Abstract
Population dynamics result from the interplay of density-independent and density-dependent processes. Understanding this interplay is important, especially for being able to predict near-term population trajectories for management. In recent years, the study of model systems—experimental, observational and theoretical—has shed considerable light on the way that the both density-dependent and -independent aspects of the environment affect population dynamics via impacting on the organism's life history and therefore demography. These model-based approaches suggest that (i) individuals in different states differ in their demographic performance, (ii) these differences generate structure that can fluctuate independently of current total population size and so can influence the dynamics in important ways, (iii) individuals are strongly affected by both current and past environments, even when the past environments may be in previous generations and (iv) dynamics are typically complex and transient due to environmental noise perturbing complex population structures. For understanding population dynamics of any given system, we suggest that ‘the devil is in the detail’. Experimental dissection of empirical systems is providing important insights into the details of the drivers of demographic responses and therefore dynamics and should also stimulate theory that incorporates relevant biological mechanism.
Keywords: environmental stochasticity, time-series, inverse problem, life history, phenotypic plasticity
1. Introduction
The world is undergoing a potentially ‘deadly anthropogenic cocktail’ (Travis 2003) of habitat destruction and climate change. Habitat destruction is realized by both a reduction in the total amount of habitat available for organisms to inhabit, and also its spatial distribution, with habitat often becoming fragmented into small, isolated patches. Climate change, driven by greenhouse gas emissions, is resulting in shifts of ‘climate envelopes’ as the mean climate changes, but will also result in changes in the variance in climatic conditions (at differing temporal scales: daily, annual, decadal) and the frequency of extreme climatic events (IPCC 2002).
Given this changing world, how can we predict how populations may respond? The vast majority of population dynamic analyses focus on using time-series methods to analyse population abundances. Using phenomenological statistical models, such as simple auto-regressive models fitted to time-series data, to predict future dynamics may not work for three reasons. Firstly, the combination of global warming, changes in greenhouse gases and habitat change is unique so the future is unprecedented in environmental novelty. Predicting out of the domain of the data is widely seen as unwise (e.g. Anon 2004; Rice 2004). Secondly, threshold effects abound in nonlinear systems and biological systems are typically nonlinear (Hastings et al. 2005). We cannot therefore expect that species that have responded in a certain way to incremental changes in habitat fragmentation or climate will continue to respond in the same way to future changes. Thirdly, estimating population parameters typically means ignoring inter-individual differences and, we are increasingly realizing, these demographic performance heterogeneities between individuals, or groups of individuals, can have considerable dynamical consequences (Coulson et al. 2001; Benton et al. 2004).
To predict how species may respond to a changing environment requires understanding the mechanisms by which the environment (its mean and variability) create the changes in the population dynamics (Sutherland & Norris 2002). Environmental effects on population dynamics are mediated through changing demographic rates as different environments lead to changes in the life history (survival, fecundity, etc.), which directly affect population size. Hence, identifying, and understanding, variation in demography between individuals is necessary to understand the way that environments will impact on dynamics: heterogeneity between individuals carries key information that should not be discarded in favour of finding a population average parameter. Our aim in this review is to examine the empirical evidence for differences between individuals, the biological mechanisms that give rise to the differences and the consequences such differences have for population dynamics. We end with some discussion of the implications that individual differences have in creating complex causation driving population dynamics. Our intention is not to review ‘how to model population dynamics’ (for that, see de Roos & Persson 2005), but to pull together the evidence for the importance of individual variability in understanding dynamics.
2. Individual variation: empirical evidence and causation
Much of our understanding of the mapping of the environment to population dynamics through demography has arisen from the study of empirical model systems (of which there is a continuum ranging from laboratory systems, like Drosophila, Callosobruchus, Sancassania1 to systems studied in depth in the field, like Soay sheep or red deer). A common finding is that populations are often structured in non-obvious ways (e.g. the detail of the structure within a stage class such as ‘juveniles’ affects the population response to perturbations). A second finding is that life histories are often plastic as different environments alter both the way that traits are expressed and the covariation between traits, which may be direct (e.g. between current growth and survival) or delayed (e.g. between current growth and future fecundity). Thus, an individual's response to a given environment depends on both the current environment and the past environment. The corollary of this is that we should expect that there are complex patterns of change in the life history as the environment (including population density) changes, making density-dependent processes more complex than often assumed. A third finding is that evolution is both common and rapid.
(a) Life cycles imply population structure
The biggest cause of heterogeneity between individuals occurs due to differences between them in their stage or age, and the importance of this for population biology has been recognized for sometime (e.g. matrix population models; Caswell 2001).
Population structure is important for two principal and related reasons: (i) the life cycle takes time to complete and (ii) different ages or stages may be affected differently by an environmental effect. This creates lagged-effects in the dynamics as the numerical response to the environmental states works its way through the life cycle (Coulson et al. 2001; Clutton-Brock & Coulson 2002). For example, experiments on soil mites Sancassania berlesei show that the same environmental state, food supply in this case, can lead to different population trajectories depending on the population structure rather than density (Benton & Beckerman 2005): adults respond to food by increasing fecundity, juveniles by growing, so adult-biased populations grow faster following a food pulse than juvenile-biased populations. Even experiments using a rotifer model system, chosen for its apparent simplicity, have shown that incorporating the age structure of the rotifer in mathematical models is necessary to match their output to empirical results (Fussman et al. 2005). Similarly, a mismatch of model predictions and observed dynamics in the responses of mites to perturbations was ascribed to not incorporating sufficient structure in the ‘juvenile’ stage (Benton et al. 2004).
(b) Plasticity, trade-offs and inter-generational effects
Organisms typically exhibit phenotypic plasticity, where the phenotype varies in response to the environment. Plasticity is probably ubiquitous though the traits affected will vary between organisms. For example, in non-seasonally forced environments individual growth rates may vary proportionately with food availability resulting in plasticity in age at maturity. However, in seasonally forced environments, the costs of not being a given size at a given time may require that growth rates vary out of proportion to food availability by changing the relative proportions of food ‘spent’ on growth versus ‘reserves’ leading to the accelerated or compensatory growth shown by many organisms following periods of food deprivation (Metcalfe & Monaghan 2001; Ali et al. 2003; Dmitriew & Rowe 2005). Such organisms may have fixed size or age at maturity but the plasticity in resource allocation strategies results in later plasticity in other traits like reproduction or senescence (Metcalfe & Monaghan 2001; Bateson et al. 2004; Forsen et al. 2004; Ozanne & Hales 2004; Yearsley et al. 2004).
