that massed training was ineffective in producing LTM regardless of training amount (up to five shocks). This result is in agreement with previous research showing that massed training is less effective than spaced training in producing LTM for habituation (Carew et al. 1972), as well as a recent report of similar findings for LTM for sensitization of T-SW (Wainwright et al. 2002). The differential effectiveness of spaced relative to massed training trials in the induction of LTM at the behavioral level is similarly reflected in SN-MN synaptic facilitation at the cellular level, where spaced applications of 5HT are significantly more effective in inducing LTF than are massed applications (Mauelshagen et al. 1998).
Under our training conditions, four trials were the minimum required to induce LTM when those trials are each spaced by 15 min. It is still unclear, however, whether four trials will prove to be a minimum under all training conditions. For example, it is possible that increasing the intensity of the shock or manipulating the spacing interval between shocks will reduce (or increase) the number of trials required for the induction of LTM. It is also unknown whether any amount of massed training is capable of inducing LTM. In this regard, at tail SN-MN synapses, Mauelshagen et al. (1998) observed LTF in a subset of preparations (∼40%) treated with 25-min massed exposures of 5HT; and LTF can be consistently induced by longer (90 min) massed 5HT exposures (Emptage and Carew 1993; Zhang et al. 1997), raising the possibility that more extensive amounts of massed training may be effective for the induction of LTM for sensitization. These important issues await further research.
Amount and Pattern of Training in Intermediate-Term Memory
Two distinct effects of training amount on the duration of ITM were observed. First, training with two shocks spaced by 15 min produced a short-lasting temporal profile that is highly similar to that observed after a single shock (Sutton et al. 2001a). Moreover, a corresponding temporal profile of SN-MN synaptic facilitation (lasting <30 min) is similarly observed after one to four spaced pulses of 5HT (Mauelshagen et al. 1996). In addition, neither STF at tail SN-MN synapses after a single 5HT pulse nor STM after a single tail shock depend on new protein synthesis (Montarolo et al. 1986; Sherff and Carew 1999; Sutton and Carew 2000; Sutton et al. 2001a). In contrast, ITM (lasting >90 min) after five tail shocks is protein synthesis-dependent (Sutton et al. 2001a), and in the present study, a highly similar temporal profile of sensitization was produced by four spaced shocks. The temporal dynamics of memory for sensitization after four to five shocks also parallel the dynamics of tail SN-MN synaptic facilitation after five pulses of 5-HT, which persists for >90 min and requires new protein synthesis (Mauelshagen et al. 1996; Sutton and Carew 2000; see also Ghirardi et al. 1995 for a similar finding with cultured SN-MN synapses).
The second effect of training amount on the duration of ITM was observed after three spaced shocks, where we found a persistent memory for sensitization enduring beyond 30 min, but significantly diminished in both magnitude and duration (E-ITM, lasting <75 min) relative to L-ITM observed after four to five shocks. As will be discussed below, the recapitulation of this diminished temporal profile across other training protocols indicates that it may reflect a unique early phase of ITM interposed between STM and L-ITM.
We also observed two distinct effects of training pattern on ITM. First, unlike the emergence of LTM after four to five shocks, the diminished form of ITM (E-ITM) after three shocks was induced both by spaced and massed patterns of training. Second, both the magnitude and duration of ITM after four to five shocks were diminished following massed relative to spaced patterns of training to a level comparable to that seen after three shocks (massed or spaced; see Fig. 7). These features indicate the possibility that the early phase of ITM (E-ITM) revealed under these different training conditions reflects a phase of memory distinct from both STM and L-ITM. Specifically, unlike STM, E-ITM persists >30 min after training and requires multiple training trials for its induction. Also, unlike L-ITM observed after four to five spaced shocks, E-ITM decays completely by 75 min, is insensitive to the pattern of training, and is not associated with the emergence of LTM 20–24 h after training.
A Cellular Correlate of E-ITM?
