Abstract
Oscillations in cytosolic free Ca2+ concentration ([Ca2+]cyt) are an important component of Ca2+-based signal transduction pathways. This fact has led us to investigate whether oscillations in [Ca2+]cyt are involved in the response of stomatal guard cells to the plant hormone abscisic acid (ABA). We show that ABA induces oscillations in guard-cell [Ca2+]cyt. The pattern of the oscillations depended on the ABA concentration and correlated with the final stomatal aperture. We examined the mechanism by which ABA generates oscillations in guard-cell [Ca2+]cyt by using 1-(6-{[17β-3-methoxyestra-1,3,5(10)-trien-17-yl]amino}hexyl)-1H-pyrrole-2,5-dione (U-73122), an inhibitor of phosphoinositide-specific phospholipase C (PI-PLC)-dependent processes in animals. U-73122 inhibited the hydrolysis of phosphatidylinositol 4,5-bisphosphate by a recombinant PI-PLC, isolated from a guard-cell-enriched cDNA library, in a dose-dependent manner. This result confirms that U-73122 is an inhibitor of plant PI-PLC activity. U-73122 inhibited both ABA-induced oscillations in [Ca2+]cyt and stomatal closure. In contrast, U-73122 did not inhibit external Ca2+-induced oscillations in guard-cell [Ca2+]cyt and stomatal closure. Furthermore, there was no effect of the inactive analogue 1-(6-{[17β-3-methoxyestra-1,3,5(10)-trien-17-yl]amino}hexyl)-2,5-pyrrolidinedione on recombinant PI-PLC activity or ABA-induced and external Ca2+-induced oscillations in [Ca2+]cyt and stomatal closure. This lack of effect suggests that the effects of U-73122 in guard cells are the result of inhibition of PI-PLC and not a consequence of nonspecific effects. Taken together, our data suggest a role for PI-PLC in the generation of ABA-induced oscillations in [Ca2+]cyt and point toward the involvement of oscillations in [Ca2+]cyt in the maintenance of stomatal aperture by ABA.
Stomata form pores in the epidermis of the leaf that allow CO2 uptake for photosynthesis and water loss via transpiration. During drought, the loss of water through transpiration is reduced in response to an increase in the levels of the plant hormone abscisic acid (ABA) in the leaves (1). ABA stimulates the efflux of K+ from the guard cells that surround the stomatal pore, resulting in a reduction in guard-cell turgor and a decrease in the width of the pore (2). An increase in cytosolic free Ca2+ concentration ([Ca2+]cyt) has been shown to be an early event in the signal transduction pathway by which ABA stimulates a reduction in guard-cell turgor (3–8). In addition, components of Ca2+-based second messenger systems found in animals have been identified in guard cells (9). However, little is known about the process by which the information required to describe the strength of the ABA stimulus is encoded in ABA-induced changes in guard-cell [Ca2+]cyt or the mechanism(s) by which these changes are generated.
It has been proposed that oscillations in [Ca2+]cyt have the potential to increase the amount of information encoded by changes in [Ca2+]cyt in plant cells through the generation of a stimulus-specific Ca2+ signature (9, 10). Studies in animals suggest that signaling information may be encoded in the period and/or the amplitude of stimulus-induced oscillations in [Ca2+]cyt (11–13). Recently, stimulus-induced oscillations and waves in [Ca2+]cyt have been observed in a number of different higher plant cells, including stomatal guard cells (14, 15), root hairs (16), staminal hairs (17), and pollen tubes (18, 19). These data suggest that oscillations may be important components of Ca2+-dependent signal transduction pathways in plants.
There is an increasing body of evidence for a role of phosphoinositide-specific phospholipase C (PI-PLC)-based signaling in plant cells (20–22). Several intermediates in animal PI-PLC-based signaling systems have been identified in stomatal guard cells (23, 24). In addition, inositol 1,4,5-trisphosphate (Ins(1,4,5)P3) and diacylglycerol have both been implicated in guard-cell signal transduction. Ins(1,4,5)P3 turnover has been shown to increase in guard-cell protoplasts on stimulation with ABA (25). The release of Ins(1,4,5)P3 in guard cells has also been reported to inhibit the plasma-membrane inward K+ channel (26) and to stimulate an increase in guard-cell [Ca2+]cyt, leading to stomatal closure (27). Diacylglycerol has been shown to be involved in light-induced stomatal opening (28). Furthermore, PI-PLC activity has been shown in guard cells (29), and recently, PI-PLC has been cloned from the guard cells of tobacco (30) and potato (22). These data point toward the presence of a PI-PLC-based signaling system in guard cells, although, to date, there have been few reports of a physiological role for this system in this cell type.
We have investigated the ability of ABA to stimulate oscillations in [Ca2+]cyt in guard cells that may encode information describing the strength of the ABA stimulus. Our results show that ABA stimulates oscillations in [Ca2+]cyt in guard cells of Commelina communis. The pattern of the oscillations correlated with the concentration of ABA and with the final stomatal aperture observed in response to ABA. Both ABA-induced oscillations in guard-cell [Ca2+]cyt and ABA-induced stomatal closure, together with the activity of a recombinant PI-PLC isolated from a guard-cell-enriched cDNA library, were inhibited by an aminosteroid known to inhibit PI-PLC-dependent processes in animal cells (31–34). We provide evidence that this inhibition is unlikely to be a consequence of any potential nonspecific effects of this inhibitor in plants. Taken together, these data suggest a physiological role for PI-PLC and oscillations in [Ca2+]cyt in the ABA signal transduction pathway in guard cells.
