Abstract
In murine models of Crohn's disease, rheumatoid arthritis and colon cancer, IL-6 (interleukin-6) signalling via the sIL-6R (soluble IL-6 receptor; termed IL-6 trans-signalling) has been shown to promote the pathology associated with these conditions. These detrimental activities can, however, be selectively blocked by soluble forms of the gp130 (glycoprotein 130) receptor. Although sgp130 (soluble gp130) therefore represents a viable therapeutic modality for the treatment of these conditions, the mass manufacture of such biologics is often expensive. The advent of molecular farming has, however, provided an extremely cost-effective strategy for the engineering of recombinant proteins. Here, we describe the expression and production of a biologically active sgp130 variant that is expressed in transgenic tobacco plants as an ELP (elastin-like peptide)-fusion protein (mini-gp130–ELP). Mini-gp130–ELP consists of the first three domains of gp130 (Ig-like domain and cytokine binding module) fused to 100 repeats of ELP. Expression of mini-gp130–ELP did not affect the growth rate or morphology of the transgenic plants, and purification was achieved using inverse transition cycling. This approach led to an overall yield of 141 μg of purified protein per g of fresh leaf weight. The purified mini-gp130–ELP specifically inhibited sIL-6R-mediated trans-signalling as measured by binding to the IL-6–sIL-6R complex and through its ability to block sIL-6R-mediated activation of STAT3 (signal transducer and activator of transcription 3) phosphorylation and proliferation in human hepatoma cells and murine pre-B-cells. Consequently, the present study validates the potential application of molecular farming in transgenic tobacco plants as a strategy for the expression and purification of therapeutically advantageous biologics such as sgp130.
Keywords: cytokine, elastin-like peptide (ELP), glycoprotein 130 (gp130), inverse transition cycling, interleukin-6 (IL-6), tobacco
Abbreviations: CaMV, cauliflower mosaic virus; CNTF, ciliary neurotrophic factor; DMEM, Dulbecco's modified Eagle's medium; EBNA, Epstein–Barr nuclear antigen; ECL, enhanced chemiluminescence; ELP, elastin-like peptide; ER, endoplasmic reticulum; FCS, foetal calf serum; gp130, glycoprotein 130; HEK-293 cell, human embryonic kidney cell; IL, interleukin; LIF, leukaemia inhibitory factor; mAb, monoclonal antibody; OSM, oncostatin M; sgp130, soluble gp130; IL-6R, IL-6 receptor; sIL-6R, soluble IL-6R; STAT, signal transducer and activator of transcription
INTRODUCTION
Cytokines are a series of small soluble polypeptides, which control cellular functions such as proliferation, differentiation and viability. Dysregulation of cytokine activity is often associated with the progression of chronic disease, and selective targeting of these proteins in various disease states has proven to be clinically beneficial. Such an approach is exemplified by the use of anti-tumour necrosis factor α regimes in rheumatoid arthritis and Crohn's disease.
Cytokines of the gp130 (glycoprotein 130) family, including IL-6 (interleukin-6), IL-11, LIF (leukaemia inhibitory factor), OSM (oncostatin M), CNTF (ciliary neurotrophic factor), cardiotropin-1, CLC (cardiotrophin-like related cytokine)/NNT-1 (neurotrophin-1)/BSF-3 (B-cell stimulating factor 3), neuropoietin and IL-27, share the membrane glycoprotein gp130 as a common signal transducer subunit (reviewed in [1,2]).
On target cells, IL-6 first binds to the IL-6R (IL-6 receptor). The resulting complex of IL-6 and IL-6R then associates with the signal transducing membrane protein gp130, thereby initiating intracellular signalling [3]. The gp130 subunit is expressed by most, if not all, cells in the body, whereas IL-6R expression is mainly limited to hepatocytes, neutrophils, monocytes/macrophages and specific lymphocyte subsets. Moreover, a sIL-6R (soluble IL-6R) has been found in various body fluids [4–11]. sIL-6R forms an agonistic complex with IL-6 that stimulates cells that only express gp130 [12,13] – a process that we have termed trans-signalling [12,14].
Interestingly, a naturally occurring sgp130 (soluble gp130) possesses antagonistic properties on IL-6 acting in complex with the sIL-6R, but not on IL-6 acting via the membrane IL-6R. At 100-fold higher concentrations, sgp130 also inhibits LIF and OSM, but not CNTF and IL-27 [15–17]. Therefore we have concluded that sgp130 is the natural inhibitor of IL-6 trans-signalling. Moreover, in mouse models of human diseases, sgp130 has been shown to be a potent candidate for therapy of, e.g., Crohn's disease, rheumatoid arthritis and colon cancer [18–21].
Following the initial report on expression of complete antibodies in transgenic plants [22], several alternative expression strategies have been described for the high-yield production of recombinant proteins in transgenic plants [23,24]. However, for many proteins, increased ER (endoplasmic reticulum) retention leads to a trapping of the recombinant protein within the plant [25,43]. Moreover, we have demonstrated a 2-fold increase in spider silk protein accumulation in tobacco leaves and a 40-fold increase in single-chain antibody accumulation in tobacco seeds after fusion to 100 repeats of an ELP (elastin-like peptide), with one peptide unit made up of Val-Pro-Gly-Xaa-Gly (Xaa is any amino acid except proline; [26–28]). This amino acid stretch is derived from the characteristic repeat motif VPGVG found in the native sequence for mammalian elastin. ELPs and ELP-fusion proteins are soluble in water below their transition temperature and become reversibly insoluble when the temperature is raised above the transition temperature (inverse transition cycling) [27]. Since we have shown that inverse transition cycling can be used to purify heat-stable spider silk proteins from transgenic tobacco leaves [26], we have now asked whether this principle could be transferred to non-heat-stable proteins with therapeutic potential. We therefore generated transgenic tobacco plants, which express a sgp130–ELP (mini-gp130–ELP) fusion protein under the transcriptional control of the plant virus promoter CaMV (cauliflower mosaic virus) 35 S. Such an approach led to the accumulation of large amounts of mini-gp130–ELP in the ER, which could be purified as a functionally active variant using inverse transition cycling.
MATERIALS AND METHODS
Cells and reagents
HepG2 cells (human hepatoma cells) were obtained from the American Type Culture Collection (Rockville, MD, U.S.A./Manassas, VA, U.S.A.). BA/F3 cells (murine pre-B-cells) stably transfected with human gp130 (Ba/F3-gp130 cells) or gp130 and IL-6R cDNAs (Ba/F3-gp130-IL-6R cells) have been previously described [29,30]. All cells were grown in DMEM (Dulbecco's modified Eagle's medium) High Glucose culture medium (PAA Laboratories, Pasching, Austria) supplemented with 10% (v/v) FCS (foetal calf serum), penicillin (60 mg/l) and streptomycin (100 mg/l) at 37 °C with 5% CO2 in a humidified atmosphere. For the cultivation of Ba/F3-gp130-IL-6R cells, the standard DMEM was supplemented with 10% FCS, penicillin (60 mg/l), streptomycin (100 mg/l) and 10 ng/ml IL-6 or 10 ng/ml Hyper-IL-6. Hyper-IL-6 is a fusion protein of IL-6 and sIL-6R connected by a flexible polypeptide linker. sgp130Fc is a fusion protein of the extracellular portion of gp130 and the Fc portion of IgG1 [16,29]. Both recombinant proteins were expressed and purified as previously described [16,29]. Hyper-IL-6–Fc is a fusion protein of the Hyper-IL-6 and the Fc portion of an IgG antibody, which was expressed in transfected EBNA (Epstein–Barr nuclear antigen)-HEK-293 cells (human embryonic kidney cells) and was directly used as conditioned supernatant. Mouse anti-STAT3 (signal transducer and activator of transcription 3) mAb (monoclonal antibody) was purchased from Cell Signaling Technology (Beverly, MA, U.S.A.), and rabbit anti-phospho-STAT3 mAb (Tyr705) was from Cell Signaling Technology. Mouse anti-human IL-6R mAb M91 was from Immunotech (Marseille, France). Mouse anti-c-Myc mAb 9E10 was from Abcam (Cambridge, U.K.). Sheep anti-mouse IgG–horseradish peroxidase and goat anti-rabbit IgG–horseradish peroxidase were from Amersham Biosciences (Little Chalfont, Bucks., U.K.). All restriction enzymes were obtained from MBI Fermentas (St. Leon-Rot, Germany). [3H]Thymidine was purchased from Amersham Biosciences (Freiburg, Germany).