Mauelshagen et al. (1996) described a rapid transition from STF (lasting <30 min) to ITF (lasting >90 min) at tail SN-MN synapses as a function of the number of pulses of 5HT applied to the bath. Specifically, one to four spaced pulses all produced STF that was similar in both magnitude and time-course (lasting <30 min in all cases), whereas five pulses of 5HT produced robust ITF lasting >90 min. Importantly, they did not observe a time course of synaptic facilitation that appeared intermediate between STF and ITF. Thus, the reduced ITM (E-ITM) we observed after three spaced shocks (or after massed training) was not predicted by the time course of SN-MN facilitation using varying numbers of 5HT pulses (Mauelshagen et al. 1996). However, other experiments do provide some evidence for this diminished intermediate-term profile at the cellular level. For example, Ghirardi et al. (1995), in addition to initially identifying and characterizing ITF at cultured SN-MN synapses, also described a phase of synaptic facilitation in the short- to intermediate-term domain that appeared unique from both STF and ITF. Whereas Mauelshagen et al. (1996) varied the number of 5HT pulses at a given concentration (50 μM), Ghirardi et al. (1995) systematically varied the concentration of 5HT leaving the number and pattern of 5HT pulses constant (five spaced pulses in all cases). They found that repeated application of low concentrations of 5HT (1–10 nM) induced a protein synthesis-independent form of synaptic facilitation, which they referred to as a “persistent form of STF” that extended beyond 30 min but was distinct from ITF (observed at higher concentrations of 5HT, 50 nM–10 μM) in four important respects: (1) it was weaker in magnitude, (2) it was less persistent, (3) it was not associated with the emergence of LTF 24 h later, and (4) it did not require protein synthesis. Thus, many of the features of the “persistent STF” described by Ghirardi and colleagues are also evident in behaviorally expressed E-ITM that we describe in the present study. Relative to L-ITM after four to five spaced shocks, E-ITM is weaker in magnitude, less persistent, and in none of the cases in which we observed E-ITM, did we subsequently find significant LTM for sensitization. These similarities raise the possibility that E-ITM reflects, in part, the unique phase of “persistent STF” described by Ghirardi et al. (1995). However, it remains to be determined whether the early phase of ITM we describe is independent of new protein synthesis.
A second example of the E-ITM profile at the cellular level comes from studies of massed versus spaced applications of 5HT in the induction of ITF and LTF at tail SN-MN synapses in the intact CNS (Mauelshagen et al. 1998). In this study, SN-MN facilitation in the intermediate-term (0.5–1 h) decayed significantly more rapidly after a continuous 25-min 5HT exposure (massed training) relative to five spaced 5-min pulses of 5HT. Moreover, preparations treated with massed 5HT, as a group, failed to show significant LTF 20–22 h after treatment, whereas significant LTF was observed after spaced 5HT exposure. Interestingly, in a subset of preparations treated with massed 5HT (∼40%) that appeared to show LTF, the SN-MN facilitation in the intermediate-term domain appeared greater in magnitude and duration relative to massed 5HT-treated preparations that failed to show LTF. These characteristics of tail SN-MN synaptic facilitation appear similar to the features of memory for sensitization after massed or spaced patterns of training observed in the present study. Thus, ITM is significantly weaker in magnitude and less prolonged after massed relative to spaced training, and LTM appears only associated with the induction of L-ITM (lasting >90 min) produced by spaced training. This relationship is especially intriguing given the fact that even L-ITM decays completely several hours before the onset of LTM. The close association between L-ITM and LTM also raises the question of whether L-ITM is a prerequisite for the induction of LTM.
The studies summarized above provide some evidence for a cellular analog of E-ITM for sensitization. However, we should note that we could distinguish E-ITM by varying the number of training trials (shocks), whereas Mauelshagen et al. (1996) found no evidence for a diminished form of ITF at tail SN-MN synapses by varying the number of applied 5HT pulses. But only one concentration of bath-applied 5HT was examined by Mauelshagen et al. (1996), and the spatiotemporal pattern of 5HT release following tail shock is likely to be significantly different than bath-applied 5HT pulses used in cellular studies. Indeed, recent chronoamperometric measurements of 5HT release in the Aplysia CNS after tail nerve shock support this idea (Marinesco and Carew 2002). Future research using chronoamperometry will help in identifying experimental parameters for use in cellular analyses that more accurately capture the features of 5HT release that occurs with behavioral training. In so doing, such studies will allow for a more rigorous examination of the relationship between different phases of memory for sensitization and their putative cellular mechanisms.