MATERIALS AND METHODS
Chemicals.
ABA, 1-(6-{[17β-3-methoxyestra-1,3,5(10)-trien-17-yl]amino}hexyl)-1H-pyrrole-2,5-dione (U-73122), and 1-(6-{[17β-3-methoxyestra-1,3,5(10)-trien-17-yl]amino}hexyl)-2,5-pyrrolidinedione (U-73343) were purchased from Calbiochem. Stock solutions of ABA were prepared in ethanol. Stock solutions of U-73122 and U-73343 were prepared in dimethyl sulfoxide. In experiments that used isolated epidermis, the final concentrations of ethanol and dimethyl sulfoxide never exceeded 0.01% and 0.05%, respectively, and were found to have no effect on stomatal aperture or guard-cell [Ca2+]cyt (data not shown).
Epidermal Strip Bioassay.
Plants of C. communis were grown from seed, and stomatal bioassay experiments were performed as described (14) with minor modifications. Freshly prepared abaxial epidermis was incubated in CO2-free 10 mM Mes/5 mM KCl, pH 6.2 (adjusted with Tris base) at 25°C (Mes/KCl) for 3 h and then transferred to fresh Mes/KCl containing no, 1 nM, or 1 μM ABA in the presence or absence of 1 μM U-73122 or U-73343. Subsequently, stomatal apertures were determined microscopically at 5-min intervals or after a 1-h incubation.
Preparation of Recombinant PI-PLC.
The PI-PLC clone NrEFPLC2, isolated from a cDNA library of Nicotiana rustica and prepared from epidermal fragments highly enriched (>95%) with guard cells (30), was expressed as a recombinant glutathione S-transferase (GST)-NrEFPLC2 fusion protein. The entire coding region of NrEFPLC2 and the first three nucleotides preceding the initiator ATG were amplified by PCR and then ligated in-frame into the SalI–NotI restriction sites within the multiple cloning sites of the expression vector pGEX-4T-2 (Amersham Pharmacia) to yield the plasmid pGEX-NrEFPLC2. The integrity of the inserted DNA was verified by dideoxy terminator sequencing of the plasmid DNA. pGEX-NrEFPLC2 and the control plasmid pGEX were used separately to transform Escherichia coli strain BL-21 (DE3) pLysS (Promega). The expression of GST-NrEFPLC2 or GST was monitored in small-scale (5-ml) cultures. E. coli transformants were grown at 28°C to OD600 = 0.4–0.6 and then induced with 1 mM isopropyl β-d-galactopyranoside for 90 min. The fusion protein produced was purified by affinity chromatography on glutathione-Sepharose 4B (Amersham Pharmacia), according to the manufacturer’s instructions. For large-scale preparations of recombinant protein for biochemical studies, overnight cultures harboring pGEX-NrEFPLC2 were diluted 1:100 in fresh culture medium and grown for 6–8 h without addition of isopropyl β-d-galactopyranoside. The recombinant protein was recovered from the soluble fraction of the cell lysate on glutathione-Sepharose 4B. The purity of the affinity-chromatography-purified GST (29 kDa) and recombinant GST-PI-PLC (95 kDa) were assessed by SDS/PAGE (35); gels were stained with Coomassie brilliant blue G-250. The purified GST-PI-PLC preferentially hydrolyzed phosphatidylinositol 4,5-bisphosphate and was activated by Ca2+ (data not shown).
PI-PLC Assay.
PI-PLC activity was measured as described (36). The reaction mixture contained 50 mM Tris-maleate (pH 6.0–6.5), 0.2 mM phosphatidyl[2-3H]inositol-4,5-bisphosphate (1,000–2,000 dpm/nmol) in micellar solution, 10 μM free Ca2+, 80 mM KCl, 1.5 mM MgCl2, and 0.1–0.5 μg of recombinant protein. The reaction was carried out for 10–30 min at 37°C. The recombinant protein was preincubated in the presence of 0–120 μM U-73122 or U-73343 for 15 min before adding the substrate lipids. The U-73122 and U-73343 stock solutions were diluted in dimethyl sulfoxide, and the PI-PLC activity was expressed as a percentage of the dimethyl sulfoxide control.
Measurement of [Ca2+]cyt.
Guard-cell [Ca2+]cyt was determined essentially as described (14). Briefly, freshly prepared epidermis of C. communis was perfused with CO2-free 10 mM Mes/15 mM KCl, pH 6.2 (adjusted with Tris base) at 25°C. Fura-2 (Calbiochem) was microinjected into the cytosol of cells by iontophoresis (3) or by pressure (37). Microinjected cells were maintained under conditions promoting stomatal opening. Measurements of [Ca2+]cyt were performed only on fura-2-loaded guard cells that met all the criteria for estimating viability and that opened to the same aperture size (6–10 μm) as those on the rest of the epidermal strip. Subsequently, guard cells in this category were perfused with Mes/KCl containing no, 1 nM, or 1 μM ABA or 1 mM CaCl2 in the presence or absence of 1 μM U-73122 or U-73343. Resting [Ca2+]cyt was determined in guard cells of open stomata perfused with Mes/KCl at the start of each experiment. Measurements were performed only on cells that exhibited a stable level of resting [Ca2+]cyt in Mes/KCl. The whole-cell fluorescence of fura-2-loaded guard cells (340- and 380-nm excitation; 510-nm emission) was quantified with a Cairn spectrophotometer system (Faversham, U.K.). Autofluorescence subtraction and calculation of the Ca2+-dependent ratio (340:380 nm) were performed online. Similar results were obtained for both in vitro and in vivo calibration of the Ca2+-dependent ratio in guard cells (5, 7). Therefore, ratios were converted into measurements of whole-cell [Ca2+]cyt concentration by using in vitro calibration curves obtained at weekly intervals.