In general, changes in demographic rates are unlikely to be independent of each other. When resources are common, increased investment in many different traits may be possible, with traits positively covarying; when resources are rare the linkage of life-history traits through the presence of trade-offs (e.g. between current traits like growth versus reproduction or traits linked across time like fecundity versus future survival) means that traits may covary negatively. As resources vary across time and space, and individuals' abilities to access resources also varies, it is likely that even in food-limited populations where trade-offs may create negative trait covariation, there will be some individuals with more resources and positive trait covariation.
The way that traits covary means that individuals that experience common environmental conditions can carry the signature of those conditions throughout life. This is especially true of exposure to early environments, which can lead to marked differences in the life histories of cohorts of organisms born at different times (Lindstrom 1999; Beckerman et al. 2002; Reid et al. 2003; Bateson et al. 2004; Loison et al. 2004; Solberg et al. 2004).
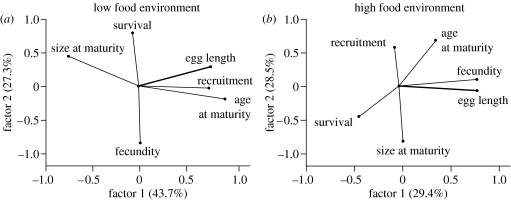
Organisms living in a variable environment may face radically different availability of resources over time (whether the resource is limited by abiotic conditions such as the weather or biotic interactions like density-dependent competitive processes). The resource allocation decisions that may have evolved are likely to lead to different relationships between life-history traits or demographic rates depending on conditions (Plaistow et al. 2006). For example, hatchling soil mites trade-off different components in different environments. Under low food conditions, hatchlings from large eggs ‘defend’ survival at the expense of growth, under high food conditions they defend fecundity, presumably by investing in reserves, at the expense of growth, and in medium food conditions they grow fast. Thus, the size of eggs individuals hatch from correlates with different traits depending on resources (figure 1). Similar context-dependent trade-offs between traits has been identified recently in other model systems such as the seed beetle Callsobruchus maculatus (Messina & Fry 2003) and woodlouse, Porcellio laevis (Lardies et al. 2004).
Figure 1.
Context-dependent life-history variation. Factor plots for a factor analysis of six life-history traits for soil mites living in environments characterized by high and low food availability. The angle between vectors reflects the correlations between the traits (less than 90°, positive correlations; greater than 90°, negative correlations; 90°, no correlation). Under low food conditions, the size of the egg an individual hatches from (egg length) is strongly positively correlated with survival to adulthood (recruitment) and age at maturity, negatively with size at maturity but uncorrelated with adult survival (survival) or fecundity. Conversely, under high food conditions, egg size is strongly correlated with fecundity and adult survival and not with juvenile survival to recruitment or size and age at maturity. Figure after fig. 4 in Plaistow et al. (2006).
One particularly important context-dependent trade-off is the parental provisioning of offspring, as this can link the environments experienced by past generations to the current generation. Parental reproductive allocation strategies can vary markedly over time as resources and other dependent variables change (such as age). Frequently this means that both the number of offspring change with conditions, and also the per capita investment in each one (Bridges & Heppell 1996; Fox et al. 1997; Kruuk et al. 1999; Potti 1999; Fox & Czesak 2000; LaMontagne & McCauley 2001; Lardies et al. 2004; Benton et al. 2005). In recent years, studies of model systems have frequently shown that patterns of maternal investment—as with conditions during early development of the offspring—have a marked effect on the later life history of the offspring (Mousseau & Fox 1998; Potti 1999; Fox et al. 2003; Alekseev & Lampert 2004; Bateson et al. 2004; Dyer 2004; Lardies et al. 2004; Meylan et al. 2004; Gendreau et al. 2005; Stillwell & Fox 2005). These patterns may typically be driven by changes in the position on trade-offs created by the initial investment in the offspring, and, as a result change the way that the organism responds to its current environment. It has recently been experimentally shown that changes in propagule size influence growth rates and therefore size and age at reproduction of the offspring, which subsequently affect the offsprings' reproductive allocation decisions (LaMontagne & McCauley 2001; Plaistow et al. 2006). This linking of the size of propagule an individual hatches from with the size of propagule the individual then produces creates inter-generational effects that can still be detected even after three generations (Plaistow et al. 2006).
(c) Evolution in empirical systems
Many empirical systems have exhibited rapid evolutionary responses to experimental conditions (Scheiner & Yampolsky 1998; Shertzer & Ellner 2002; Friedenberg 2003; Heath et al. 2003; Houle & Rowe 2003; Prasad et al. 2003; Conover et al. 2005; Fussman et al. 2005; Mueller et al. 2005; Tuda & Shimada 2005) and, perhaps more rarely, field-based systems have shown evolutionary responses (Coulson et al. 2003; Reale et al. 2003; Olsen et al. 2004; Simmons & Thomas 2004). Such rapid or ‘contemporary’ evolution (Stockwell et al. 2003) has considerable implications for predicting population dynamics as the mean parameters in population models may therefore change in response to changing environments both due to plasticity and a response to selection. The possibility of contemporary evolution in management thinking is therefore important (Law 2000; Ashley et al. 2003; Conover et al. 2005).
3. The dynamical consequences of individual heterogeneities
(a) Population structure per se
Structured population models (e.g. matrix population models; Caswell 2001) have long been a route to link information on life-history traits, through demographic rates, to population consequences. At their simplest, they relate the demographic rates (i.e. age- or stage-structured parameters) to asymptotic population growth rate, λ. For example, sensitivity analyses relate independent changes in each parameter to their effect on λ (Caswell 2001). Life table response experiments consider the change in demographic rates caused by some, typically experimental, manipulation and how these affect the population growth rate, λ (Caswell 1989, 2001). Such analyses have largely been conducted in laboratories, especially in ecotoxicology (e.g. Hansen et al. 1999) but can also be applied to field populations (e.g. Dobson & Oli 2001). These matrix approaches are, however, sensitive to the assumptions of population structure in terms of the dimension of the projection matrix (Enright et al. 1995; De Matos & Matos 1998; Easterling et al. 2000; Yearsley & Fletcher 2002). In addition, as a typical empirical finding is that traits covary, simple sensitivity analysis of λ (Caswell 2001), where traits are assumed to vary independently, may be a poor basis for estimating the links between demography and dynamics (Coulson et al. 2005). The problem of discretizing continuous variation into a small number of stage classes is avoided within the integral projection matrix framework (Easterling et al. 2000; Ellner & Ress 2006), or the equivalent integro-physiologically structured models (Diekmann et al. 1998, 2001), which allow ‘stages’ to be effectively composed of individuals.