Multiphasic Organization of Memory Processing
Although distinctions are often drawn between STM and LTM, evidence in a number of systems has emerged that this two-phase notion of memory does not adequately capture the features of memory retention across all temporal domains. Rather, several lines of evidence indicate that memory retention is composed of a number of additional phases that are fundamentally distinct. For example, in addition to memory for sensitization, the temporal dynamics of memory for learning about inedible foods in Aplysia can also be separated into at least four phases by varying the training parameters (Botzer et al. 1998). In fact, a number of studies in a range of species have shown apparent discontinuities in memory retention across temporal domains that may reflect a transition between distinct memory phases (Kamin 1957, 1963; Riege and Cherkin 1971; Sanders and Barlow 1971; Tallarico 1973; Gibbs and Ng 1979; Rosenzweig et al. 1993; Izquierdo et al. 1998; Ploner et al. 1998; Gerber and Menzel 2000; Sutton et al. 2001a). In other cases, the temporal dynamics of memory appear continuous, but detailed mechanistic analyses have revealed multiple overlapping memory phases. For example, using a genetic approach in Drosophila, it has been possible to dissect at least four phases of memory (Tully et al. 1990, 1994) from an overall retention curve that appears continuous. Moreover, pharmacological disruption of memory retention in Drosophila (Xia et al. 1998), chicks (Gibbs and Ng 1977; Rosenzweig et al. 1993), and rats (Frieder and Allweis 1978, 1982) have all generated strong evidence for distinct intermediate phases of memory. In a similar vein, different forms of neuronal plasticity thought to underlie memory can also be separated into multiple phases by either their time course, mechanism, or both (Montarolo et al. 1986; Frey et al. 1988; Nguyen et al. 1994; Ghirardi et al. 1995; Mauelshagen et al. 1996; Crow et al. 1997; Winder et al. 1998; Crow et al. 1999; Sutton and Carew 2000).
That memory in laboratory animals can show a U-shaped profile over time (often referred to as the “Kamin effect,” after the initial observations by Kamin [1957]) seems somewhat counterintuitive. Nevertheless, this general phenomenon, in which periods of high memory retention are intercalated with periods of lesser retention, is highly conserved across species (see above), including humans (Tallarico 1973; Ploner et al. 1998). Moreover, this effect has been observed in both appetitively motivated (e.g., Gerber and Menzel 2000) and aversively motivated (e.g., Rosenzweig et al. 1993) behaviors, as well as in memory based on nonassociative learning (Sutton et al. 2001a), classical conditioning (e.g., Gerber and Menzel 2000), and instrumental conditioning (e.g., Kamin 1957, 1963). Thus, it is unlikely that the biphasic profile of memory retention observed across all of these studies owes to a specific feature of the task or species examined. But these collective observations raise the following question: Is the biphasic profile expressed at the level of behavioral performance indicative of biphasic memory processing? One line of evidence that argues strongly in favor of this idea is that memory processing before and after the “dip” in retention can be subserved by distinct mechanisms (Rosenzweig et al. 1993; Sutton et al. 2001a). For example, in Aplysia, the dip between ITM and LTM for sensitization is associated with a transition in macromolecular synthesis requirements indicating that the temporal discontinuity, in this case, reflects distinct nonoverlapping memory phases.
Because memories can endure from seconds to years, there are potentially far more numerous memory phases than have been identified at present. In the limit, it is possible that each broad temporal domain (e.g., STM, ITM, and LTM) will be composed of a number of different subphases. Whereas this idea at present is speculative, there is already some evidence in Aplysia that the long-term domain of memory for sensitization may be composed of separable phases. For example, O'Leary et al. (1995) found that the time window during which protein synthesis was required for long-term (24 h) structural remodeling of Aplysia SNs was extended several hours beyond that required for LTF (24 h) at SN-MN synapses described by Montarolo and colleagues (1986). These results raised the possibility that at least one component of LTF (and LTM) may be independent of structural plasticity. Indeed, four spaced blocks of training trials over 1 d fail to produce gross morphological changes in tail SNs (Wainwright et al. 2002), yet this same training procedure induces both LTM (>24 h) for sensitization of T-SW and LTF of tail SN-MN synapses (Cleary et al. 1998). Consistent with these results, recent studies of cultured SN-MN synapses have shown that 5HT-induced LTF evident at 24 h can be divided into two phases: one that extends to at least 72 h and is associated with growth of SN varicosities and one that does not persist beyond 48 h and is independent of growth (Casadio et al. 1999). In the present context, these results have two interesting implications. First, the differentiation of distinct phases of LTF raises the possibility that multiple phases of LTM may be distinguishable based on similar criteria. Given that memory for sensitization can extend for several weeks after training (Pinsker et al. 1973), the long-term temporal domain of memory is sufficiently broad to encompass a number of distinct phases. Indeed, Frost and colleagues (1985) have shown that both the magnitude and duration of memory for sensitization from 1–7 days after training can be progressively enhanced by increasing amounts of training. Moreover, Wainwright et al. (2002) have found that more extensive behavioral training (over 4 days) does produce morphological changes in tail SNs, and the magnitude of LTM (at 24 h) after this extended training regimen is enhanced relative to 1 d of training (which failed to induce morphological changes). An important question now to be resolved is whether the enhanced LTM for sensitization evident after extensive training is strictly dependent on the emergence of this morphological plasticity.