RESULTS
ABA Induces Oscillations in Guard-Cell [Ca2+]cyt.
The effects of ABA on [Ca2+]cyt in guard cells perfused with Mes/KCl [i.e., 5 mM external K+ concentration ([K+]ext)] are shown in Fig. 1. Under these conditions, ABA induced a concentration-dependent reduction in stomatal aperture (Fig. 1A Inset); the stomatal apertures observed in response to no, 10 nM, and 1 μM ABA were 12.9 ± 0.3 μm, 10.4 ± 0.3 μm, and 0.8 ± 0.5 μm, respectively. Resting [Ca2+]cyt was determined at the start of each experiment, during perfusion with Mes/KCl. In the majority of cells (67% of cells tested), the resting [Ca2+]cyt remained constant, with values ranging from 250 nM to 400 nM (n = 48 cells); these values are similar to the level reported previously for guard cells under conditions of “low” (<50 mM) [K+]ext (5). Measurements were performed only on cells that exhibited a stable level of resting [Ca2+]cyt.
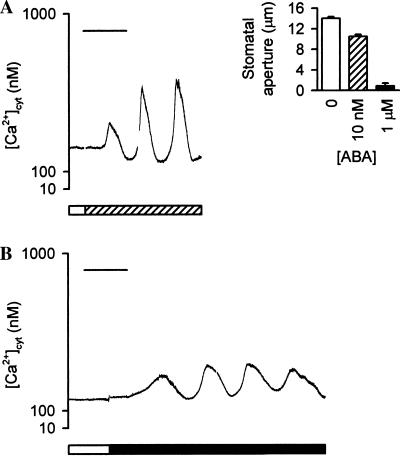
Figure 1.
ABA induces oscillations in [Ca2+]cyt in guard cells of C. communis perfused with Mes/KCl. (A and B) The cytosol of guard cells of closed stomata was microinjected with fura-2, and the stomata opened >6 μm. Resting [Ca2+]cyt was determined during perfusion with Mes/KCl (open bars). Subsequently, guard cells were perfused with Mes/KCl containing 10 nM ABA (A, hatched bar) or 1 μM ABA (B, solid bar). Representative traces are shown. (Bars = 15 min.) (Inset) Stomatal responses to ABA in Mes/KCl. Isolated epidermis of C. communis was incubated in Mes/KCl for 3 h under conditions promoting stomatal opening and then transferred to Mes/KCl containing no (open bar), 10 nM (hatched bar), or 1 μM (solid bar) ABA for 1 h. Values are the means of 120 measurements ± SEM.
ABA stimulated oscillations in [Ca2+]cyt in guard cells perfused with Mes/KCl. All cells that exhibited a stable level of resting [Ca2+]cyt displayed this oscillatory behavior (n = 48 cells). There was a lag period of 1–15 min, during which [Ca2+]cyt increased gradually, before oscillations in [Ca2+]cyt were observed. Cell-to-cell variation in the duration of this lag period may reflect differences in the Ca2+-buffering capacity of the cell resulting from variations in the concentration of fura-2 loaded into the cytosol (7), or cell-to-cell variation may reflect the physiological address of the cell (which reflects the cell’s physiological and developmental history; refs. 9 and 10). Once established, the period and amplitude of the oscillations remained constant in the presence of the stimulus for up to 3 h. Both 10 nM and 1 μM ABA induced oscillations in [Ca2+]cyt that were slightly asymmetric, with amplitudes of 200–600 nM (Fig. 1). The period of the oscillations depended on the concentration of ABA; 10 nM ABA induced oscillations with a mean period of 11.0 ± 0.9 min (n = 22 cells; Fig. 1A), whereas 1 μM ABA induced oscillations with a mean period of 18.0 min ± 0.9 min (n = 31 cells; Fig. 1B). There was little difference in the mean value for the total increase in [Ca2+]cyt integrated over the period of one oscillation (14) in cells perfused with 10 nM or 1 μM ABA (2,834 ± 37 nM⋅min and 3,028 ± 46 nM⋅min, respectively).
In 33% of cells tested (n = 24 cells), perfusion with Mes/KCl in the absence of ABA resulted in oscillations in [Ca2+]cyt (data not shown). These data suggest the existence of two populations of guard cells in C. communis. Interestingly, in Arabidopsis, 30% of the guard-cell population has been shown to undergo spontaneous transitions in membrane potential to a hyperpolarized state (38). Furthermore, a reduction in [K+]ext has been reported to cause hyperpolarization of the plasma membrane in Arabidopsis guard cells (39). Plasma-membrane hyperpolarization has been observed to cause oscillations in guard-cell [Ca2+]cyt (15). Therefore, the present data suggest that a population of hyperpolarized guard cells, similar to that in Arabidopsis, also exists in C. communis; in addition, the data suggest that perfusion with Mes/KCl (i.e., 5 mM [K+]ext) causes additional hyperpolarization of the plasma membrane of these cells to such an extent that they display oscillations in [Ca2+]cyt in the absence of ABA. Oscillations of this type were abolished when guard cells were returned to the slightly higher concentration of [K+]ext present during guard-cell microinjection and stomatal opening (15 mM KCl), providing support for this hypothesis. These data correlate well with the results of previous studies investigating hyperpolarization-induced oscillations in guard-cell [Ca2+]cyt (15). Subtle variations in physiological address (9, 10) may underlie the existence of these two populations of guard cells. These variations will influence the nature of the stimulus-induced Ca2+ signature profoundly. A “simple” pattern of oscillations provides the best opportunity for analyzing the mechanism(s) involved in the generation of the oscillatory behavior. Consequently, in the present study, we have focused on the 67% of guard cells that exhibited a stable level of resting [Ca2+]cyt when perfused with Mes/KCl as being most likely to generate a simple pattern of oscillations in [Ca2+]cyt in response to ABA.