Construction of the binary vector for mini-gp130–ELP under the control of the CaMV 35 S promoter for the transformation of tobacco plants
The cytokine-binding portion (D1–D3) of the cDNA coding for sgp130Fc-opt (see below) was amplified by PCR using the sense-strand oligonucleotide primer gp130-Δsignal (5′-GACGTCACATGTTTAGCAGAGCTGCTGGATCCTTGC-3′) and the gp130-NaeI (5′-CAGTGCCGGCTCTGTCCTCGTAGGTGATGC-3′) anti-sense oligonucleotide primer. The plasmid pCR-Script-sgp130Fc-opt served as a template (GeneArt, Munich, Germany). Here, the codon usage of sgp130Fc was adapted to the codon bias for Cricetulus griseus (Chinese hamster) genes (for sequence information of the cDNA coding for mini-gp130–ELP and the protein sequence of mini-gp130–ELP, see Supplementary Figures 1A and 1B at http://www.BiochemJ.org/bj/398/bj3980577add.htm). The purified PCR product was digested with AflIII and NaeI and cloned into the plasmid pRTRA7/3-35S-anti-KRES-c-myc-100xELP [27], which was linearized with AflIII and NaeI. The generation of the 100xELP-fusion protein was described previously [26]. The resulting plasmid pRTRA7/3-35S-mini-gp130-ELP was digested with HindIII, and the fragment containing the expression cassette [35 S promoter/LeB4 signal peptide/mini-gp130/c-Myc tag/ELP/KDEL (ER-retention signal)/CaMV 35 S terminator] was cloned into the binary plasmid pCB301-Kan. The vector pCB301-Kan is based on the vector pCB301 [31] and was produced by the transfer of a BglII–BamHI T-DNA fragment containing the kanamycin resistance gene of the pBIN19 vector [32].
Transformation of tobacco
The mini-gp130–ELP encoding construct was transferred into Agrobacterium tumefaciens C58C1 (pGV2260) by electroporation. Tobacco (Nicotiana tabacum cv. SNN) leaf discs were transformed as described elsewhere [33]. Regenerated transgenic plants were grown in vitro on Murashige–Skoog medium containing 100 mg/l kanamycin. Regenerated plants were grown to maturity in the greenhouse and were screened for high expression by Western-blot analysis using the anti-c-Myc mAb 9E10.
Purification of recombinant mini-gp130–ELP
Green leaves from transgenic tobacco plants were ground in a mortar under liquid nitrogen. PBS (50 ml) was added to 10 g of ground leaves and the suspension was stirred for 5 min at room temperature (21 °C). The extract was cleared by centrifugation at 8000 g for 30 min. The supernatant was filtered [Syringe Filter 0.22 μm pore diameter (Roth, Karlsruhe, Germany)] and sodium chloride was added to a final concentration of 2 M. The solution was incubated in a water bath at 40 °C for 30 min to allow for aggregation of the ELP-fusion proteins. The aggregates were precipitated by centrifugation at 8000 g for 30 min at 40 °C. The precipitate was dissolved on a vertical shaker at 200 rev./min in 50 ml of PBS for 15 min at 20 °C. Insoluble material was removed by centrifugation at 8000 g for 15 min at 20 °C. The supernatant was supplemented with sodium chloride to a final concentration of 2 M. The solution was again incubated in a water bath at 40 °C for 30 min. The precipitate was removed by centrifugation at 8000 g for 30 min at 40 °C. The precipitate was dissolved on a vertical shaker at 200 rev./min in 2 ml of PBS for 15 min at 20 °C. Insoluble material was removed by centrifugation at 8000 g for 15 min at 20 °C.
Size-exclusion chromatography
The mini-gp130–ELP protein was further purified on a calibrated HiPrep 26/60 Sephacryl S-300 High Resolution column (Amersham Biosciences, Germany) using PBS as the mobile phase with a constant flow rate of 1.0 ml/min. For calibration, the high-molecular-mass standards (Amersham Biosciences, Germany) were used (see Supplementary Figures 4A and 4B at http://www.BiochemJ.org/bj/398/bj3980577add.htm). Fractions of 2.5 ml were collected, analysed by SDS/PAGE, pooled and concentrated.
Protein concentration
The method of Waxman et al. [34] was used to determine the protein concentration. The molar absorption coefficient (ϵ) of mini-gp130–ELP at 280 nm was calculated to be 64890 litres·mol−1·cm−1. The absorption spectra were recorded in the range of 240–320 nm (Supplementary Figure 5A at http://www.BiochemJ.org/bj/398/bj3980577add.htm).
Edman sequencing
The purified mini-sgp130–ELP (10 μg) dissolved in 10 μl of PBS was sent for N-terminal sequence determination by automated Edman degradation to Sequence Laboratories Göttingen (Seqlab, Göttingen, Germany).
Immunoprecipitation
Purified mini-gp130–ELP protein was incubated with Hyper-IL-6–Fc in DMEM containing 10% FCS overnight at 4 °C followed by addition of 50 μl of Protein A–Sepharose (50% slurry; CL-4B from Amersham Biosciences, Germany) for at least 1 h at 4 °C. Immunoprecipitates were washed twice with washing buffer (10 mM Tris, pH 7.6, 150 mM NaCl, 0.2% Tween 20 and 2 mM EDTA) and once with PBS prior to addition of Laemmli sample buffer [2×Laemmli: 100 mM Tris, pH 6.8, 4% (w/v) SDS, 200 mM dithiothreitol, 20% (v/v) glycerol and 0.1% Bromophenol Blue] and boiling at 95 °C for 5 min.
ELISA
Microtitre plates (Greiner Microlon, Solingen, Germany) were coated with purified mini-gp130–ELP (5 μg/ml) in PBS. The plate was incubated overnight at room temperature. After blocking with 3% (w/v) BSA in PBS for 2 h, 100 μl aliquots of Hyper IL-6 (0.1, 0.5 and 1 μg/ml) [with or without antagonistic sgp130Fc (5 μg/ml)] were added. The plate was incubated for 1 h at room temperature. Hyper-IL-6 bound to the plate was detected by the non-neutralizing mAb M91 (working concentration 0.1 μg/ml) followed by anti-mouse IgG–horseradish peroxidase (1:1000) diluted in PBS/3% BSA. The enzymatic reaction was performed with soluble peroxidase substrate (BM Blue POD from Roche, Mannheim, Germany) at 37 °C for 30 min, and the absorbance was measured at 450 nm on an SLT Rainbow plate reader from Tecan (Maennedorf, Switzerland).
Immunoblotting and ECL (enhanced chemiluminescence) detection
For Western blotting, proteins separated by SDS/PAGE were transferred on to PVDF membranes (Hybond-P from Amersham Biosciences, Germany) by a semi-dry electroblotting procedure. Membranes were blocked in a solution of TBS (Tris-buffered saline; 10 mM Tris, pH 8, and 150 mM NaCl) supplemented with 0.02% Tween 20 and 5% (w/v) skimmed milk powder, and probed overnight with the indicated antibodies at 4 °C, followed by incubation at 21 °C for 1 h with horseradish peroxidase-conjugated secondary antibody. Immunoreactive proteins were detected using a chemiluminescence kit (ECL® plus Western Blotting Detection system; Amersham Biosciences, Germany) following the manufacturer's instructions. Band intensities were quantified using ImageJ software (National Institutes of Health, Bethesda, MD, U.S.A.).