A second implication of these recent studies in Aplysia is that the presence (or absence) of morphological plasticity, and perhaps the extent of structural remodeling, may prove to be a distinguishing feature of independent memory phases across species and/or tasks. This is an important consideration, given that our present ability to dissect memory retention into component phases is constrained by a limited number of defining criteria. For example, whereas macromolecular synthesis requirements can broadly distinguish three distinct phases of memory for sensitization, this single criterion cannot distinguish between memory phases that share these requirements. In this regard, it is noteworthy that both forms of LTF identified by Casadio and colleagues (1999) have important mechanistic features in common (e.g., they are both CREB-dependent) and are only distinguishable at 24 h by the presence or absence of growth. If the extent of morphological plasticity proves to be a “signature” of distinct LTM phases, this would greatly facilitate analyses of the overall organization of memory processing in the long-term domain.
Finally, this general discussion also raises the question of the nature of the criteria necessary to validly distinguish among unique memory phases. Whereas the notion of a “phase” necessarily implies temporal features, the temporal persistence of memory alone does not appear sufficient to characterize a particular phase. For example, Sutton et al. (2001b) have recently identified a second (activity-dependent) form of memory for sensitization in Aplysia that is expressed in the same temporal domain as L-ITM described in the present paper (see also, Sutton et al. 2001a); however, unlike the form of L-ITM examined in the present study, this form of sensitization does not require protein synthesis. In addition, Menzel et al. (2001) have recently shown that 1–2 d memory for conditioning of the proboscis extension reflex in honeybees after spaced training is significantly diminished by protein synthesis inhibitors, whereas 1–2 d memory after massed training is not. Thus, whereas different temporal phases of memory may have distinct mechanisms, time alone is not sufficient to characterize the properties of memory existing in a given temporal domain. Rather, an elucidation of specific molecular signatures appears necessary to fully capture the features of particular forms of memory and their multiphasic organization.
Given the discussion above, do our results warrant the classification of the early decaying intermediate-term profile as a distinct phase of memory? We think not, at least not yet. On the one hand, the E-ITM temporal profile is consistent across several different training protocols, and its induction has rules that are distinct from those for STM (e.g., E-ITM requires multiple training trials) and L-ITM (e.g., sensitivity to training pattern). On the other hand, the cellular and molecular mechanisms critical for the E-ITM profile are still unknown. Thus, we cannot rule out the possibility that it represents an extended form of STM or a diminished form of ITM at the mechanistic level. In our view, distinguishing among memory “phases” is only instructive insofar as it captures fundamental differences in the means by which memory processing is accomplished. Whereas the characteristics of the E-ITM profile certainly indicate that it may reflect a distinct memory phase, only an analysis of its underlying mechanism can provide conclusive evidence necessary for this determination.
MATERIALS AND METHODS
Behavioral Procedures
Wild-caught adult Aplysia californica (supplied by Marinus, Long Beach, CA, or Marine Specimens Unlimited, Pacific Palisades, CA) weighing >250 g were housed and tested in individual chambers. At least 4 d before testing, animals were anesthetized (by cooling), and the parapodia were removed to enhance visibility of the siphon. To prevent excessive inking during training, the accessible extent of the ink gland was also removed at this time. Animals were food deprived for 2 d before testing to minimize variability attributable to satiation effects on defensive withdrawal (Advokat 1980).
In all experiments, the duration of siphon withdrawal was measured by an observer who was blind to the training history of the animal. Before training in all experiments, three pretests (with an intertrial-interval ITI = 15 min) were conducted to ensure a stable baseline of reflex duration. In each of these tests, the tail (∼1 cm from its posterior tip) was stimulated with a water jet (0.5 sec duration), which elicited siphon withdrawal that typically lasted between 5 and 10 sec in duration. Animals that did not show stable responses (any pretest greater than ±20% of the mean) during the pretests were not used in the experiments (<10% of all animals tested). For training, tail shocks (AC, 1.5 sec duration) were delivered to a site immediately posterior to the convergence of the parapodia (see Fig. 1A) through a hand-held electrode. Because this anterior region of the tail retains a relatively fixed position during tail withdrawal (a consequence of tail shock), this same region can be repeatedly shocked even at very short intervals. The nominal current across the electrode was 100 mA, although much of this current is shunted by the sea water. The sites on the tail for training and testing were sufficiently separated to activate subpopulations of tail SNs with nonoverlapping receptive fields (Walters et al. 1983). In all experiments, the beginning of training was offset among different groups to provide temporal alignment of the last shock in all groups. Six post-tests of tail-elicited siphon withdrawal (T-SW; elicited in the same fashion as the pretests described above) were conducted from 15–90 min after training at 15-min intervals to examine STM and ITM. Three additional post-tests (ITI = 15 min) were also conducted 20–24 h after training to examine LTM. Previous studies from our laboratory have shown that the magnitude of LTM is stable over this 20–24-h post-training testing interval (Sutton et al. 2001a).