Population structure directly influences population dynamics because of the time it takes for the organisms to complete the life cycle. This is important as different stages may respond to perturbations in different ways, but also because time lags may generally destabilize dynamics. For example, generation cycles, created by asymmetric cohort competition, occur in many well-studied laboratory organisms (Knell 1998; Briggs et al. 2000; Wearing et al. 2004; Costantino et al. 2005).
(b) Individual heterogeneities
Thinking about population dynamics has typically concentrated on population average demographic rates. Increasingly, there is realization that variation between individuals is both common and dynamically important (e.g. Pfister & Stevens 2003; de Roos & Persson 2005). Depending on the details of the density dependence, cohort variation can lead to increased variability in the population dynamics or decreased variability in the dynamics (Lindstrom & Kokko 2002; Beckerman et al. 2003). Plasticity in the time to reach maturity or longevity can lead to biologically important dynamical lags being context-dependent rather than of a fixed time-step (e.g. age at maturity is density dependent in mites, so in a discrete model the lag between fecundity and adult numbers is not fixed; Benton & Beckerman 2005; Benton et al. 2005). Such plasticity in the dynamical lags means that population structure varies with time as cohorts of individuals may progress through their life history in unison or diverge depending on conditions. Also, the way individuals respond to a particular environmental state (such as food supply) may depend on the current environment (including density) and their ‘condition’, itself a function of previous environments. Population regulation therefore becomes very complex as different traits will be affected by competition at different times and at different densities (both within and between age classes) and under different conditions (Dobson & Oli 2001; Clutton-Brock & Coulson 2002; Benton et al. 2004), and indirect effects may be common as stages which are reduced in density may lead to compensatory increases in the density of other stages (Moe et al. 2002; Cameron & Benton 2004). Additionally, we also expect that traits such as dispersal will typically be condition dependent so that dispersal rates will be context-dependent with the inevitable population consequences this leads to (Bowler & Benton 2005).
(c) Inter-generational effects
The influence of maternal effects on population dynamics, especially of cyclic species, has been much discussed and biological information has been used to parameterize models to investigate the effect (Bridges & Heppell 1996; Crone 1997; Turchin & Hanski 2001; Kendall et al. 2005). Linking from the biology of maternal effects directly to population dynamics has been attempted by looking for signatures of past conditions in animals in the field—typically without success (Myers et al. 1998; Ergon et al. 2001a,b; Banks & Powell 2004). Recently, using the soil mite experimental system, Benton et al. (2005) show that populations initiated with eggs of different sizes showed markedly different population trajectories, confirming that maternal effects can be dynamically important.
4. Consequences of individual variation for theory and analysis
In the natural world, chance events (amount of food, weather, etc.) interact with more deterministic biological rules to generate the emergent behaviour of population dynamics. Thus, understanding any population time-series requires incorporating environmental variation into the population biology and coping with the inherent structure created by the lags as individuals progress through the life cycle (Costantino et al. 2005; Hastings et al. 2005). Even very simple nonlinear, equilibrium, population models can exhibit highly complex dynamics when exposed to stochastic forcing.
(a) Time-series analysis
Understanding the role of the life history in population dynamics requires estimating the contributions of individual heterogeneities (both within and between ages or stages), environmental variance and density dependence to population fluctuations. Lande et al. (in press) have recently shown that within a time-series analytical framework (i) estimating properties such as the environmental variance without taking into account the lags created by stage structure can lead to considerable biases and (ii) it is possible to remove much of the complexity in the age-structured analysis by analysing temporal patterns in the population's total reproductive value rather than the coupled age-structured time-series (i.e. simplify a multivariate analysis into a univariate one). In this way, it is possible to estimate the environmental variance and therefore the total strength of density dependence (in terms of approach speed to equilibrium), and therefore have the tool available to project forward the population dynamics.
(b) Similar time-series can arise from different mechanisms
Typical analyses of population time-series have tried to extract biological information by fitting models to data. Recent work, investigating the complexity of the interaction between stochasticity and deterministic behaviour in structured population models, shows biologically similar models can give rise to different dynamical behaviour, and conversely, time-series with similar statistical descriptions can arise from very different processes (Greenman & Benton 2003, 2004, 2005a,b). Greenman & Benton analyse simple structured, density-dependent models at equilibrium. With such models, noise can interact with the density-dependence and have little effect or can lead to highly complex dynamics. A number of different factors influence the interaction between noise and determinism, including (i) the closeness of stability boundaries in parameter space (where the dynamics bifurcates from stable to unstable) and their type, (ii) the structure of the model, which can affect the transmission of the noise from stage to stage and (iii) the structure of the environmental noise (e.g. whether it is ‘white’, ‘red’—with low-frequency fluctuations or ‘blue’—with high-frequency fluctuations) which can modify the way noise is transmitted through the structure of the model and therefore the properties of the dynamics. Finally, the dynamics of any system depend on the dynamical attractor. The dynamical behaviour can be complex if there is the potential for multiple different attractors within the same area of parameter space. Noise, even demographic noise, can cause jumping between different dynamical attractors. This is most famously illustrated by the coupled theoretical–empirical studies on Tribolium by Costantino et al. (2005). The chaotic population dynamics exhibited by the beetles are mechanistically created by stochasticity bouncing the system between different dynamical attractors; such stochastic switching between attractors can also be seen with less complex, periodic dynamics (Henson et al. 1999, 2002, 2003; Cushing et al. 2003; King et al. 2004; Costantino et al. 2005) as well as in disease dynamics (Keeling et al. 2001; Rohani et al. 2002). But even with stable dynamics, there may exist nearby unstable dynamical features (such as Arnol'd tongues; Greenman & Benton 2004) which can, under noise, create intermittent and complex patterns in the dynamics.