Proliferation assays
Ba/F3-gp130-IL-6R cells were washed three times with sterile PBS and resuspended in DMEM containing 10% FCS at 5×103 cells/well of a 96-well plate. The cells were cultured in a final volume of 100 μl with additional cytokines as indicated in the legend of Figure 4(C) for 72 h and subsequently pulse-labelled with 0.25 μCi of [3H]thymidine for 4 h. The specific radioactivity of [3H]thymidine was 25 Ci/mmol. Cells were harvested on a glass fibre filter (Filtermat A from Wallac, Turku, Finland) and the filter was microwave-baked for 2 min. Subsequently, the filter was soaked in 4.5 ml of liquid-scintillation cocktail (Betaplate Scint from Wallac). [3H]Thymidine incorporated into cellular DNA was determined by scintillation counting using the MicroBeta TriLux counter from PerkinElmer (Wellesley, MA, U.S.A.). Bioassays were performed with each value being determined in triplicate.
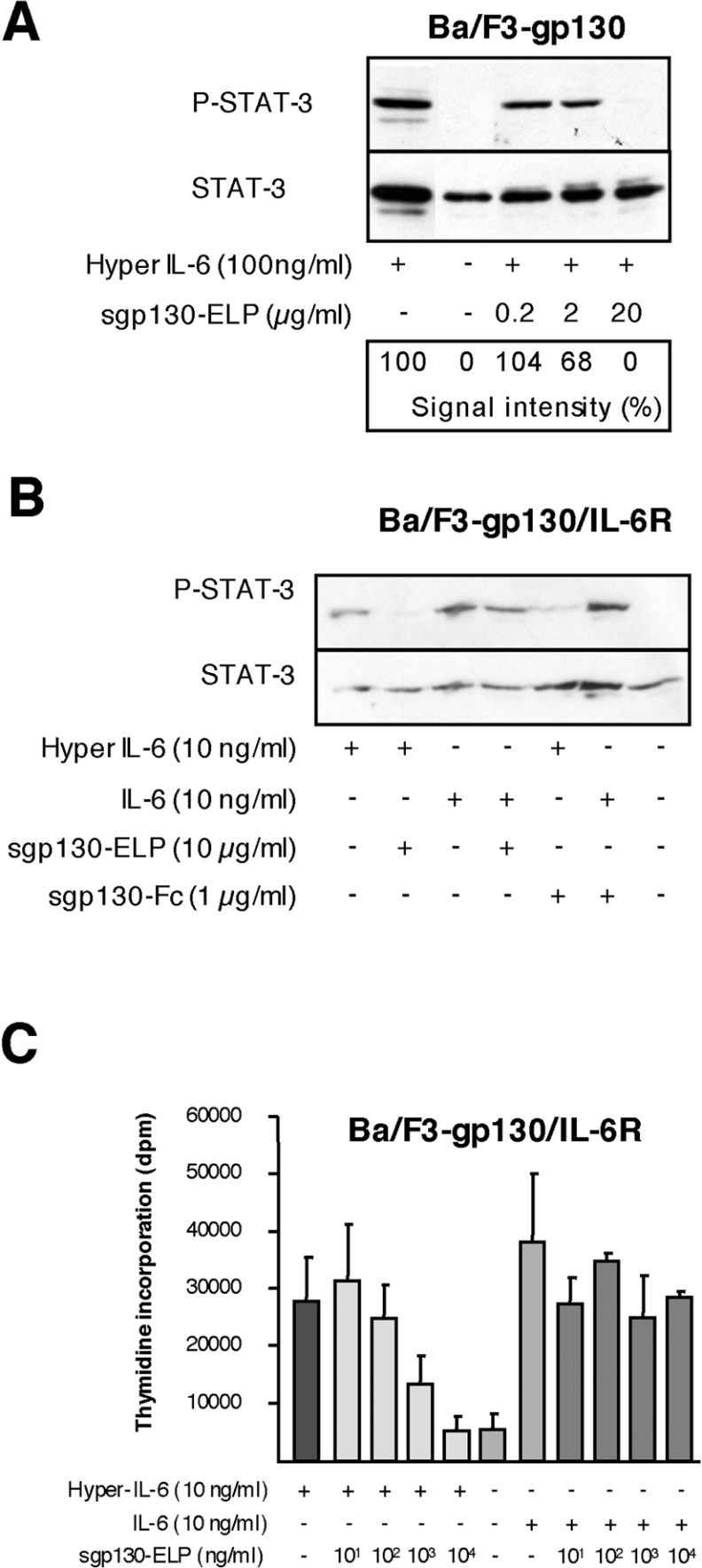
Figure 4. Plant-produced mini-gp130–ELP leads to an inhibition of Hyper-IL-6-induced Ba/F3-gp130/IL-6R proliferation.
(A) Inhibition of Hyper-IL-6-induced STAT3 phosphorylation by mini-gp130–ELP in Ba/F3-gp130 cells. Ba/F3-gp130 cells were stimulated with 100 ng/ml of IL-6 or Hyper-IL-6 for 5 min in the presence or absence of mini-gp130–ELP (0.2, 2 and 20 μg/ml). Unstimulated cells were used as controls. STAT3 phosphorylation was detected by Western blotting using anti-phospho-STAT3-specific antibodies. Western blotting against STAT3 served as loading control. (B) Inhibition of Hyper-IL-6-induced STAT3 phosphorylation by mini-gp130–ELP in Ba/F3-gp130/IL-6R cells. Ba/F3-gp130/IL-6R cells were stimulated with 10 ng/ml of IL-6 or Hyper-IL-6 for 5 min in the presence or absence of mini-gp130–ELP. Unstimulated cells were used as controls. STAT3 phosphorylation was detected by Western blotting using anti-phospho-STAT3-specific antibodies. Western blotting against STAT3 served as loading control. (C) Equal amounts of stably transfected Ba/F3-gp130-IL-6R-cells were cultured for 3 days in the presence of IL-6 or Hyper-IL-6 in combination with increasing amounts of mini-gp130–ELP. Proliferation was measured by pulse-labelling the cells after 72 h with [3H]thymidine for 4 h. Cells were harvested, and incorporated radioactivity was measured by scintillation counting. Bioassays were performed with each value being determined in triplicate.