Modulation of Stomatal Aperture and the Oscillatory Behavior.
Changing the concentration of ABA resulted in an alteration in both stomatal aperture and the pattern of the ABA-induced oscillations in [Ca2+]cyt (Fig. 2). Incubation of isolated epidermis of C. communis in Mes/KCl containing 10 nM ABA resulted in a final stomatal aperture of ≈10.5 μm within 40 min (Fig. 2A). When the concentration of ABA was raised to 1 μM ABA, there was an immediate change in stomatal aperture, resulting in “fully closed” stomata (i.e., stomatal apertures of ≈1 μm) within 15 min (Fig. 2A).
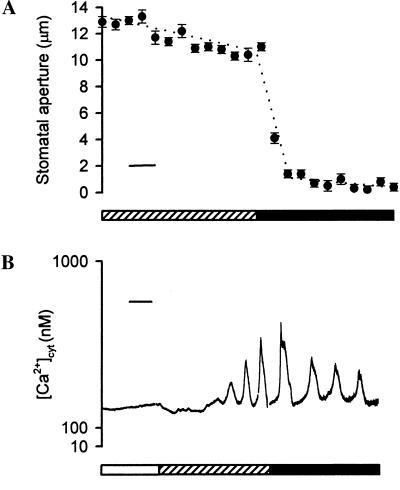
Figure 2.
The pattern of ABA-induced oscillations in [Ca2+]cyt correlates with the final stomatal aperture. (A) Isolated epidermis of C. communis was incubated in Mes/KCl for 3 h under conditions promoting stomatal opening and then transferred to Mes/KCl containing 10 nM ABA (hatched bar). After 1 h, the ABA concentration was increased to 1 μM (solid bar). Stomatal apertures were recorded every 5 min throughout the experiment. Values are the means of 120 measurements ± SEM. (Bar = 10 min.) (B) Fura-2-loaded guard cells of open (>6 μm) stomata of C. communis were perfused with Mes/KCl, and the resting [Ca2+]cyt was determined (open bar). Subsequently, guard cells were perfused with Mes/KCl containing 10 nM ABA (hatched bar). After the characteristic oscillations in [Ca2+]cyt were established, the concentration of ABA was increased to 1 μm (solid bar). A representative trace is shown. (Bar = 15 min.)
Alterations in stomatal aperture were reflected in a change in the period of the ABA-induced oscillations in [Ca2+]cyt. When guard cells of open stomata exhibiting characteristic oscillations in response to Mes/KCl containing 10 nM ABA were perfused with 1 μM ABA, there was an immediate change in the pattern of oscillations; oscillations typical of the higher concentration of ABA were established rapidly (n = 5 cells; Fig. 2B). As shown in Fig. 2B, the period of the oscillations established after the transfer to 1 μM ABA (mean period 18.0 min ± 0.9 min; 15 min in the cell shown) was longer than the period of those observed in response to 10 nM ABA in the same cell (mean period 11.0 ± 0.9 min; 12 min in the cell shown). The establishment of the pattern of oscillations characteristic of 1 μM ABA correlated with the maintenance of stomata in the fully closed configuration associated with the higher ABA concentration (Fig. 2A).
PI-PLC Contributes to the Generation of ABA-Induced Oscillations in Guard-Cell [Ca2+]cyt.
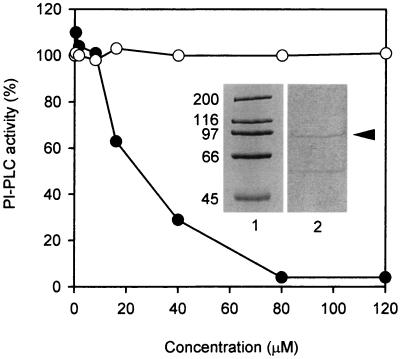
The inhibitor U-73122 and its inactive analogue U-73343 have been used widely to study PI-PLC-dependent processes in animal cells (31–34). Fig. 3 shows the effect of U-73122 and U-73343 on a PI-PLC isolated from a guard-cell-enriched cDNA library of N. rustica (22, 30) and expressed as a recombinant GST fusion protein. The purified GST-PI-PLC appeared as a discrete band running at ≈95 kDa when analyzed by SDS/PAGE (Fig. 3 Inset); the specific activity of the GST-PI-PLC was 1,000 times higher than that of the comparable GST prepared from the control pGEX plasmid (0.7 μmol⋅min−1·mg−1 and 0.7 nmol⋅ min−1·mg−1, respectively). The hydrolysis of phosphatidylinositol 4,5-bisphosphate by this recombinant plant PI-PLC was inhibited by U-73122 in a concentration-dependent manner. The IC50 for the enzyme was 23 μM U-73122, and complete inhibition of hydrolytic activity was observed at 80 μM U-73122. Importantly, U-73343 had no effect on the activity of the recombinant plant PI-PLC. These data show that U-73122 is an inhibitor of plant PI-PLC activity and, more specifically, an inhibitor of a “guard-cell” PI-PLC.
Figure 3.