Simple structured models therefore show that, in fluctuating environments, fluctuations in population size can be complex and different combinations of environmental noise and model structure can give rise to similar patterns (or vice versa). It may then be difficult to infer either biological model structure or the structure of the environmental driver from examination of the time-series generated by the structured models (Greenman & Benton 2005a,b). For example, a low-frequency (red) component in the dynamics (from which one might be tempted to infer a multi-year trend in an environment; Bjørnstad et al. 1999) could also be due to (i) ‘red’ environmental noise affecting a stable system far from the stability boundaries, (ii) blue, white or red noise affecting a system which is close to a red or ‘extinction’ boundary of the stability area and (iii) blue, white or red noise exciting a system close to a stability boundary but being filtered through the model structure to appear as though the system is near the red boundary. Similarly, the correlations in fluctuations between stages in the model (e.g. age classes) or populations (in a trophic model) can take on any value depending on details of the noise colour and the model structure. In principle, two spatially separate populations could be excited by different regimes of noise into the same patterns of dynamics, leading to population synchrony, or the same noise could lead to very different dynamics (depending on which stability boundary the deterministic attractors lie closest too) and no synchrony. Thus, finding dynamics in synchrony and correlated with a proposed environmental driver cannot necessarily allow strong inference that the driver is actually driving either the dynamics or the synchrony, or conversely finding dynamics out of synchrony or uncorrelated with a putative environmental driver does not necessarily mean the driver is unimportant (Lundberg et al. 2002; Laakso et al. 2003; Scott & Grant 2004; Royama 2005).
(c) Evolutionary models
Given the possibility that parameters will vary over time through selection (as well as plastic responses to the environment and density) there is the potential for evolutionary–ecological feedback. This is an area that requires the development of new theory as, typically, evolutionary modelling assumes a separation of time-scales for ecological and evolutionary dynamics (Geritz et al. 1998).
5. Discussion
Population dynamics arise from the sum of all individuals' responses to the environment, which, in turn are often contingent upon the individuals' previous environments. For a number of empirical systems, we are beginning to understand the biological mechanisms that link the environment to population dynamics, understanding often gained from coupled empirical experimentation and development of theory. What conclusions can we draw from this developing body of work?
Firstly, the biological mechanisms linking environment, through the life history, to the dynamics is complex. We will never be able to experimentally dissect the inherent complexity for any arbitrary species that we want to predict the dynamics for. However, just as behavioural ecology has been remarkably successful in understanding the adaptive rules by which organisms make ‘decisions’, empirical demographic studies are supporting the expectation that apparently complex demography may be underlain by a few simple rules, exemplified by trade-offs. Detailed understanding of a few model systems is suggesting both phenomena in common (e.g. the influence of past environments on current performance) and mechanisms that make sense of them (e.g. resource allocation trade-offs). Thus, commonality across well-studied systems will contribute to a general understanding of the likely mechanisms underlying the demographic responses to the environment that will be important for any poorly known species.
Secondly, there is, rightly, a desire to make ecological models as general as possible by making them simple. As discussed earlier, even simple models can give complex outputs in a variable world, so their utility in predicting future dynamics or being applied to any specific system may be limited. Given the current gap between theory and empirical results (Kareiva 1989; Holyoak & Lawler 2005), there is perhaps an imperative to ensure that models incorporate mechanism rather than just describe phenomena, and that they contain the necessary detail to model the dynamics rather than the maximum sufficient detail to have a tractable model. This in turn raises issues as to the definition of ‘necessary’. There is a temptation to consider a model as capturing the ‘correct’ mechanism generating the dynamics if the model predicts dynamics that are similar to (or even not significantly different from) the observations. However, given multiple causative routes to similar dynamics, a ‘well-fitting’ model may not be a useful predictive tool if the biological mechanism is mis-specified. Commonality of mechanisms identified from detailed empirical studies can help guide and motivate thinking about the biological processes for more poorly studied systems.
Thirdly, the empirical results show that the details underlying population dynamical responses to a fluctuating environment that is changing in both its mean quality (due to anthropogenic causes: habitat loss, fragmentation, degradation, biological invasions, climate change, etc.) and patterns of fluctuation will be complex. Therefore, extracting mean demographic parameters using simple statistical modelling of time-series may provide some insight, but is unlikely to lead to full understanding as many of the determinants of individual performance are linked in complex ways to past environments. This may not always be the case (Hallett et al. 2004), but low-powered statistical approaches fitting few parameters are perhaps more likely to not properly encompass the biological mechanism rather than to capture it properly. Techniques are available that allow the statistical fitting of complex models to data (for example, composite likelihood fitting methods; A. A. King, P. Rohani, S. Lele & M. Pascual 2005, personal communication). So, in analogy to normal procedures for fitting generalized linear models, consideration should be made to fitting a full model and simplifying to a minimal adequate model rather than fitting an initial simple model.
Recent empirical and theoretical work suggests that details matter—in terms of the model structure, the way that density dependence operates the amount of inter-individual heterogeneity, the variable lags created by delayed life history and inter-generational effects and the differential susceptibilities and responses of different individuals to environmental variability. With the potential of evolutionary responses to the rapid environmental changes affecting the world, then we should think in terms of the world being dominated by transient effects (at different temporal scales) rather than being more of a (noisy) equilibrium world (Fox & Gurevitch 2000; Stephens et al. 2002; King et al. 2004; Lande et al. in press). The consequence of this complexity is that to understand the effects of changes in the environment, where the system will not be at equilibrium, one needs to incorporate some mechanistic details into the model (i.e. move beyond purely numerical response models and incorporate numbers and states). This is possible within an analytical framework (de Roos & Persson 2005) but may result in an individual-based simulation model even at the expense of analytical tractability (Stephens et al. 2002).
Empirical dissection of model systems is showing the complexity of causation of population dynamics, but it is also indicating general mechanisms which map the environment, through the life history, to the dynamics. The traditional approach—that empirical work tests existing theory—underplays the role of empirical work in setting the agenda for the development of theory (Schmitz 2001). There is a real need to develop models than can predict population dynamics in a changing world. The complexity of causation in population dynamics that is being identified in empirical work, along with the insights from theory that different mechanisms can give rise to dynamics with similar characteristics, should provide a challenge to develop modelling approaches that can incorporate sufficiently detailed mechanism.