RESULTS
The mini-gp130–ELP-fusion protein is expressed in high amounts in leaves of transgenic tobacco plants and can be purified by inverse transition cycling
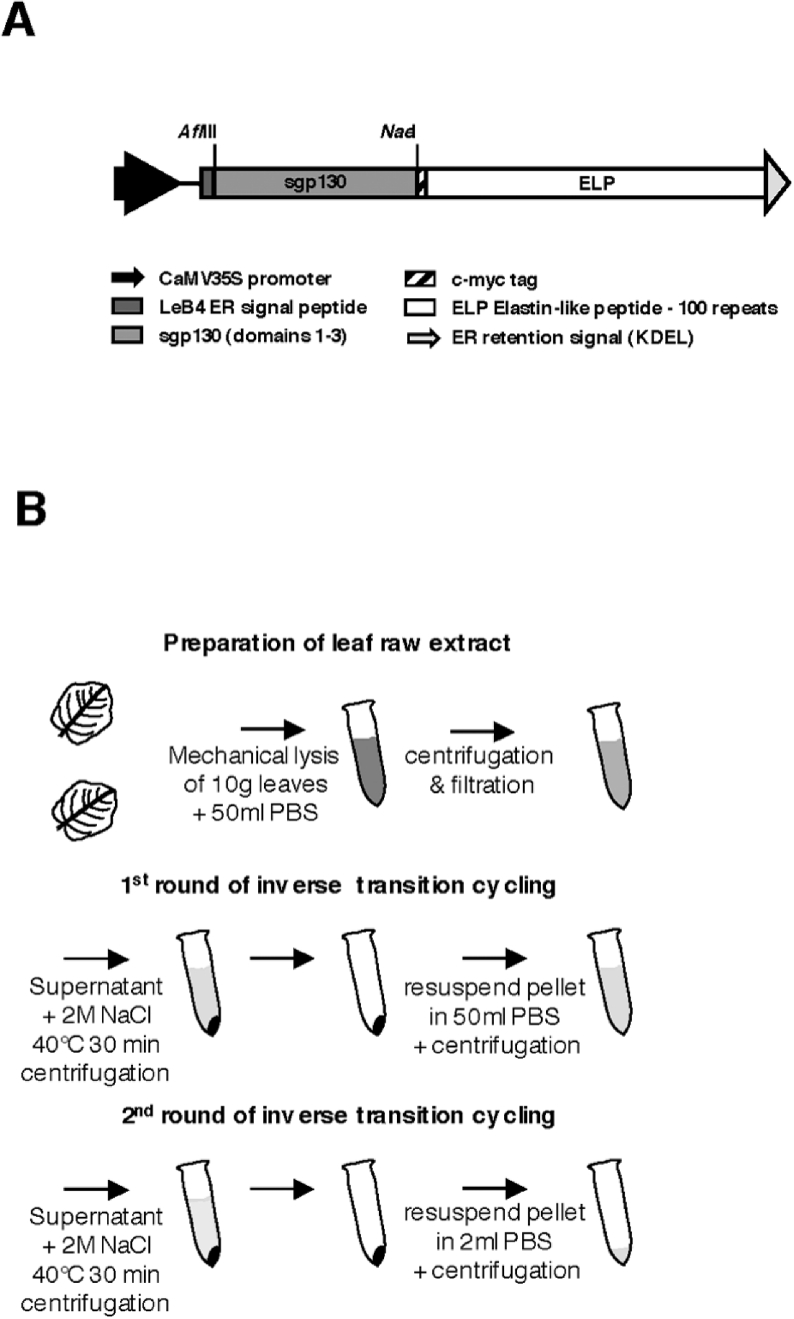
A cDNA coding for the cytokine-binding domains D1–D3 of gp130 was fused to a cDNA encoding 100 repeats of the ELP and placed under the control of the plant virus promoter CaMV 35 S (Figure 1A). For sequence information, see Supplementary Figures 1(A) and 1(B).
Figure 1. Expression and purification of mini-gp130–ELP.
(A) Schematic representation of the plant expression cassette and (B) illustration of the purification of ELP-fusion proteins from plant leaves by two rounds of ‘inverse transition cycling’.
We used ELP units that were made up of the pentapeptide Val-Pro-Gly-Xaa-Gly, where Xaa is valine, glycine or alanine residue [26]. The LeB4 signal peptide and the KDEL signal at the C-terminus caused ER retention. KDEL-mediated ER retention was chosen because maintenance of single-chain Fv fragments and spider silk proteins as ELP-fusion proteins in the ER resulted in high-level accumulation in transgenic tobacco seeds and leaves respectively [26,27]. The expression of mini-gp130–ELP without an ER-retention signal in transgenic tobacco plants was not examined. A c-Myc tag was used for detection of mini-gp130–ELP in Western blotting. Transgenic tobacco plants were generated by leaf disc transformation [33], and leaves were harvested and tested by Western blotting (results not shown). High-producers were chosen (12 out of 25 for mini-gp130–ELP; Supplementary Figure 3A at http://www.BiochemJ.org/bj/398/bj3980577add.htm) and proteins were extracted from leaves with PBS. Protein expression of recombinant mini-gp130–ELP could already be detected after SDS/PAGE separation and Coomassie Blue staining of raw leaf extracts (Figure 2A, lanes 1 and 2). Recombinant mini-gp130–ELP was purified by two rounds of inverse transition cycling as described in the Materials and methods section (Figures 1B and 2A, lanes 3–8).
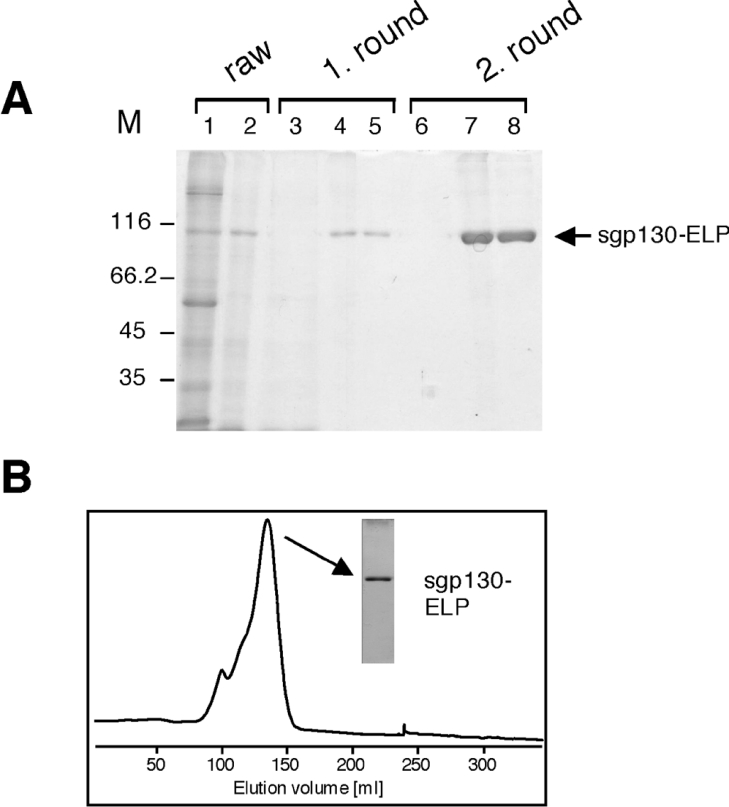
Figure 2. Purification of mini-gp130–ELP by ‘inverse transition cycling’.
(A) Lane 1: raw extract of leaf material ground in a mortar under liquid nitrogen and extracted in PBS; lane 2: centrifuged and filtered (0.2 μm pore size) raw extract; lane 3: supernatant after 1st round of inverse transition cycling; lane 4: soluble proteins after 1st round of inverse transition cycling; lane 5: soluble proteins after 1st round of inverse transition cycling cleared by centrifugation; lane 6: supernatant after 2nd round of inverse transition cycling; lane 7: soluble proteins after 2nd round of inverse transition cycling (concentrated by a factor of 25 when compared with raw extract); lane 8: soluble proteins after 2nd round of inverse transition cycling cleared by centrifugation (concentrated by a factor of 25 when compared with raw extract). Proteins were separated on an SDS/10% polyacrylamide gel and stained with Coomassie Blue. Lane M, molecular mass markers (kDa). (B) Size-exclusion chromatography of pure mini-gp130–ELP (2 ml) after two rounds of transition cycling on a calibrated HiPrep 26/60 Sephacryl S-300 High Resolution column using PBS as the mobile phase with a constant flow rate of 1.0 ml/min. Fractions of 2.5 ml were collected. Proteins were separated on an SDS/10% polyacrylamide gel and silver-stained.