Experimental Protocol
Experimental groups were distinguished on the basis of the amount and/or pattern of tail shocks used during training; otherwise, behavioral testing was identical across all groups (see above). In experiments examining the amount of training, animals received either no shocks, two shocks, three shocks, four shocks, or five shocks all with the same pattern (15-min ITI). One additional group also received two shocks with an ITI of 60 min, such that both the first and second shocks were in temporal register with the first and fifth shocks of the five-shock group. In experiments examining the pattern of training, animals received either a spaced pattern (15-min ITI) of tail shocks or a massed pattern (1-sec ITI). In these experiments, the ITI is defined as the temporal interval between the offset of one trial and the onset of the next. These two training patterns were compared across three different amounts of training: three shocks, four shocks, or five shocks.
Data Analysis
In all experiments, reflex duration was used as a dependent measure because it can be objectively quantified in freely moving animals. The duration of siphon withdrawal was defined as the elapsed time from stimulus onset to the initial relaxation of the siphon from the contracted position. Baseline duration of T-SW was determined by the average of the three pretests. Whereas the duration of T-SW is stable in nontrained animals (see Fig. 2), the distribution of responses following sensitization training was typically not normally distributed. Thus, to be conservative, all data were analyzed with nonparametric statistics and are reported as medians (±interquartile range) to accurately express the central tendency and dispersion of each group. To examine memory for sensitization within a group, baseline duration of T-SW, duration of T-SW in each of the six 15–90-min post-tests, and the average duration of T-SW across the three long-term tests were analyzed with a Friedman test. Following a significant overall effect (p < 0.05, two-tailed), individual time points were compared against baseline using a Wilcoxon test to assess significant sensitization. Differences in the magnitude of ITM for sensitization between groups were examined with Mann-Whitney U tests of normalized T-SW duration averaged across tests in which significant sensitization was expressed in both groups. For this analysis, the 15-min post-training time-point was not included to avoid contamination with STM (lasting <30 min).
Acknowledgments
We thank Adam Bristol, Martha Bagnall, and John Byrne for helpful comments on an earlier draft of this paper. This work was supported by a Natural Sciences and Engineering Research Council of Canada PGSB award to M.A.S. and National Institute of Mental Health Grant RO1 MH-14–1083 to T.J.C.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL tcarew@uci.edu; FAX (949) 824-2447.
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/lm.44802.
REFERENCES
- Advokat C. Modulation of defensive reflexes in Aplysia californica by appetitive stimulation. Behav Neural Biol. 1980;28:253–265. doi: 10.1016/s0163-1047(80)92259-1. [DOI] [PubMed] [Google Scholar]
- Botzer D, Markovich S, Susswein AJ. Multiple memory processes following training that a food is inedible in Aplysia. Learn Mem. 1998;5:204–219. [PMC free article] [PubMed] [Google Scholar]
- Carew TJ, Castellucci VF, Kandel ER. An analysis of dishabituation and sensitization of the gill-withdrawal reflex in Aplysia. Int J Neurosci. 1971;2:79–98. doi: 10.3109/00207457109146995. [DOI] [PubMed] [Google Scholar]
- Carew TJ, Pinsker HM, Kandel ER. Long-term habituation of a defensive withdrawal reflex in Aplysia. Science. 1972;175:451–454. doi: 10.1126/science.175.4020.451. [DOI] [PubMed] [Google Scholar]
- Carew TJ, Ide J, Masters SE, Sutton MA. Amount and pattern of training in the induction of intermediate- and long-term memory in Aplysia. Soc Neurosci Abs. 2001;954:13. doi: 10.1101/lm.44802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadio A, Martin KC, Giustetto M, Zhu H, Chen M, Bartsch D, Bailey CH, Kandel ER. A transient, neuron-wide form of CREB-mediated long-term facilitation can be stabilized at specific synapses by local protein synthesis. Cell. 1999;99:221–237. doi: 10.1016/s0092-8674(00)81653-0. [DOI] [PubMed] [Google Scholar]