Endnote
See Advances in Ecological Research, vol. 37 (2005) for recent reviews of several laboratory systems.
Present address: Department of Animal and Plant Sciences, University of Sheffield, Sheffield S10 2TN, UK.
References
- Alekseev V, Lampert W. Maternal effects of photoperiod and food level on life history characteristics of the cladoceran Daphnia pulicaria Forbes. Hydrobiologia. 2004;526:225–230. doi:10.1023/B:HYDR.0000041600.16226.12 [Google Scholar]
- Ali M, Nicieza A, Wootton R.J. Compensatory growth in fishes: a response to growth depression. Fish Fish. 2003;4:147–190. [Google Scholar]
- [Anon] Biology students find holes in gap study. Nature. 2004;432:147. doi: 10.1038/432147c. [DOI] [PubMed] [Google Scholar]
- Ashley M.V, Willson M.F, Pergams O.R.W, O'Dowd D.J, Gende S.M, Brown J.S. Evolutionarily enlightened management. Biol. Conserv. 2003;111:115–123. doi:10.1016/S0006-3207(02)00279-3 [Google Scholar]
- Banks P.B, Powell F. Does maternal condition or predation risk influence small mammal population dynamics? Oikos. 2004;106:176–184. doi:10.1111/j.0030-1299.2004.12679.x [Google Scholar]
- Bateson P, et al. Developmental plasticity and human health. Nature. 2004;430:419–421. doi: 10.1038/nature02725. doi:10.1038/nature02725 [DOI] [PubMed] [Google Scholar]
- Beckerman A, Benton T.G, Ranta E, Kaitala V, Lundberg P. Population dynamic consequences of delayed life-history effects. Trends Ecol. Evol. 2002;17:263–269. doi:10.1016/S0169-5347(02)02469-2 [Google Scholar]
- Beckerman A.P, Benton T.G, Lapsley C.T, Koesters N. Talking 'bout my generation: environmental variability and cohort effects. Am. Nat. 2003;162:754–767. doi: 10.1086/381056. doi:10.1086/381056 [DOI] [PubMed] [Google Scholar]
- Benton T.G, Beckerman A.P. Population dynamics in a noisy world: lessons from a mite experimental system. Adv. Ecol. Res. 2005;37:143–181. [Google Scholar]
- Benton T.G, Cameron T.C, Grant A. Population responses to perturbations: predictions and responses from laboratory mite populations. J. Anim. Ecol. 2004;73:983–995. doi:10.1111/j.0021-8790.2004.00859.x [Google Scholar]
- Benton T.G, Plaistow S.J, Beckerman A.P, Lapsley C.T, Littlejohns S. Changes in maternal investment in eggs affects population dynamics. Proc. R. Soc. B. 2005;272:1351–1356. doi: 10.1098/rspb.2005.3081. doi:10.1098/rspb.2005.3081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjørnstad O.N, Fromentin J.M, Stenseth N.C, Gjosaeter J. Cycles and trends in cod populations. Proc. Natl Acad. Sci. USA. 1999;96:5066–5071. doi: 10.1073/pnas.96.9.5066. doi:10.1073/pnas.96.9.5066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler D.E, Benton T.G. Causes and consequences of animal dispersal strategies: relating individual behaviour to spatial dynamics. Biol. Rev. 2005;80:205–225. doi: 10.1017/s1464793104006645. doi:10.1017/S1464793104006645 [DOI] [PubMed] [Google Scholar]
- Bridges T.S, Heppell S. Fitness consequences of maternal effects in Streblospio benedicti (Annelida: Polychaeta) Am. Zool. 1996;36:132–146. [Google Scholar]
- Briggs C.J, Sait S.M, Begon M, Thompson D.J, Godfray H.C.J. What causes generation cycles in populations of stored-product moths? J. Anim. Ecol. 2000;69:352–366. doi:10.1046/j.1365-2656.2000.00398.x [Google Scholar]
- Cameron T.C, Benton T.G. Stage-structured harvesting and its effects: an empirical investigation using soil mites. J. Anim. Ecol. 2004;73:996–1006. doi:10.1111/j.0021-8790.2004.00886.x [Google Scholar]
- Caswell H. Analysis of life table response experiments. 1. Decomposition of effects on population growth rates. Ecol. Model. 1989;46:221–237. doi:10.1016/0304-3800(89)90019-7 [Google Scholar]
- Caswell H. 2nd edn. Sinauer Associates Inc; Sunderland, MA: 2001. Matrix population models. [Google Scholar]
- Clutton-Brock T.H, Coulson T. Comparative ungulate dynamics: the devil is in the detail. Phil. Trans. R. Soc. B. 2002;357:1285–1298. doi: 10.1098/rstb.2002.1128. doi:10.1098/rstb.2002.1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover D.O, Arnott S.A, Walsh M.R, Munch S.B. Darwinian fishery science: lessons from the Atlantic silverside (Menidia menidia) Can. J. Fish. Aquat. Sci. 2005;62:730–737. doi:10.1139/f05-069 [Google Scholar]
- Costantino R.F, Desharnais R.A, Cushing J.M, Dennis B, Henson S.M, King A.A. Nonlinear stochastic population dynamics: the flour beetle Tribolium as an effective tool of discovery. Adv. Ecol. Res. 2005;37:101–141. [Google Scholar]
- Coulson T, Catchpole E.A, Albon S.D, Morgan B.J.T, Pemberton J.M, Clutton-Brock T.H, Crawley M.J, Grenfell B.T. Age, sex, density, winter weather, and population crashes in Soay sheep. Science. 2001;292:1528–1531. doi: 10.1126/science.292.5521.1528. doi:10.1126/science.292.5521.1528 [DOI] [PubMed] [Google Scholar]
- Coulson T, Kruuk L.E.B, Tavecchia G, Pemberton J.M, Clutton-Brock T.H. Estimating selection on neonatal traits in red deer using elasticity path analysis. Evolution. 2003;57:2879–2892. doi: 10.1111/j.0014-3820.2003.tb01528.x. [DOI] [PubMed] [Google Scholar]
- Coulson T, Gaillard J.M, Festa-Bianchet M. Decomposing the variation in population growth into contributions from multiple demographic rates. J. Anim. Ecol. 2005;74:789–801. doi:10.1111/j.1365-2656.2005.00975.x [Google Scholar]