U-73122 reduces the activity of a recombinant plant PI-PLC, whereas the inactive analogue U-73343 has no effect on the activity. The PI-PLC clone isolated from a guard-cell-enriched cDNA library of N. rustica was expressed as a recombinant GST fusion protein. This protein appeared as a discrete band when analyzed by SDS/PAGE [Inset, lane 1, molecular mass markers of 200, 116, 97, 66, and 45 kDa; lane 2, 1 μg of affinity-chromatography-purified GST-PI-PLC (arrowhead)]. After a 15-min preincubation with increasing concentrations of U-73122 (closed circles) or U-73343 (open circles), the activity of the recombinant plant PI-PLC was determined with phosphatidylinositol 4,5-bisphosphate as the substrate. Values were expressed as a percentage of the activity in the absence of U-73122 or U-73343; 100% of PI-PLC activity represents 300–500 nmol⋅min−1 per mg of protein.
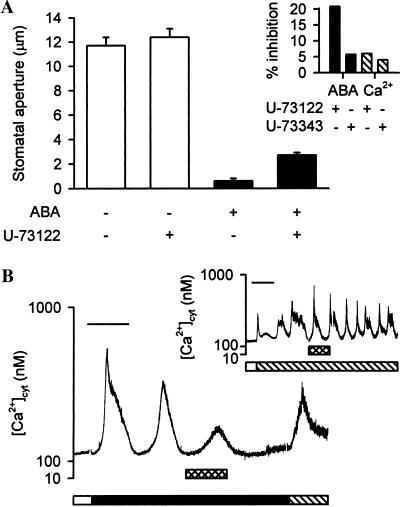
ABA-induced stomatal closure and oscillations in guard-cell [Ca2+]cyt in response to ABA were both inhibited by U-73122 (Fig. 4). In bioassay experiments, inclusion of 1 μM U-73122 in the Mes/KCl during the first 15 min of the ABA treatment inhibited the effect of 1 μM ABA on stomatal aperture by approximately 20% (Fig. 4A Inset); the final stomatal apertures in the absence and presence of U-73122 were 0.6 ± 0.2 μm and 2.7 ± 0.2 μm, respectively (Fig. 4A). A similar effect was observed on stomatal closure in response to 10 nM ABA (data not shown). There was no effect of U-73122 alone on stomatal aperture (Fig. 4A).
Figure 4.
ABA-induced stomatal closure and oscillations in guard-cell [Ca2+]cyt are inhibited by U-73122. (A) Isolated epidermis of C. communis was incubated in Mes/KCl for 3 h under conditions promoting stomatal opening and then transferred to Mes/KCl containing no ABA (open bars) or 1 μM ABA (solid bars) for 15 min in the absence or presence of 1 μM U-73122. Subsequently, the epidermis was incubated for a further 45 min in fresh Mes/KCl containing no or 1 μM ABA, respectively, in the absence of U-73122. Values are the means of 80 measurements ± SEM. (A Inset) Percentage inhibition of 1 μM ABA-induced (solid bars) or 1 mM external Ca2+ ([Ca2+]ext)-induced (hatched bars) stomatal closure by 1 μM U-73122 or U-73343. ([Ca2+]ext is a potent closing stimulus that induces oscillations in guard-cell [Ca2+]cyt; ref 14.) Values are calculated from the means of 180 measurements. (B) Fura-2-loaded guard cells of open (>6 μm) stomata of C. communis were perfused with Mes/KCl, and the resting level of [Ca2+]cyt was determined (open bar). Subsequently, guard cells were perfused with Mes/KCl containing 1 μM ABA (solid bar). After the characteristic oscillations in [Ca2+]cyt had been established, cells were given a 15-min pulse of 1 μM U-73122 (crosshatched bar). Perfusion with Mes/KCl containing 1 mM CaCl2 in the absence of ABA (hatched bar) at the end of each experiment showed that cells were still viable and were capable of maintaining Ca2+ homeostasis after exposure to U-73122. A representative trace is shown. (Bar = 15 min.) (B Inset) The effect of 1 μM U-73122 on oscillations in guard-cell [Ca2+]cyt in response to [Ca2+]ext. Resting [Ca2+]cyt was determined in fura-2-loaded guard cells of open (>6 μm) stomata of C. communis at the start of each experiment, during perfusion with Mes/KCl (open bars). Subsequently, guard cells were perfused with Mes/KCl containing 1 mM CaCl2 (hatched bar). U-73122 was given as a 15-min pulse (crosshatched bar). A representative trace is shown. (Bar = 15 min.)
U-73122 had a marked effect on ABA-induced oscillations in [Ca2+]cyt (n = 5 cells). When cells exhibiting characteristic oscillations in response to Mes/KCl containing 1 μM ABA were treated with a 15-min pulse of 1 μM U-73122, there was a change in the oscillatory behavior (Fig. 4B); there was an initial residual increase in [Ca2+]cyt, after which [Ca2+]cyt returned to resting levels (250–400 nM). Guard-cell [Ca2+]cyt subsequently remained at resting levels, even after the removal of U-73122. A similar effect was observed on oscillations in [Ca2+]cyt in response to 10 nM ABA (data not shown). All of the cells treated with U-73122 exhibited a regulated increase in [Ca2+]cyt in response to 1 mM CaCl2, indicating that they were still viable and were capable of maintaining Ca2+ homeostasis (14) (Fig. 4B).