- Castellucci VF, Blumenfeld H, Goelet P, Kandel ER. Inhibitor of protein synthesis blocks long-term behavioral sensitization in the isolated gill-withdrawal reflex of Aplysia. J Neurobiol. 1989;20:1–9. doi: 10.1002/neu.480200102. [DOI] [PubMed] [Google Scholar]
- Cleary LJ, Lee WL, Byrne JH. Cellular correlates of long-term sensitization in Aplysia. J Neurosci. 1998;18:5988–5998. doi: 10.1523/JNEUROSCI.18-15-05988.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow T, Siddiqi V, Dash PK. Long-term enhancement but not short-term in Hermissenda is dependent on mRNA synthesis. Neurobiol Learn Mem. 1997;68:340–347. doi: 10.1006/nlme.1997.3779. [DOI] [PubMed] [Google Scholar]
- Crow T, Xue-Bian J-J, Siddiqi V. Protein synthesis-dependent and mRNA synthesis-independent intermediate phase of memory in Hermissenda. J Neurophysiol. 1999;82:495–500. doi: 10.1152/jn.1999.82.1.495. [DOI] [PubMed] [Google Scholar]
- Ebbinghaus H. Uber das Gedachtnis. New York, NY: Dover; 1885. [Google Scholar]
- Emptage NJ, Carew TJ. Long-term synaptic facilitation in the absence of short-term facilitation in Aplysia neurons. Science. 1993;262:253–256. doi: 10.1126/science.8211146. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Tighe TJ. Contextual conditioning with massed versus distributed unconditional stimuli in the absence of conditional stimuli. J Exp Psychol. 1988;14:187–199. [PubMed] [Google Scholar]
- Frey U, Krug M, Reymann KG, Matthies H. Anisomycin, an inhibitor of protein synthesis, blocks late phases of LTP phenomena in the hippocampal CA1 region in vitro. Brain Res. 1988;452:57–65. doi: 10.1016/0006-8993(88)90008-x. [DOI] [PubMed] [Google Scholar]
- Frieder B, Allweis C. Transient hypoxic-amnesia: evidence for a triphasic memory-consolidating mechanism with parallel processing. Behav Neural Biol. 1978;22:178–189. doi: 10.1016/s0091-6773(78)92200-9. [DOI] [PubMed] [Google Scholar]
- ————— Memory consolidation: Further evidence for the four-phase model from the time courses of diethyldithiocarbamate and ethacrynic acid amnesias. Physiol Behav. 1982;29:1071–1075. doi: 10.1016/0031-9384(82)90300-6. [DOI] [PubMed] [Google Scholar]
- Frost WN, Castellucci VF, Hawkins RD, Kandel ER. Monosynaptic connections made by the sensory neurons of the gill- and siphon-withdrawal reflex in Aplysia participate in the storage of long-term memory for sensitization. Proc Natl Acad Sci. 1985;82:8266–8269. doi: 10.1073/pnas.82.23.8266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber B, Menzel R. Contextual modulation of memory consolidation. Learn Mem. 2000;7:151–158. doi: 10.1101/lm.7.3.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghirardi M, Montarolo PG, Kandel ER. A novel intermediate stage in the transition between short- and long-term facilitation in the sensory to motor synapse of Aplysia. Neuron. 1995;14:413–420. doi: 10.1016/0896-6273(95)90297-x. [DOI] [PubMed] [Google Scholar]
- Gibbs ME, Ng KT. Psychobiology of memory: Towards a model of memory formation. Biobehav Rev. 1977;1:113–136. [Google Scholar]
- ————— Behavioural stages in memory formation. Neurosci Lett. 1979;13:279–283. doi: 10.1016/0304-3940(79)91507-6. [DOI] [PubMed] [Google Scholar]
- Goldsmith JR, Byrne JH. Bag cell extract inhibits tail-siphon withdrawal reflex, suppresses long-term but not short-term sensitization and attenuates sensory-to-motor neuron synapses in Aplysia. J Neurosci. 1993;13:1688–1700. doi: 10.1523/JNEUROSCI.13-04-01688.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermitte G, Pedreira ME, Tomsic D, Maldonado H. Context shifts and protein synthesis inhibition disrupt long-term habituation after spaced, but not massed, training in the crab Chasmagnathus. Learn Mem. 1999;71:34–49. doi: 10.1006/nlme.1998.3858. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Barros DM, de Souza TM, de Souza MM, Iquierdo LA, Medina JH. Mechanisms for memory types differ. Nature. 1998;393:635–636. doi: 10.1038/31371. [DOI] [PubMed] [Google Scholar]