- Crone E.E. Parental environmental effects and cyclical dynamics in plant populations. Am. Nat. 1997;150:708–729. doi: 10.1086/286090. doi:10.1086/286090 [DOI] [PubMed] [Google Scholar]
- Cushing J.M, Costantino R.F, Dennis B, Desharnais R.A, Henson S.M. Academic Press; London, UK: 2003. Chaos in ecology: experimental non-linear dynamics. [Google Scholar]
- De Matos M.B, Matos D.M.S. Mathematical constraints on transition matrix elasticity analysis. J. Ecol. 1998;86:706–708. doi:10.1046/j.1365-2745.1998.00294.x [Google Scholar]
- de Roos A.M, Persson L. Unstructured population models: do population-level assumptions yield general theory? In: Cuddington K, Beisner B, editors. Ecological paradigms lost: routes of theory change. Academic Press; London, UK: 2005. pp. 31–62. [Google Scholar]
- Diekmann O, Gyllenberg M, Metz J.A.J, Thieme H.R. On the formulation and analysis of general deterministic structured population models. I. Linear theory. J. Math. Biol. 1998;36:349–388. doi: 10.1007/s002850170002. doi:10.1007/s002850050104 [DOI] [PubMed] [Google Scholar]
- Diekmann O, Gyllenberg M, Huang H, Kirkilionis M, Metz J.A.J, Thieme H.R. On the formulation and analysis of general deterministic structured population models. II. Nonlinear theory. J. Math. Biol. 2001;43:157–189. doi: 10.1007/s002850170002. doi:10.1007/s002850170002 [DOI] [PubMed] [Google Scholar]
- Dmitriew C, Rowe L. Resource limitation, predation risk and compensatory growth in a damselfly. Oecologia. 2005;142:150–154. doi: 10.1007/s00442-004-1712-2. doi:10.1007/s00442-004-1712-2 [DOI] [PubMed] [Google Scholar]
- Dobson F.S, Oli M.K. The demographic basis of population regulation in Columbian ground squirrels. Am. Nat. 2001;158:236–247. doi: 10.1086/321322. doi:10.1086/321322 [DOI] [PubMed] [Google Scholar]
- Dyer A.R. Maternal and sibling factors induce dormancy in dimorphic seed pairs of Aegilops triuncialis. Plant Ecol. 2004;172:211–218. doi:10.1023/B:VEGE.0000026339.61069.33 [Google Scholar]
- Easterling M.R, Ellner S.P, Dixon P.M. Size-specific sensitivity: applying a new structured population model. Ecology. 2000;81:694–708. [Google Scholar]
- Ellner S.P, Rees M. Integral projection models for species with complex demography. Am. Nat. 2006;167:410–428. doi: 10.1086/499438. doi:10.1086/499438 [DOI] [PubMed] [Google Scholar]
- Enright N.J, Franco M, Silvertown J. Comparing plant life histories using elasticity analysis—the importance of life-span and the number of life-cycle stages. Oecologia. 1995;104:79–84. doi: 10.1007/BF00365565. doi:10.1007/BF00365565 [DOI] [PubMed] [Google Scholar]
- Ergon T, Lambin X, Stenseth N.C. Life-history traits of voles in a fluctuating population respond to the immediate environment. Nature. 2001a;411:1043–1045. doi: 10.1038/35082553. doi:10.1038/35082553 [DOI] [PubMed] [Google Scholar]
- Ergon T, Mackinnon J.L, Stenseth N.C, Boonstra R, Lambin X. Mechanisms for delayed density-dependent reproductive traits in field voles, Microtus agrestis: the importance of inherited environmental effects. Oikos. 2001b;95:185–197. doi:10.1034/j.1600-0706.2001.950201.x [Google Scholar]
- Forsen T, Osmond C, Eriksson J.G, Barker D.J.P. Growth of girls who later develop coronary heart disease. Heart. 2004;90:20–24. doi: 10.1136/heart.90.1.20. doi:10.1136/heart.90.1.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C.W, Czesak M.E. Evolutionary ecology of progeny size in arthropods. Annu. Rev. Entomol. 2000;45:341–369. doi: 10.1146/annurev.ento.45.1.341. doi:10.1146/annurev.ento.45.1.341 [DOI] [PubMed] [Google Scholar]
- Fox G.A, Gurevitch J. Population numbers count: tools for near-term demographic analysis. Am. Nat. 2000;156:242–256. doi: 10.1086/303387. doi:10.1086/303387 [DOI] [PubMed] [Google Scholar]
- Fox C.W, Thakar M.S, Mousseau T.A. Egg size plasticity in a seed beetle: an adaptive maternal effect. Am. Nat. 1997;149:149–163. doi:10.1086/285983 [Google Scholar]
- Fox C.W, Bush M.L, Wallin W.G. Maternal age affects offspring lifespan of the seed beetle, Callosobruchus maculatus. Funct. Ecol. 2003;17:811–820. doi:10.1111/j.1365-2435.2003.00799.x [Google Scholar]
- Friedenberg N.A. Experimental evolution of dispersal in spatiotemporally variable microcosms. Ecol. Lett. 2003;6:953–959. doi:10.1046/j.1461-0248.2003.00524.x [Google Scholar]
- Fussman G.F, Ellner S.P, Hairston N.G, Jr, Jones L.E, Shertzer K.W, Yoshida T. Ecological and evolutionary dynamics of experimental plankton communities. Adv. Ecol. Res. 2005;37:221–243. [Google Scholar]
- Gendreau Y, Cote S.D, Festa-Bianchet M. Maternal effects on post-weaning physical and social development in juvenile mountain goats (Oreamnos americanus) Behav. Ecol. Sociobiol. 2005;58:237–246. doi:10.1007/s00265-005-0938-2 [Google Scholar]
- Geritz S.A.H, Kisdi E, Meszena G, Metz J.A.J. Evolutionarily singular strategies and the adaptive growth and branching of the evolutionary tree. Evol. Ecol. 1998;12:35–57. doi:10.1023/A:1006554906681 [Google Scholar]
- Greenman J.V, Benton T.G. The amplification of environmental noise in population models: causes and consequences. Am. Nat. 2003;161:225–239. doi: 10.1086/345784. doi:10.1086/345784 [DOI] [PubMed] [Google Scholar]
- Greenman J.V, Benton T.G. Large amplification in stage-structured models: Arnol'd tongues revisited. J. Math. Biol. 2004;48:647–671. doi: 10.1007/s00285-004-0264-8. doi:10.1007/s00285-004-0264-8 [DOI] [PubMed] [Google Scholar]