In contrast, U-73122 had little effect on stomatal responses to [Ca2+]ext, a potent closing stimulus that induces oscillations in guard-cell [Ca2+]cyt (ref. 14; Fig. 4). In bioassay experiments, the inclusion of 1 μM U-73122 in the Mes/KCl during the initial 15 min of treatment with 1 mM CaCl2 did not inhibit significantly (P < 0.005) [Ca2+]ext-induced stomatal closure (Fig. 4A Inset). Furthermore, oscillations in guard-cell [Ca2+]ext in response to the addition of Mes/KCl containing 1 mM CaCl2 were not inhibited by a 15-min pulse of 1 μM U-73122 (n = 7 cells; Fig. 4B Inset). Changes in the pattern of the oscillations from asymmetrical to symmetrical in form were observed in response to treatment with U-73122, although this effect was not consistent between successive oscillations (Fig. 4B Inset) or between cells. Importantly, treatment with the inactive analogue U-73343 at 1 μM for 15 min had no significant effect (P < 0.005) on ABA- or [Ca2+]ext-induced stomatal closure (Fig. 4A Inset) or on the oscillations in [Ca2+]cyt stimulated by ABA or [Ca2+]ext (data not shown). Taken together, these data suggest that the inhibition by U-73122 of ABA-induced stomatal closure and of the oscillations in guard-cell [Ca2+]cyt in response to ABA is a result of the inhibition of PI-PLC rather than a consequence of nonspecific effects of this inhibitor.
DISCUSSION
In this paper, we provide evidence for a physiological role of PI-PLC and oscillations in [Ca2+]cyt in the ABA signal transduction pathway in guard cells. ABA stimulated oscillations in [Ca2+]cyt in guard cells of C. communis perfused with 5 mM [K+]ext (Fig. 1). Under these conditions, ABA induced a concentration-dependent reduction in stomatal aperture (Fig. 1A Inset). Previously, the effects of ABA on [Ca2+]cyt have been studied in guard cells perfused with 50 mM [K+]ext; these effects vary from no detectable change to transient and sustained increases ranging from 100 to 1,000 nM above resting levels (3–8). Recent studies suggest that the apoplastic K+ concentration in plants is between 5 and 10 mM (40). A reduction in the concentration of [K+]ext from 50 to 5 mM has been shown to cause hyperpolarization of the plasma membrane in Arabidopsis guard cells (39). Hyperpolarization of the plasma membrane has been reported to facilitate ABA-induced increases in guard-cell [Ca2+]cyt (4, 15) through ABA-activated Ca2+-permeable channels (4). Taken together, these studies suggest that the membrane potential of guard cells perfused with 5 mM [K+]ext will tend to favor the generation of changes in guard-cell [Ca2+]cyt in response to ABA, including ABA-induced oscillations in [Ca2+]cyt.
The aminosteroid U-73122, which has been used widely to investigate PI-PLC-dependent processes in animal cells (31–34), inhibited the activity of a recombinant plant PI-PLC, isolated from a guard-cell-enriched cDNA library, in a concentration-dependent manner (Fig. 3). Importantly, the inactive analogue U-73343 had no effect on the activity of the recombinant plant PI-PLC. These data suggest an effect of U-73122 on PI-PLC in guard cells. Both ABA-induced oscillations in [Ca2+]cyt (Fig. 4B) and stomatal closure in response to ABA (Fig. 4A) were inhibited by U-73122. In contrast, U-73122 failed to inhibit both [Ca2+]ext-induced oscillations in guard-cell [Ca2+]cyt (Fig. 4B Inset) and stomatal closure in response to [Ca2+]ext (Fig. 4A Inset). Therefore, the inhibition of ABA-induced stomatal closure and of oscillations in guard-cell [Ca2+]cyt in response to ABA by U-73122 is unlikely to be a consequence of potential nonspecific effects of this inhibitor in plants. Importantly, there was no effect of the inactive analogue U-73343 on ABA- and [Ca2+]ext-induced stomatal closure or oscillations in [Ca2+]cyt (data not shown), providing an additional control against potential nonspecific effects of this inhibitor. These data suggest a role for PI-PLC in the generation of ABA-induced oscillations in guard-cell [Ca2+]cyt and the response of stomata to ABA.
There is an increasing body of evidence to suggest a role for PI-PLC-based signaling in the transduction of extracellular signals in plants (20–22). For example, in guard cells, Ins(1,4,5)P3 has been shown to inhibit the plasma-membrane inward K+ channel (26) and to stimulate an increase in guard-cell [Ca2+]cyt leading to stomatal closure (27), whereas diacylglycerol has been shown to enhance light-induced stomatal opening (28). In addition, more than 10 plant PI-PLC clones have been reported, including those from the guard cells of tobacco (30) and potato (22). Importantly, turnover of Ins(1,4,5)P3 has been shown in response to environmental stimuli such as auxin (41), ABA (25), and light (42, 43). Furthermore, the expression of genes encoding a PI-PLC from Arabidopsis have also been shown to be induced by environmental stress (21). These data, together with those of the present study, imply the presence of a functional PI-PLC-based signal transduction pathway in plants that is involved in the response to environmental stimuli such as ABA.
In our guard-cell experiments, different concentrations of ABA produced their own distinctive Ca2+ signature. The pattern of the oscillations depended on the ABA concentration and correlated with the final stomatal aperture (Fig. 2). This result supports the hypothesis that oscillations in [Ca2+]cyt enable the generation of an increase in [Ca2+]cyt in plants that reflects the strength of the stimulus (9, 10). Stomata closed rapidly in Mes/KCl in response to ABA; treatment with 1 μM ABA resulted in a steady-state stomatal aperture within 15 min (Fig. 2A). In contrast, there was a lag period of 1–15 min, during which [Ca2+]cyt increased gradually, before oscillations in [Ca2+]cyt were established (Figs. 1, 2, and 4). Furthermore, the period of the ABA-induced oscillations in [Ca2+]cyt was relatively long (10 nM ABA, 11.0 ± 0.9 min; 1 μM ABA, 18.0 min ± 0.9 min) compared with the time course of stomatal closure. Therefore, we propose that oscillations in [Ca2+]cyt are more likely to be involved in the maintenance of a steady-state stomatal aperture, depending on the strength of the ABA stimulus, rather than the induction of stomatal closure per se.