Finally, 2 ml of pure mini-gp130–ELP protein from 10 g of green leaves was further purified by size-exclusion chromatography on a calibrated HiPrep 26/60 Sephacryl S-300 High Resolution column (for calibration curve, see Supplementary Figure 4). We determined the molecular mass of monomeric mini-gp130–ELP by SDS/PAGE analysis to be 100 kDa. The difference from the calculated molecular mass of mini-gp130–ELP (77.4 kDa) is likely due to glycosylation within the gp130 portion of the mini-gp130–ELP-fusion protein. Since most of the mini-gp130–ELP behaved on the column like a 240 kDa protein as estimated by comparison with a calibration curve of molecular mass standards, it is likely that most of the mini-gp130–ELP formed at least dimers (large peak in Figure 2B). A smaller fraction formed even higher ordered oligomers (650 kDa), whereas monomeric mini-gp130–ELP could not be detected. On the other hand, it could not be excluded that the presence of the ELP region resulted in an elongated protein that was eluted in the position expected for a globular protein of higher molecular mass.
Mini-gp130–ELP from the major peak was collected and the concentration was quantified by UV spectroscopy using the method of Waxman et al. [34] (see Supplementary Figure 5A). Therefore the final yield of purified mini-gp130–ELP was 141 μg of purified protein per g of leaf fresh weight (Supplementary Figure 5B).
The correct cleavage of the LeB4 signal peptide is important, since the N-terminus of gp130 is needed for efficient binding to the IL-6–IL-6R complex [36,37]. The four N-terminal amino acids of the mature mini-gp130–ELP protein were determined by automated Edman sequencing (performed by Seqlab) to be ELLD, which corresponds to the wild-type sequence of mature gp130. Purified mini-gp130–ELP protein was routinely stored at −20 °C but was also stable at 4 °C and room temperature without detectable loss of soluble recombinant protein integrity (Supplementary Figures 2A and 2B at http://www.BiochemJ.org/bj/398/bj3980577add.htm).
Mini-gp130–ELP is biologically active and inhibits exclusively IL-6 trans-signalling, but not classical IL-6 signalling
Purified mini-gp130–ELP (4 μg) was incubated at 21 °C for 1 h with a molar excess of Hyper-IL-6–Fc and precipitated by Protein A–Sepharose. Hyper-IL-6 is a fusion protein of IL-6 and the sIL-6R connected by a flexible linker [29], which can directly bind to gp130. Here, Hyper-IL-6 was additionally fused to the Fc portion of an IgG antibody to allow for direct precipitation with Protein A–Sepharose. Hyper-IL-6–Fc was produced in EBNA-HEK-293 cells and was directly used as conditioned supernatant. As depicted in Figure 3(A), mini-gp130–ELP was precipitated by Hyper-IL-6–Fc (lane 2), whereas no mini-gp130–ELP remained in the cleared supernatant after immunoprecipitation with Hyper-IL-6–Fc (lane 1). Moreover, mini-gp130–ELP could not be precipitated by Protein A–Sepharose alone (lane 3: supernatant; lane 4: immunoprecipitated protein). Since it was very likely that mini-gp130–ELP formed dimers at higher protein concentrations (Figure 2B), we cannot exclude that these dimers also existed at the lower mini-gp130–ELP concentrations used for the immunoprecipitation and for the following experiments. Therefore it is conceivable that the interaction of one out of two sgp130 molecules of the mini-gp130–ELP dimer with Hyper-IL-6 is necessary for complete immunoprecipitation of mini-gp130–ELP. Since there was no detectable mini-gp130–ELP remaining in the supernatant after immunoprecipitation with Hyper-IL-6–Fc, we conclude that at least 50% of the mini-gp130–ELP molecules are biologically active in terms of binding to the complex of IL-6 and sIL-6R.
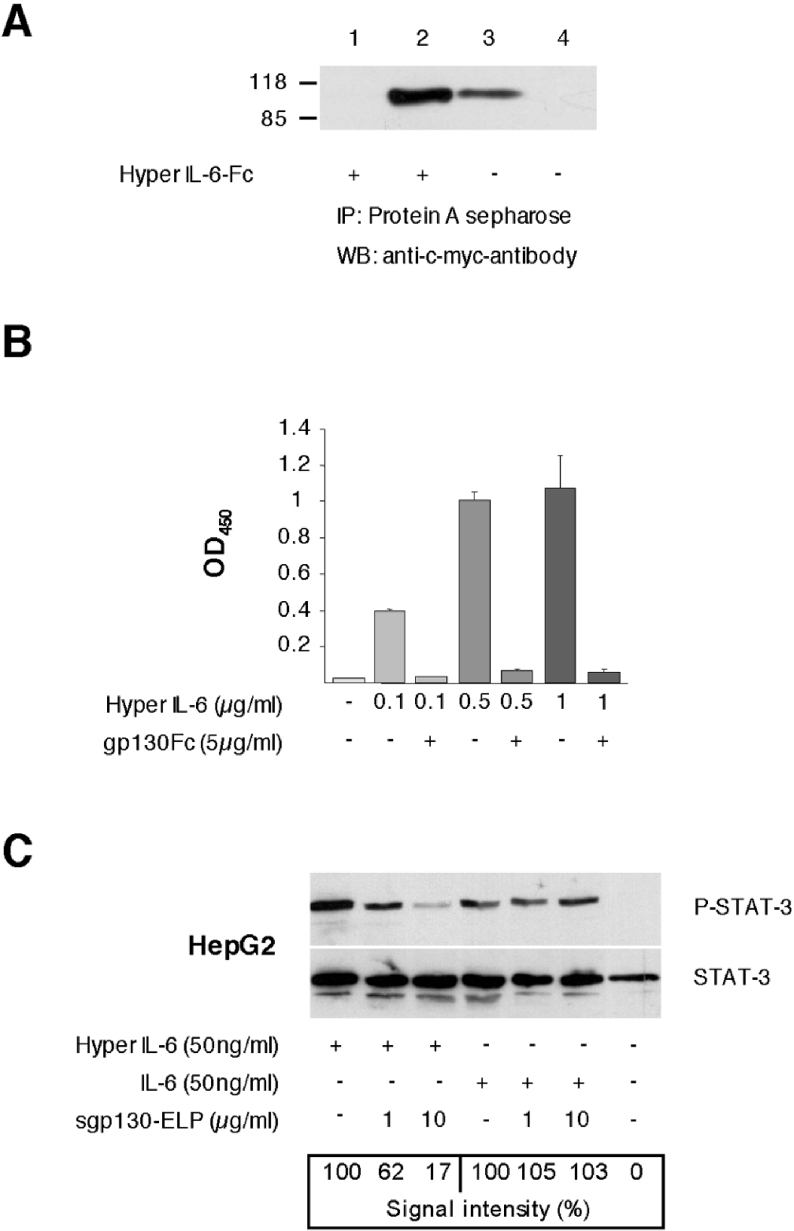
Figure 3. Plant-produced mini-gp130–ELP is biologically active.
(A) Immunoprecipitation of mini-gp130–ELP by Hyper-IL-6–Fc. Mini-gp130–ELP (4 μg) was co-incubated at 21 °C for 1 h with conditioned culture supernatant containing Hyper-IL-6–Fc or with culture supernatant without Hyper-IL-6–Fc. Precipitated proteins and supernatants were separated by SDS/PAGE and blotted on to a PVDF membrane. Proteins were detected with the c-Myc-specific antibody 9E10 and visualized by ECL detection. Lane 1: supernatant after precipitation of mini-gp130–ELP with Hyper-IL-6–Fc. Lane 2: pellet after precipitation of mini-gp130–ELP with Hyper-IL-6–Fc. Lane 3: supernatant after precipitation of mini-gp130–ELP without Hyper-IL-6–Fc. Lane 4: pellet after precipitation of mini-gp130–ELP without Hyper-IL-6–Fc. (B) Binding of Hyper-IL-6 to mini-gp130–ELP and competition with sgp130Fc as measured by ELISA. Microtitre plates were coated with mini-gp130–ELP (5 μg/ml) in PBS. After blocking with 3% BSA in PBS, 100 μl aliquots of Hyper IL-6 (0.1, 0.5 and 1 μg/ml) [with or without antagonistic sgp130Fc (5 μg/ml)] were added. Hyper-IL-6 bound to the plate was detected by the non-neutralizing anti-IL-6R mAb M91 followed by anti-mouse Ig–peroxidase conjugate. The enzymatic reaction was performed with soluble peroxidase substrate at 37 °C for 30 min and the absorbance (OD) was measured at 450 nm. (C) Inhibition of Hyper-IL-6-induced STAT3 phosphorylation by mini-gp130–ELP in human hepatoma cells (HepG2). HepG2 cells were stimulated with 50 ng/ml IL-6 or Hyper-IL-6 for 5 min in the presence or absence of mini-gp130–ELP. Unstimulated cells were used as controls. STAT3 phosphorylation was detected by Western blotting using anti-phospho-STAT3-specific antibodies. Western blotting against STAT3 served as loading control.