- Greenman J.V, Benton T.G. The frequency spectrum of structured discrete time population models: its properties and their ecological implications. Oikos. 2005a;110:369–389. doi:10.1111/j.0030-1299.2005.13652.x [Google Scholar]
- Greenman J.V, Benton T.G. The impact of environmental fluctuations on structured discrete time population models: resonance, synchrony and threshold behaviour. Theor. Popul. Biol. 2005b;68:217–235. doi: 10.1016/j.tpb.2005.06.007. doi:10.1016/j.tpb.2005.06.007 [DOI] [PubMed] [Google Scholar]
- Hallett T.B, Coulson T, Pilkington J.G, Clutton-Brock T.H, Pemberton J.M, Grenfell B.T. Why large-scale climate indices seem to predict ecological processes better than local weather. Nature. 2004;430:71–75. doi: 10.1038/nature02708. doi:10.1038/nature02708 [DOI] [PubMed] [Google Scholar]
- Hansen F, Forbes V.E, Forbes T.L. Using elasticity analysis of demographic models to link toxicant effects on individuals to the population level: an example. Funct. Ecol. 1999;13:157–162. doi:10.1046/j.1365-2435.1999.00299.x [Google Scholar]
- Hastings A, Arzberger P, Bolker B, Collins S, Ives A.R, Johnson N.A, Palmer M.A. Quantitative bioscience for the 21st century. Bioscience. 2005;55:511–517. [Google Scholar]
- Heath D.D, Heath J.W, Bryden C.A, Johnson R.M, Fox C.W. Rapid evolution of egg size in captive salmon. Science. 2003;299:1738–1740. doi: 10.1126/science.1079707. doi:10.1126/science.1079707 [DOI] [PubMed] [Google Scholar]
- Henson S.M, Costantino R.F, Cushing J.M, Dennis B, Desharnais R.A. Multiple attractors, saddles, and population dynamics in periodic habitats. Bull. Math. Biol. 1999;61:1121–1149. doi: 10.1006/bulm.1999.0136. doi:10.1006/bulm.1999.0136 [DOI] [PubMed] [Google Scholar]
- Henson S.M, Costantino R.F, Desharnais R.A, Cushing J.M, Dennis B. Basins of attraction: population dynamics with two stable 4-cycles. Oikos. 2002;98:17–24. doi:10.1034/j.1600-0706.2002.980102.x [Google Scholar]
- Henson S.M, King A.A, Costantino R.F, Cushing J.M, Dennis B, Desharnais R.A. Explaining and predicting patterns in stochastic population systems. Proc. R. Soc. B. 2003;270:1549–1553. doi: 10.1098/rspb.2003.2420. doi:10.1098/rspb.2003.2420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holyoak M, Lawler S.P. The contribution of laboratory experiments on protists to understanding population and metapopulation dynamics. Adv. Ecol. Res. 2005;37:245–271. [Google Scholar]
- Houle D, Rowe L. Natural selection in a bottle. Am. Nat. 2003;161:50–67. doi: 10.1086/345480. doi:10.1086/345480 [DOI] [PubMed] [Google Scholar]
- IPCC Climate change and biodiversityIPCC Technical Paper V2002IPCC; Geneva, Switzerland [Google Scholar]
- Kareiva P. Renewing the dialogue between ecological theory and experiments in ecology. In: Roughgarden J, May R.M, Levin S.A, editors. Perspectives in ecological theory. Princeton University Press; Princeton, NJ: 1989. pp. 68–88. [Google Scholar]
- Keeling M.J, Rohani P, Grenfell B.T. Seasonally forced disease dynamics explored as switching between attractors. Physica D. 2001;148:317–335. doi:10.1016/S0167-2789(00)00187-1 [Google Scholar]
- Kendall B.E, Ellner S.P, McCauley E, Wood S.N, Briggs C.J, Murdoch W.M, Turchin P. Population cycles in the pine looper moth: dynamical tests of mechanistic hypotheses. Ecol. Monogr. 2005;75:259–276. [Google Scholar]
- King A.A, Costantino R.F, Cushing J.M, Henson S.M, Desharnais R.A, Dennis B. Anatomy of a chaotic attractor: subtle model-predicted patterns revealed in population data. Proc. Natl Acad. Sci. USA. 2004;101:408–413. doi: 10.1073/pnas.2237266100. doi:10.1073/pnas.2237266100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knell R.J. Generation cycles. Trends Ecol. Evol. 1998;13:186–190. doi: 10.1016/S0169-5347(97)01321-9. doi:10.1016/S0169-5347(97)01321-9 [DOI] [PubMed] [Google Scholar]
- Kruuk L.E.B, Clutton-Brock T.H, Rose K.E, Guinness F.E. Early determinants of lifetime reproductive success differ between the sexes in red deer. Proc. R. Soc. B. 1999;266:1655–1661. doi: 10.1098/rspb.1999.0828. doi:10.1098/rspb.1999.0828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakso J, Kaitala V, Ranta E. Non-linear biological responses to disturbance: consequences on population dynamics. Ecol. Model. 2003;162:247–258. doi:10.1016/S0304-3800(02)00385-X [Google Scholar]
- LaMontagne J.M, McCauley E. Maternal effects in Daphnia: what mothers are telling their offspring and do they listen? Ecol. Lett. 2001;4:64–71. doi:10.1046/j.1461-0248.2001.00197.x [Google Scholar]
- Lande, R., Engen, S., Saether, B.-E. & Coulson, T. N. In press. Using reproductive value to estimate environmental variance and density dependence in age-structured populations. Am. Nat
- Lardies M.A, Carter M.J, Bozinovic F. Dietary effects on life history traits in a terrestrial isopod: the importance of evaluating maternal effects and trade-offs. Oecologia. 2004;138:387–395. doi: 10.1007/s00442-003-1447-5. doi:10.1007/s00442-003-1447-5 [DOI] [PubMed] [Google Scholar]
- Law R. Fishing, selection, and phenotypic evolution. ICES J. Mar. Sci. 2000;57:659–668. doi:10.1006/jmsc.2000.0731 [Google Scholar]