A similar relationship between the strength of the stimulus, the form of the Ca2+ signal, and the magnitude of the physiological response has been reported for oscillations in guard-cell [Ca2+]cyt induced by [Ca2+]ext (14). Agonist-induced oscillations in [Ca2+]cyt have also been studied extensively in animals (11–13). For a given agonist and cell type, the pattern of oscillations in [Ca2+]cyt is a direct function of the agonist concentration. Therefore, the use of oscillations in [Ca2+]cyt to generate a Ca2+ signature that encodes information regarding the strength of a stimulus seems to be an important feature of Ca2+-based signaling that is conserved between plants and animals.
It has been proposed that information may be encoded in both the period and the amplitude of oscillations in [Ca2+]cyt (9–12). In the present study, the period of the ABA-induced oscillations in [Ca2+]cyt was characteristic of the strength of the ABA stimulus. The mean period of the oscillations in guard-cell [Ca2+]cyt in response to 10 nM ABA was 11.0 ± 0.9 min, whereas the mean period of oscillations induced by 1 μM ABA was 18.0 min ± 0.9 min. These data suggest that it is the period of the ABA-induced oscillations in guard-cell [Ca2+]cyt that encodes information in the Ca2+ signature responsible for describing the strength of the ABA stimulus. It is only possible to speculate about the molecular mechanism(s) by which this information is decoded. Recently, it has been shown that calmodulin-dependent protein kinase II, which is a ubiquitous enzyme target of Ca2+ signaling pathways in animals, can decode the information encoded in the period or frequency of Ca2+ spikes into distinct levels of kinase activity in vitro (13). If this decoding also occurs in vivo, it may result in the differential activation of signaling elements downstream of Ca2+. It would be interesting to determine whether a similar mechanism exists in plants.
Our data provide evidence for a role of PI-PLC in the generation of ABA-induced oscillations in guard-cell [Ca2+]cyt and suggest that oscillations in [Ca2+]cyt have the potential to encode the information required to maintain a steady-state stomatal aperture, depending on the strength of the ABA stimulus. However, to achieve an optimum stomatal aperture, guard cells must integrate signals from a range of often conflicting stimuli, many of which use Ca2+ as a second messenger (9, 10). This fact raises important questions regarding the mechanisms(s) by which guard cells distinguish between different Ca2+-mobilizing stimuli. In animals, the pattern of agonist-induced oscillations in [Ca2+]cyt varies, depending on both the strength and the type of the stimulus allowing the generation of a stimulus-specific Ca2+ signature (11–13). It has been proposed that oscillations in [Ca2+]cyt may perform the same function in Ca2+-based signal transduction in plants (9, 10). A detailed study of stimulus-induced changes in [Ca2+]cyt in plants is required to test this hypothesis; such a study would use the same cell type under identical conditions.
Acknowledgments
I.S. and C.P. were funded by grants from the Biotechnology and Biological Sciences Research Council (U.K.). L.T.M. was funded through a studentship from the University of Lancaster (U.K.). M.R.M. is grateful to The Royal Society of the U.K. for the award of a University Research Fellowship.
Footnotes
Abbreviations: ABA, abscisic acid; [Ca2+]cyt, cytosolic free Ca2+ concentration; [Ca2+]ext, external Ca2+ concentration; [K+]ext, external K+ concentration; GST, glutathione S-transferase; Ins(1,4,5)P3, inositol 1,4,5-trisphosphate; PI-PLC, phosphoinositide-specific phospholipase C; U-73122, 1-(6-{[17β-3-methoxyestra-1,3,5(10)-trien-17-yl]amino}hexyl)-1H-pyrrole-2,5-dione; U-73343, 1-(6-{[17β-3-methoxyestra-1,3,5(10)-trien-17-yl]amino}hexyl)-2,5-pyrrolidinedione.