- Kamin LJ. The retention of an incompletely learned avoidance response. J Comp Physiol Psychol. 1957;50:457–460. doi: 10.1037/h0044226. [DOI] [PubMed] [Google Scholar]
- ————— The retention of an incompletely learned avoidance response: Some further analyses. J Comp Physiol Psychol. 1963;56:719–722. doi: 10.1037/h0043941. [DOI] [PubMed] [Google Scholar]
- Lefebvre L, Sabourin M. Effects of spaced and massed repeated elicitation on tonic immobility in the goldfish (Carassius auratus) Behav Neural Biol. 1977;21:300–305. [Google Scholar]
- Levenson J, Byrne JH, Eskin A. Levels of serotonin in the hemolymph of Aplysia are modulated by light/dark cycles and sensitization training. J Neurosci. 1999;19:8094–8103. doi: 10.1523/JNEUROSCI.19-18-08094.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson J, Endo S, Kategaya LS, Fernandez RI, Brabham DG, Chin J, Byrne JH, Eskin A. Long-term regulation of neuronal high-affinity glutamate and glutamine uptake in Aplysia. Proc Natl Acad Sci. 2000;97:12858–12863. doi: 10.1073/pnas.220256497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinesco, S. and Carew, T.J. 2002. Serotonin release evoked by tail-nerve stimulation in the central nervous system of Aplysia: Characterization and relationship to heterosynaptic plasticity. J. Neurosci. (in press). [DOI] [PMC free article] [PubMed]
- Mauelshagen J, Parker GR, Carew TJ. Dynamics of induction and expression of long-term synaptic facilitation in Aplysia. J Neurosci. 1996;16:7099–7108. doi: 10.1523/JNEUROSCI.16-22-07099.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauelshagen J, Sherff CM, Carew TJ. Differential induction of long-term synaptic facilitation by spaced and massed applications of serotonin at sensory neuron synapses of Aplysia californica. Learn Mem. 1998;5:246–256. [PMC free article] [PubMed] [Google Scholar]
- Menzel R, Manz G, Menzel R, Greggers U. Massed and spaced learning in honeybees: The role of CS, US, the intertrial interval, and the test interval. Learn Mem. 2001;8:198–208. doi: 10.1101/lm.40001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer AR, Emptage NJ, Carew TJ. Pharmacological dissociation of modulatory effects of serotonin in Aplysia sensory neurons. Science. 1991;254:1811–1813. doi: 10.1126/science.1662413. [DOI] [PubMed] [Google Scholar]
- Montarolo PG, Goelet P, Castellucci VF, Morgan J, Kandel ER, Schacher S. A critical period for macromolecular synthesis in long-term heterosynaptic facilitation in Aplysia. Science. 1986;234:1249–1254. doi: 10.1126/science.3775383. [DOI] [PubMed] [Google Scholar]
- Muzzio IA, Ramirez RR, Talk AC, Matzel LD. Interactive contributions of intracellular calcium and protein phosphatases to massed-trials learning deficits in Hermissenda. Behav Neurosci. 1999;113:103–117. doi: 10.1037//0735-7044.113.1.103. [DOI] [PubMed] [Google Scholar]
- Nguyen PV, Abel T, Kandel ER. Requirement of a critical period of transcription for induction of a late phase of LTP. Science. 1994;265:1104–1107. doi: 10.1126/science.8066450. [DOI] [PubMed] [Google Scholar]
- O'Leary FA, Byrne JH, Cleary LJ. Long-term structural remodeling in Aplysia sensory neurons requires de novo protein synthesis during a critical time period. J Neurosci. 1995;15:3519–3525. doi: 10.1523/JNEUROSCI.15-05-03519.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsker H, Carew TJ, Hening W, Kandel ER. Long-term sensitization of a defensive withdrawal reflex in Aplysia californica. Science. 1973;182:1039–1042. doi: 10.1126/science.182.4116.1039. [DOI] [PubMed] [Google Scholar]
- Ploner CJ, Gaymard B, Rivaud S, Agid Y, Pierrot-Deseilligny C. Temporal limits of spatial working memory in humans. Eur J Neurosci. 1998;10:794–797. doi: 10.1046/j.1460-9568.1998.00101.x. [DOI] [PubMed] [Google Scholar]
- Riege WH, Cherkin A. One-trial learning and biphasic time course of performance in the goldfish. Science. 1971;172:966–968. doi: 10.1126/science.172.3986.966. [DOI] [PubMed] [Google Scholar]
- Rosenzweig MR, Bennet EL, Columbo PJ, Lee DW, Serrano PA. Short-term intermediate-term and long-term memories. Behav Brain Res. 1993;57:193–198. doi: 10.1016/0166-4328(93)90135-d. [DOI] [PubMed] [Google Scholar]