An ELISA was developed to detect the binding of mini-gp130–ELP to the IL-6–sIL-6R-complex. Mini-gp130–ELP protein was surface-adsorbed and increasing amounts of Hyper IL-6 were added, followed by detection via the anti-IL-6R antibody M91 (Figure 3B). The mAb M91 directed against the human IL-6R was chosen for the immunochemical detection of Hyper IL-6. M91 recognizes the Ig-like domain of IL-6R and therefore does not affect binding of Hyper-IL-6 to mini-gp130–ELP. Minigp130–ELP alone did not cross-react with the detection antibodies. Binding of Hyper-IL-6 to mini-gp130–ELP could be competed by a molar excess of sgp130Fc. sgp130Fc consists of the complete extracellular part of gp130 (domains 1–6) fused to a dimerization domain (Fc part of a human IgG-antibody) [16]. These experiments clearly show that mini-gp130–ELP binds to Hyper-IL-6, which can be competed with the sgp130Fc protein.
Next, we asked whether mini-gp130–ELP could inhibit IL-6- or Hyper-IL-6-induced STAT3 phosphorylation. Human hepatoma cells (HepG2) were stimulated with 50 ng/ml of either IL-6 or Hyper-IL-6. We have shown previously that sgp130Fc exclusively inhibits the IL-6–sIL-6R complex without affecting the activity of IL-6 [16]. As shown in Figure 3(C), increasing amounts of mini-gp130–ELP led to a dose-dependent inhibition of Hyper-IL-6-induced STAT3 phosphorylation. On the other hand, mini-gp130–ELP was not able to inhibit STAT-3 phosphorylation when the cells were stimulated with IL-6 alone. These results indicate that mini-gp130–ELP specifically inhibits IL-6 signals that were mediated by the sIL-6R but not by the membrane-bound IL-6R.
The IL-3-dependent murine pro-B-cell line Ba/F3 is the only mammalian cell line known that completely lacks gp130 expression [38]. However, if these cells are transfected with gp130 (Ba/F3-gp130 cells), their reliance on IL-3 can be circumvented by stimulation with Hyper-IL-6 [29,39–41]. Ba/F3-gp130 cells were stimulated with a fixed concentration of Hyper-IL-6 in the presence of increasing amounts of mini-gp130–ELP. Minigp130–ELP inhibited Hyper-IL-6-induced STAT3 phosphorylation of Ba/F3-gp130 cells (Figure 4A). To prove that mini-gp130–ELP selectively blocked sIL-6R-mediated responses, an identical experiment was performed in Ba/F3 cells transfected with cDNA for both gp130 and the IL-6R (Ba/F3-gp130-IL-6R cells). These cells respond to either IL-6 or Hyper-IL-6; as shown in Figure 4(B), mini-gp130–ELP selectively blocked Hyper-IL-6-induced activation of STAT3, but not the IL-6-mediated phosphorylation of STAT3 in these cells. The activity of sgp130Fc was used as a comparative control protein. Moreover, the proliferation of Ba/F3-gp130-IL-6R cells stimulated by Hyper-IL-6 could be blocked by mini-gp130–ELP in a dose-dependent manner (Figure 4C). As expected, mini-gp130–ELP had no inhibitory effect on the proliferation of Ba/F3-gp130-IL-6R cells treated with IL-6 alone. Thus mini-gp130–ELP specifically inhibits IL-6 trans-signalling, but not classical IL-6-signalling.
DISCUSSION
Plant expression systems have the potential to be used for the production of safe and biologically active recombinant proteins. It has been estimated that proteins can be produced 10–50 times cheaper in plants than in Escherichia coli [42]. Therefore the combination of safe and low-cost protein production with a protein-intrinsic purification strategy would be very desirable. So far, there have been examples of high-yield production of recombinant proteins in transgenic plants, mainly in the field of molecular medicine such as antibodies, enzymes and vaccines [42,44,45]. Moreover, health risks that may arise from contamination of recombinant proteins with potential human pathogens or toxins are minimized by molecular farming [46]. Recently, an improvement of the production of recombinant single-chain antibodies and spider silk proteins respectively in seeds and leaves has been achieved by ER retention of ELP-fusion constructs [26,27]. Furthermore, inverse transition cycling of a spider silk ELP-fusion protein has been used for purification from leaf extracts [26]. Spider silk proteins are heat-stable and therefore represent an ideal model for inverse transition cycling, since the temporary heat treatment did not lead to protein denaturation. Initially, we generated transgenic tobacco plants accumulating sgp130Fc or sgp130Fc–ELP in the ER of leaves. It turned out that these proteins of approx. 800 and 1300 amino acids respectively were produced very poorly (<0.01 and 0.05% of total soluble protein respectively; Supplementary Figure 3A). Therefore we decided to generate transgenic plants, which accumulate a minimal mini-gp130–ELP-fusion protein in the ER of leaves. Mini-gp130–ELP only contains the cytokine-binding portion of gp130 (domains 1–3303 amino acids) fused to 100 repeats of ELP and was found to be produced in high amounts in transgenic tobacco leaves by ER retention (compare Supplementary Figures 3A and 3B). So far, it has not been examined whether ELP-fusion proteins, which are expressed in the cytoplasm or in plastids, will also result in increased protein accumulation compared with the non-fusion proteins.
Inverse transition cycling was applied to purify the mini-gp130–ELP to homogeneity. It has previously been shown that for sgp130Fc, a 2–5-fold molar excess was sufficient to inhibit IL-6/sIL-6R-mediated BA/F3-gp130 cell proliferation by 50% [16]. In sgp130Fc, the complete extracellular part of gp130 (domains 1–6) was fused to a dimerization domain (Fc part of a human IgG antibody). Thereby sgp130Fc mimics the receptor signal complex on the membrane, which also exists as a preformed dimer [47]. Interestingly, the preformed sgp130Fc dimer has a 10-fold higher efficiency to inhibit IL-6/sIL-6R-mediated responses than the sgp130 monomer [16]. For mini-gp130–ELP, an 80-fold molar excess inhibited IL-6/sIL-6R-mediated BA/F3-gp130 cell proliferation by 50%. Therefore we conclude that plant-produced mini-gp130–ELP antagonized the activity of Hyper-IL-6 less efficiently than the preformed sgp130Fc dimer but with efficiency comparable with a sgp130 monomer purified from mammalian cell-culture supernatants [16]. In this respect, mini-gp130–ELP resembles the functional properties of the highly truncated spliced variant of sgp130 known as gp130-RAPS (splicing variant of the gp130 mRNA; soluble gp130 receptor), which consists of D1–D3 of gp130 plus a unique C-terminal amino acid sequence [48,49]. Interestingly, mini-gp130–ELP very likely formed dimers, which in contrast with the preformed sgp130Fc dimer are non-directional, since mini-gp130–ELP exhibited antagonistic properties comparable with the monomeric sgp130 and not with dimeric sgp130Fc.