References
- 1.Leung J, Giraudat J. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:199–222. doi: 10.1146/annurev.arplant.49.1.199. [DOI] [PubMed] [Google Scholar]
- 2.MacRobbie E A C. J Exp Bot. 1997;48:515–528. doi: 10.1093/jxb/48.Special_Issue.515. [DOI] [PubMed] [Google Scholar]
- 3.McAinsh M R, Brownlee C, Hetherington A M. Nature (London) 1990;343:186–188. [Google Scholar]
- 4.Schroeder J I, Hagiwara S. Proc Natl Acad Sci USA. 1990;87:9305–9309. doi: 10.1073/pnas.87.23.9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilroy S, Fricker M D, Read N D, Trewavas A J. Plant Cell. 1991;3:333–344. doi: 10.1105/tpc.3.4.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Irving H R, Gehring C A, Parish R W. Proc Natl Acad Sci USA. 1992;89:1790–1794. doi: 10.1073/pnas.89.5.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McAinsh M R, Brownlee C, Hetherington A M. Plant Cell. 1992;4:1113–1122. doi: 10.1105/tpc.4.9.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allan A C, Fricker M D, Ward J L, Beale M H, Trewavas A J. Plant Cell. 1994;6:1319–1328. doi: 10.1105/tpc.6.9.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McAinsh M R, Brownlee C, Hetherington A M. Physiol Plant. 1997;100:16–29. [Google Scholar]
- 10.McAinsh M R, Hetherington A M. Trends Plant Sci. 1998;3:32–36. [Google Scholar]
- 11.Clapham D E. Cell. 1995;80:259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- 12.Berridge M J. Nature (London) 1997;386:759–760. doi: 10.1038/386759a0. [DOI] [PubMed] [Google Scholar]
- 13.De Koninck P, Schulman H. Science. 1998;279:227–230. doi: 10.1126/science.279.5348.227. [DOI] [PubMed] [Google Scholar]
- 14.McAinsh M R, Webb A A R, Taylor J E, Hetherington A M. Plant Cell. 1995;7:1207–1219. doi: 10.1105/tpc.7.8.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grabov A, Blatt M R. Proc Natl Acad Sci USA. 1998;95:4778–4783. doi: 10.1073/pnas.95.8.4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ehrhardt D W, Wais R, Long S R. Cell. 1996;85:673–681. doi: 10.1016/s0092-8674(00)81234-9. [DOI] [PubMed] [Google Scholar]
- 17.Tucker E B, Boss W F. Plant Physiol. 1996;111:459–467. doi: 10.1104/pp.111.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franklin-Tong V E, Drøbak B K, Allan A C, Watkins P A K, Trewavas A J. Plant Cell. 1996;8:1305–1321. doi: 10.1105/tpc.8.8.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holdaway-Clark T L, Feijó J A, Hackett G R, Kunkel J G, Hepler P K. Plant Cell. 1997;9:1999–2010. doi: 10.1105/tpc.9.11.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drøbak B K. Biochem J. 1992;288:697–712. doi: 10.1042/bj2880697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirayama T, Ohoto C, Mizoguchi T, Shinozaki K. Proc Natl Acad Sci USA. 1995;92:3903–3907. doi: 10.1073/pnas.92.9.3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kopka J, Pical C, Gray J E, Müller-Röber B. Plant Physiol. 1998;116:239–250. doi: 10.1104/pp.116.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parmar P N, Brearley C A. Plant J. 1993;4:255–263. [Google Scholar]
- 24.Parmar P N, Brearley C A. Plant J. 1995;8:425–433. [Google Scholar]
- 25.Lee Y S, Choi Y B, Shu S, Lee J, Assmann S M, Joe C O, Kelleher J F, Crain R C. Plant Physiol. 1996;110:987–996. doi: 10.1104/pp.110.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blatt M R, Thiel G, Trentham D R. Nature (London) 1990;346:766–769. doi: 10.1038/346766a0. [DOI] [PubMed] [Google Scholar]
- 27.Gilroy S, Read N D, Trewavas A J. Nature (London) 1990;346:769–771. doi: 10.1038/346769a0. [DOI] [PubMed] [Google Scholar]
- 28.Lee Y, Assmann S M. Proc Natl Acad Sci USA. 1991;88:2127–2131. doi: 10.1073/pnas.88.6.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brearley C A, Parmar P N, Hanke D E. Biochem J. 1997;324:123–131. doi: 10.1042/bj3240123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pical C, Kopka J, Müller-Röber B, Hetherington A M, Gray J E. Plant Physiol. 1997;114:748. [Google Scholar]
- 31.Bleasdale J E, Thakur R, Gremban R S, Bundy G L, Fitzpatrick F A, Smith R J, Bunting S. J Pharmacol Exp Ther. 1990;255:756–768. [PubMed] [Google Scholar]
- 32.Smith R J, Sam L M, Justen J M, Bundy G L, Bala G A, Bleasdale J E. J Pharmacol Exp Ther. 1990;253:688–697. [PubMed] [Google Scholar]
- 33.Zheng L X, Krsmanovic L Z, Vergara L A, Catt K J, Stojilkovic S S. Proc Natl Acad Sci USA. 1997;94:1573–1578. doi: 10.1073/pnas.94.4.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stauffer T P, Ahn S, Meyer T. Curr Biol. 1998;8:343–346. doi: 10.1016/s0960-9822(98)70135-6. [DOI] [PubMed] [Google Scholar]
- 35.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 36.Pical C, Sandelius A S, Melin P-M, Sommarin M. Plant Physiol. 1992;100:1296–1303. doi: 10.1104/pp.100.3.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leckie C P, McAinsh M R, Allen G J, Sanders D, Hetherington A M. Proc Natl Acad Sci USA. 1998;95:15837–15842. doi: 10.1073/pnas.95.26.15837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roelfsema M R G. Ph.D. thesis. Groningen, The Netherlands: University of Groningen; 1997. [Google Scholar]
- 39.Roelfsema M R G, Prins B A H. Planta. 1997;202:18–27. doi: 10.1007/s004250050098. [DOI] [PubMed] [Google Scholar]
- 40.Mühling K H, Sattelmacher B. J Exp Bot. 1997;48:1609–1614. [Google Scholar]
- 41.Ettlinger C, Lehle L. Nature (London) 1988;331:176–178. doi: 10.1038/331176a0. [DOI] [PubMed] [Google Scholar]
- 42.Morse M J, Crain R C, Satter R L. Proc Natl Acad Sci USA. 1987;84:7075–7078. doi: 10.1073/pnas.84.20.7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morse M J, Crain R C, Coté G G, Satter R L. Plant Physiol. 1989;89:724–727. doi: 10.1104/pp.89.3.724. [DOI] [PMC free article] [PubMed] [Google Scholar]