- Salafia WR, Mis WF, Terry WS, Bartosiak RS, Daston AP. Conditioning of the nictitating membrane response of the rabbit (Orycyolagus cuniculus) as a function of intertrial interval. Animal Learn Behav. 1973;1:109–115. [Google Scholar]
- Sanders GD, Barolow JJ. Variations in retention performance during long-term memory formation. Nature. 1971;232:203–204. doi: 10.1038/232203a0. [DOI] [PubMed] [Google Scholar]
- Scholz KP, Byrne JH. Long-term sensitization in Aplysia: Biophysical correlates in tail sensory neurons. Science. 1987;235:685–687. doi: 10.1126/science.2433766. [DOI] [PubMed] [Google Scholar]
- Sherff CM, Carew TJ. Coincident induction of long-term synaptic facilitation in Aplysia: Cooperativity between cell bodies and remote synapses. Science. 1999;285:1911–1914. doi: 10.1126/science.285.5435.1911. [DOI] [PubMed] [Google Scholar]
- Smolen P, Baxter DA, Byrne JH. Frequency selectivity, multistability, and oscillations emerge from models of genetic regulatory systems. Am J Physiol. 1998;274:C531–C542. doi: 10.1152/ajpcell.1998.274.2.C531. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Carew TJ. Parallel molecular pathways mediate expression of distinct forms of intermediate-term facilitation at tail sensory-motor synapses in Aplysia. Neuron. 2000;26:219–231. doi: 10.1016/s0896-6273(00)81152-6. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Masters SE, Bagnall MW, Carew TJ. Molecular mechanisms underlying a unique intermediate phase of memory in Aplysia. Neuron. 2001a;31:143–154. doi: 10.1016/s0896-6273(01)00342-7. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Bagnall MW, Carew TJ. Molecular mechanisms of site-specific memory for sensitization in Aplysia. Soc Neurosci Abs. 2001b;954:12. [Google Scholar]
- Tallarico PT. A musical investigation of the Kamin effect. J Res Music Education. 1973;21:153–161. [Google Scholar]
- Tully T, Boynton SC, Brandes C, Dura J-M, Mihalek R, Preat T, Villela A. Genetic dissection of memory formation in Drosophila melanogaster. Cold Spring Harbor Symp Quant Biol. 1990;55:203–211. doi: 10.1101/sqb.1990.055.01.022. [DOI] [PubMed] [Google Scholar]
- Tully T, Preat T, Boynton SC, Del Vecchio M. Genetic dissection of consolidated memory in Drosophila. Cell. 1994;79:35–47. doi: 10.1016/0092-8674(94)90398-0. [DOI] [PubMed] [Google Scholar]
- Wainwright, M.L., Zhang, H., Byrne, J.H., and Cleary, L.J. 2002. Localized neuronal outgrowth induced by long-term sensitization training in Aplysia. J. Neurosci. in press. [DOI] [PMC free article] [PubMed]
- Walters ET, Byrne JH, Carew TJ, Kandel ER. Mechanoafferent neurons innervating tail of Aplysia. II. Modulation by sensitizing stimulation. J Neurophysiol. 1983;50:1543–1559. doi: 10.1152/jn.1983.50.6.1543. [DOI] [PubMed] [Google Scholar]
- Winder DG, Mansuy IM, Osman M, Moallem TM, Kandel ER. Genetic and pharmacological evidence for a novel, intermediate phase of long-term potentiation suppressed by calcineurin. Cell. 1998;92:25–37. doi: 10.1016/s0092-8674(00)80896-x. [DOI] [PubMed] [Google Scholar]
- Wu G-Y, Deisseroth K, Tsien RW. Spaced stimuli stabilize MAPK pathway activation and its effects on dendritic morphology. Nat Neurosci. 2001;4:151–158. doi: 10.1038/83976. [DOI] [PubMed] [Google Scholar]
- Xia S-Z, Feng C-H, Guo A-K. Multiple-phase model of memory consolidation confirmed by behavioral and pharmacological analyses of operant conditioning in Drosophila. Pharmacol Biochem Behav. 1998;60:809–816. doi: 10.1016/s0091-3057(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Yin JCP, Wallach JS, Del Vecchio M, Wilder EL, Zhou H, Quinn WG, Tully T. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell. 1994;79:49–58. doi: 10.1016/0092-8674(94)90399-9. [DOI] [PubMed] [Google Scholar]
- Yin JCP, Del Vecchio M, Zhou H, Tully T. CREB as a memory modulator: Induced expression of dCREB2 activator isoform enhances long-term memory in Drosophila. Cell. 1995;81:107–115. doi: 10.1016/0092-8674(95)90375-5. [DOI] [PubMed] [Google Scholar]
- Zhang F, Endo S, Cleary LJ, Eskin A, Byrne JH. Role of transforming growth factor-β in long-term synaptic facilitation in Aplysia. Science. 1997;275:1318–1320. doi: 10.1126/science.275.5304.1318. [DOI] [PubMed] [Google Scholar]