In summary, we have generated transgenic tobacco plants accumulating high amounts of biologically active mini-gp130–ELP in the ER, which could be purified to homogeneity by inverse transition cycling. The results reported in the present study are encouraging and will stimulate the generation of constructs with a cleavable ELP portion, which can then be dimerized artificially and in a directed fashion, e.g. by free accessible C-terminal cysteine residues. This strategy might lead to plant-produced mini-gp130-cytokine antagonists with even higher activity.
Online data
Acknowledgments
We thank Christine Helmold and Stefanie Schnell for excellent technical help and Simon Jones (Department of Medical Biochemistry and Immunology, School of Medicine, Cardiff University, Cardiff, Wales, U.K.) for discussions and the help with the preparation of this paper. The work in the laboratory of S.R.-J. and J.S. was supported by grants from the Deutsche Forschungsgemeinschaft (Bonn, Germany). U.C. was supported by the Pharma Planta Project supported by the European Community.
References
- 1.Heinrich P. C., Behrmann I., Muller-Newen G., Schaper F., Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem. J. 1998;334:297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones S., Rose-John S. The role of soluble receptors in cytokine biology: the agonistic properties of the sIL-6R/IL-6 complex. Biochim. Biophys. Acta. 2002;1592:251–264. doi: 10.1016/s0167-4889(02)00319-1. [DOI] [PubMed] [Google Scholar]
- 3.Rose-John S. Coordination of interleukin-6 biology by membrane bound and soluble receptors. Adv. Exp. Med. Biol. 2001;495:145–151. doi: 10.1007/978-1-4615-0685-0_19. [DOI] [PubMed] [Google Scholar]
- 4.Lust J. A., Donovan K. A., Kline M. P., Greipp P. R., Kyle R. A., Maihle N. J. Isolation of an mRNA encoding a soluble form of the human interleukin-6 receptor. Cytokine. 1992;4:96–100. doi: 10.1016/1043-4666(92)90043-q. [DOI] [PubMed] [Google Scholar]
- 5.Rose-John S., Heinrich P. C. Soluble receptors for cytokines and growth factors: generation and biological function. Biochem. J. 1994;300:281–290. doi: 10.1042/bj3000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Müllberg J., Althoff K., Jostock T., Rose-John S. The importance of shedding of membrane proteins for cytokine biology. Eur. Cytokine Netw. 2000;11:27–38. [PubMed] [Google Scholar]
- 7.Althoff K., Reddy P., Peschon J., Voltz N., Rose-John S., Müllberg J. Contribution of the amino acid sequence at the cleavage site to the cleavage pattern of transmembrane proteins. Eur. J. Biochem. 2000;267:2624–2631. doi: 10.1046/j.1432-1327.2000.01278.x. [DOI] [PubMed] [Google Scholar]
- 8.Althoff K., Müllberg J., Aasland D., Voltz N., Kallen K.-J., Grötzinger J., Rose-John S. Recognition sequences and structural elements contribute to shedding susceptibility of membrane proteins. Biochem. J. 2001;353:663–672. doi: 10.1042/0264-6021:3530663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hundhausen C., Misztela D., Berkhout T. A., Broadway N., Saftig P., Reiss K., Hartmann D., Fahrenholz F., Postina R., Matthews V., et al. The disintegrin-like metalloproteinase ADAM10 is involved in constitutive cleavage of CX3CL1 (fractalkine) and regulates CX3CL1-mediated cell–cell adhesion. Blood. 2003;102:1186–1195. doi: 10.1182/blood-2002-12-3775. [DOI] [PubMed] [Google Scholar]
- 10.Matthews V., Schuster B., Schütze S., Kallen K.-J., Rose-John S. Cholesterol depletion of the plasma membrane triggers shedding of the human interleukin-6 receptor by TACE and independently of PKC. J. Biol. Chem. 2003;278:38829–38839. doi: 10.1074/jbc.M210584200. [DOI] [PubMed] [Google Scholar]
- 11.Abel S., Hundhausen C., Mentlein R., Schulte A., Berkhout T. A., Broadway N., Hartmann D., Sedlacek R., Dietrich S., Muetze B., et al. The transmembrane CXC-chemokine ligand 16 is induced by IFN-gamma and TNF-alpha and shed by the activity of the disintegrin-like metalloproteinase ADAM10. J. Immunol. 2004;172:6362–6372. doi: 10.4049/jimmunol.172.10.6362. [DOI] [PubMed] [Google Scholar]
- 12.Mackiewicz A., Schooltink H., Heinrich P. C., Rose-John S. Complex of soluble human IL-6-receptor/IL-6 up-regulates expression of acute-phase proteins. J. Immunol. 1992;149:2021–2027. [PubMed] [Google Scholar]
- 13.Taga T., Hibi M., Hirata Y., Yamasaki K., Yasukawa K., Matsuda T., Hirano T., Kishimoto T. Interleukin-6 triggers the association of its receptor with a possible signal transducer, gp130. Cell. 1989;58:573–581. doi: 10.1016/0092-8674(89)90438-8. [DOI] [PubMed] [Google Scholar]
- 14.Jones S. A., Richards P. J., Scheller J., Rose-John S. IL-6 transsignaling: the in vivo consequences. J. Interferon Cytokine Res. 2005;25:241–253. doi: 10.1089/jir.2005.25.241. [DOI] [PubMed] [Google Scholar]
- 15.Hui W., Bell M., Carroll G. Soluble glycoprotein 130 (gp130) attenuates OSM- and LIF-induced cartilage proteoglycan catabolism. Cytokine. 2000;12:151–155. doi: 10.1006/cyto.1999.0550. [DOI] [PubMed] [Google Scholar]
- 16.Jostock T., Müllberg J., Özbek S., Atreya R., Blinn G., Voltz N., Fischer M., Neurath M. F., Rose-John S. Soluble gp130 is the natural inhibitor of soluble IL-6R transsignaling responses. Eur. J. Biochem. 2001;268:160–167. doi: 10.1046/j.1432-1327.2001.01867.x. [DOI] [PubMed] [Google Scholar]
- 17.Scheller J., Schuster B., Holscher C., Yoshimoto T., Rose-John S. No inhibition of IL-27 signaling by soluble gp130. Biochem. Biophys. Res. Commun. 2005;326:724–728. doi: 10.1016/j.bbrc.2004.11.098. [DOI] [PubMed] [Google Scholar]
- 18.Atreya R., Mudter J., Finotto S., Müllberg J., Jostock T., Wirtz S., Schütz M., Bartsch B., Holtmann M., Becker C., et al. Blockade of IL-6 transsignaling abrogates established experimental colitis in mice by suppression of the antiapoptotic resistance of lamina propria T cells. Nat. Med. 2000;6:583–588. doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- 19.Nowell M. A., Richards P. J., Horiuchi S., Yamamoto N., Rose-John S., Topley N., Williams A. S., Jones S. A. Soluble IL-6 receptor governs IL-6 activity in experimental arthritis: blockade of arthritis severity by soluble glycoprotein 130. J. Immunol. 2003;171:3202–3209. doi: 10.4049/jimmunol.171.6.3202. [DOI] [PubMed] [Google Scholar]
- 20.Becker C., Fantini M. C., Schramm C., Lehr H. A., Wirtz S., Nikolaev A., Burg J., Strand S., Kiesslich R., Huber S., et al. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity. 2004;21:491–501. doi: 10.1016/j.immuni.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 21.Becker C., Fantini M. C., Wirtz S., Nikolaev A., Lehr H. A., Galle P. R., Rose-John S., Neurath M. F. IL-6 signaling promotes tumor growth in colorectal cancer. Cell Cycle. 2005;4:217–220. [PubMed] [Google Scholar]
- 22.Hiatt A., Cafferkey R., Bowdish K. Production of antibodies in transgenic plants. Nature (London) 1989;342:76–78. doi: 10.1038/342076a0. [DOI] [PubMed] [Google Scholar]
- 23.Schillberg S., Fischer R., Emans N. Molecular farming of recombinant antibodies in plants. Cell. Mol. Life Sci. 2003;60:433–445. doi: 10.1007/s000180300037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma J. K.-C., Drake P., Christou P. The production of pharmaceutical proteins in plants. Nat. Rev. Genet. 2003;4:794–805. doi: 10.1038/nrg1177. [DOI] [PubMed] [Google Scholar]
- 25.Artsaenko O., Peisker M., zur Nieden U., Fiedler U., Weiler E. W., Muntz K., Conrad U. Expression of a single-chain Fv antibody against abscisic acid creates a wilty phenotype in transgenic tobacco. Plant J. 1995;8:745–750. doi: 10.1046/j.1365-313x.1995.08050745.x. [DOI] [PubMed] [Google Scholar]
- 26.Scheller J., Henggeler D., Viviani A., Conrad U. Purification of spider silk-elastin from transgenic plants and application for human chondrocyte proliferation. Transgenic Res. 2004;13:51–57. doi: 10.1023/b:trag.0000017175.78809.7a. [DOI] [PubMed] [Google Scholar]
- 27.Scheller J., Leps M., Conrad U. Forcing single-chain variable fragment production in tobacco seeds by fusion to elastin-like polypeptides. Plant. Biotech. J. 2006;4:243–247. doi: 10.1111/j.1467-7652.2005.00176.x. [DOI] [PubMed] [Google Scholar]
- 28.Scheller J., Conrad U. Plant-based material, protein and biodegradable plastic. Curr. Opin. Plant Biol. 2005;8:188–196. doi: 10.1016/j.pbi.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 29.Fischer M., Goldschmitt J., Peschel C., Kallen K. J., Brakenhoff J. P. J., Wollmer A., Grötzinger J., Rose-John S. A designer cytokine with high activity on human hematopoietic progenitor cells. Nat. Biotechnol. 1997;15:142–145. doi: 10.1038/nbt0297-142. [DOI] [PubMed] [Google Scholar]
- 30.Vollmer P., Oppmann B., Voltz N., Fischer M., Rose-John S. A role for the immunoglobulin-like domain of the human IL-6 receptor: intracellular protein transport and shedding. Eur. J. Biochem. 1999;263:438–446. doi: 10.1046/j.1432-1327.1999.00511.x. [DOI] [PubMed] [Google Scholar]
- 31.Xiang C., Han P., Lutziger I., Wang K., Oliver D. J. A mini binary vector series for plant transformation. Plant Mol. Biol. 1999;40:711–717. doi: 10.1023/a:1006201910593. [DOI] [PubMed] [Google Scholar]
- 32.Bevan M. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 1984;12:8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zambriski P., Joos H., Gentello J., Leemans J., Van Montagu M., Schell J. Ti-plasmid vector for introduction of DNA into plant cells without altering their normal regeneration capacity. EMBO J. 1983;2:2111–2123. doi: 10.1002/j.1460-2075.1983.tb01715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waxman E., Rusinova E., Hasselbacher C. A., Schwartz G. P., Laws W. R., Ross J. B. Determination of the tryptophan:tyrosine ratio in proteins. Anal. Biochem. 1993;210:425–428. doi: 10.1006/abio.1993.1220. [DOI] [PubMed] [Google Scholar]
- 35. Reference deleted.
- 36.Chow D.-C., He X.-L., Snow A. L., Rose-John S., Garcia K. C. Structure of an extracellular gp130-cytokine receptor signalling complex. Science. 2001;291:2150–2155. doi: 10.1126/science.1058308. [DOI] [PubMed] [Google Scholar]
- 37.Hammacher A., Richardson R. T., Layton J. E., Smith D. K., Angus L. J., Hilton D. J., Nicola N. A., Wijdenes J., Simpson R. J. The immunoglobulin-like module of gp130 is required for signaling by interleukin-6, but not by leukemia inhibitory factor. J. Biol. Chem. 1998;273:22701–22707. doi: 10.1074/jbc.273.35.22701. [DOI] [PubMed] [Google Scholar]
- 38.Hibi M., Murakami M., Saito M., Hirano T., Taga T., Kishimoto T. Molecular cloning and expression of an IL-6 signal transducer, gp130. Cell. 1990;63:1149–1157. doi: 10.1016/0092-8674(90)90411-7. [DOI] [PubMed] [Google Scholar]
- 39.Palacios R., Steinmetz M. IL-3-dependent mouse clones that express B-220 surface antigen, contain Ig genes in germ-line configuration, and generate B lymphocytes in vivo. Cell. 1985;41:727–734. doi: 10.1016/s0092-8674(85)80053-2. [DOI] [PubMed] [Google Scholar]
- 40.Gearing D. P., Ziegler S. F., Comeau M. R., Friend D., Thoma B., Cosman D., Park L., Mosley B. Proliferative responses and binding properties of hematopoietic cells transfected with low-affinity receptors for leukemia inhibitory factor, oncostatin M, and ciliary neurotrophic factor. Proc. Natl. Acad. Sci. U.S.A. 1994;91:1119–1123. doi: 10.1073/pnas.91.3.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horsten U., Muller Newen G., Gerhartz C., Wollmer A., Wijdenes J., Heinrich P. C., Grotzinger J. Molecular modeling-guided mutagenesis of the extracellular part of gp130 leads to the identification of contact sites in the interleukin-6 (IL-6).IL-6 receptor.gp130 complex. J. Biol. Chem. 1997;272:23748–23757. doi: 10.1074/jbc.272.38.23748. [DOI] [PubMed] [Google Scholar]
- 42.Scheller J., Guhrs K. H., Grosse F., Conrad U. Production of spider silk proteins in tobacco and potato. Nat. Biotechnol. 2001;19:573–577. doi: 10.1038/89335. [DOI] [PubMed] [Google Scholar]
- 43.Artsaenko O., Kettig B., Fiedler U., Conrad U., Düring K. Potato tubers as a biofactory for recombinant antibodies. Mol. Breeding. 1998;4:313–319. [Google Scholar]
- 44.Larrick J. W., Thomas D. W. Producing proteins in transgenic plants and animals. Curr. Opin. Biotechnol. 2001;12:411–418. doi: 10.1016/s0958-1669(00)00236-6. [DOI] [PubMed] [Google Scholar]
- 45.Richter L. J., Thanavala Y., Arntzen C. J., Mason H. S. Production of hepatitis B surface antigen in transgenic plants for oral immunization. Nat. Biotechnol. 2001;18:1167–1171. doi: 10.1038/81153. [DOI] [PubMed] [Google Scholar]
- 46.Daniell H., Streatfield S. J., Wycoff K. Medical molecular farming: production of antibodies, biopharmaceuticals and edible vaccines in plants. Trends Plant Sci. 2001;6:219–226. doi: 10.1016/S1360-1385(01)01922-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giese B., Roderburg C., Sommerauer M., Wortmann S. B., Metz S., Heinrich P. C., Muller-Newen G. Dimerization of the cytokine receptors gp130 and LIFR analysed in single cells. J. Cell Sci. 2005;118:5129–5140. doi: 10.1242/jcs.02628. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka M., Kishimura M., Ozaki S., Osakada F., Hashimoto H., Okubo M., Murakami M., Naka K. Cloning of novel soluble gp130 and detection of its neutralizing autoantibodies in rheumatoid arthritis. J. Clin. Invest. 2001;106:137–144. doi: 10.1172/JCI7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Richards P. J., Nowell M. A., Horiuchi S., McLoughlin R. M., Fielding C. A., Grau S., Yamamoto N., Ehrmann M., Rose-John S., Williams A. S., et al. Functional characterization of a soluble gp130 isoform and its therapeutic capacity in an experimental model of inflammatory arthritis. Arthritis Rheum. 2006;54:1662–1672. doi: 10.1002/art.21818. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.