- Lindstrom J. Early development and fitness in birds and mammals. Trends Ecol. Evol. 1999;14:343–348. doi: 10.1016/s0169-5347(99)01639-0. [DOI] [PubMed] [Google Scholar]
- Lindstrom J, Kokko H. Cohort effects and population dynamics. Ecol. Lett. 2002;5:338–344. doi:10.1046/j.1461-0248.2002.00317.x [Google Scholar]
- Loison A, Solberg E.J, Yoccoz N.G, Langvatn R. Sex differences in the interplay of cohort and mother quality on body mass of red deer calves. Ecology. 2004;85:1992–2002. [Google Scholar]
- Lundberg P, Ripa J, Kaitala V, Ranta E. Visibility of demography—modulating noise in population dynamics. Oikos. 2002;96:379–382. doi:10.1034/j.1600-0706.2002.960219.x [Google Scholar]
- Messina F.J, Fry J.D. Environment-dependent reversal of a life history trade-off in the seed beetle Callosobruchus maculatus. J. Evol. Biol. 2003;16:501–509. doi: 10.1046/j.1420-9101.2003.00535.x. doi:10.1046/j.1420-9101.2003.00535.x [DOI] [PubMed] [Google Scholar]
- Metcalfe N.B, Monaghan P. Compensation for a bad start: grow now, pay later? Trends Ecol. Evol. 2001;16:254–260. doi: 10.1016/s0169-5347(01)02124-3. doi:10.1016/S0169-5347(01)02124-3 [DOI] [PubMed] [Google Scholar]
- Meylan S, De Fraipont M, Clobert J. Maternal size and stress and offspring philopatry: an experimental study in the common lizard (Lacerta vivipara) Ecoscience. 2004;11:123–129. [Google Scholar]
- Moe S.J, Stenseth N.C, Smith R.H. Density-dependent compensation in blowfly populations give indirectly positive effects of a toxicant. Ecology. 2002;83:1597–1603. [Google Scholar]
- Mousseau T.A, Fox C.W. Oxford University Press; Oxford, UK: 1998. Maternal effects as adaptations. [Google Scholar]
- Mueller L.D, Rauser C.L, Rose M.R. Population dynamics, life history and demography: lessons from Drosophila. Adv. Ecol. Res. 2005;37:77–99. [Google Scholar]
- Myers J.H, Boettner G, Elkinton J. Maternal effects in gypsy moth: only sex ratio varies with population density. Ecology. 1998;79:305–314. [Google Scholar]
- Olsen E.M, Heino M, Lilly G.R, Morgan M.J, Brattey J, Ernande B, Dieckmann U. Maturation trends indicative of rapid evolution preceded the collapse of northern cod. Nature. 2004;428:932–935. doi: 10.1038/nature02430. doi:10.1038/nature02430 [DOI] [PubMed] [Google Scholar]
- Ozanne S.E, Hales C.N. Lifespan—catch-up growth and obesity in male mice. Nature. 2004;427:411–412. doi: 10.1038/427411b. doi:10.1038/427411b [DOI] [PubMed] [Google Scholar]
- Pfister C.A, Stevens F.R. Individual variation and environmental stochasticity: implications for matrix model predictions. Ecology. 2003;84:496–510. [Google Scholar]
- Plaistow S.J, Lapsley C.T, Benton T.G. Context-dependent intergenerational effects: the interaction between past and present environments and its effect on population dynamics. Am. Nat. 2006;167:206–215. doi: 10.1086/499380. [DOI] [PubMed] [Google Scholar]
- Potti J. Maternal effects and the pervasive impact of nestling history on egg size in a passerine bird. Evolution. 1999;53:279–285. doi: 10.1111/j.1558-5646.1999.tb05353.x. [DOI] [PubMed] [Google Scholar]
- Prasad N.G, Dey S, Shakarad M, Joshi A. The evolution of population stability as a by-product of life-history evolution. Proc. R. Soc. B. 2003;270:S84–S86. doi: 10.1098/rsbl.2003.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reale D, Mcadam A.G, Boutin S, Berteaux D. Genetic and plastic responses of a northern mammal to climate change. Proc. R. Soc. B. 2003;270:591–596. doi: 10.1098/rspb.2002.2224. doi:10.1098/rspb.2002.2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid J.M, Bignal E.M, Bignal S, McCracken D.I, Monaghan P. Environmental variability, life-history covariation and cohort effects in the red-billed chough Pyrrhocorax pyrrhocorax. J. Anim. Ecol. 2003;72:36–46. doi:10.1046/j.1365-2656.2003.00673.x [Google Scholar]
- Rice K. Sprint research runs into a credibility gap. Nature. 2004;432:147. doi: 10.1038/432147b. doi:10.1038/432147b [DOI] [PubMed] [Google Scholar]
- Rohani P, Keeling M.J, Grenfell B.T. The interplay between determinism and stochasticity in childhood diseases. Am. Nat. 2002;159:469–481. doi: 10.1086/339467. doi:10.1086/339467 [DOI] [PubMed] [Google Scholar]
- Royama T. Moran effect on nonlinear population processes. Ecol. Monogr. 2005;75:277–293. [Google Scholar]
- Scheiner S.M, Yampolsky L.Y. The evolution of Daphnia pulex in a temporally varying environment. Genet. Res. 1998;72:25–37. doi:10.1017/S0016672398003322 [Google Scholar]
- Schmitz O.J. From interesting details to dynamical relevance: toward more effective use of empirical insights in theory construction. Oikos. 2001;94:39–50. doi:10.1034/j.1600-0706.2001.11312.x [Google Scholar]
- Scott F, Grant A. Visibility of the impact of environmental noise: a response to Kaltala and Ranta. Proc. R. Soc. B. 2004;271:1119–1124. doi: 10.1098/rspb.2004.2710. doi:10.1098/rspb.2004.2710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shertzer K.W, Ellner S.P. State-dependent energy allocation in variable environments: life history evolution of a rotifer. Ecology. 2002;83:2181–2193. [Google Scholar]
- Simmons A.D, Thomas C.D. Changes in dispersal during species' range expansions. Am. Nat. 2004;164:378–395. doi: 10.1086/423430. doi:10.1086/423430 [DOI] [PubMed] [Google Scholar]
- Solberg E.J, Loison A, Gaillard J.M, Heim M. Lasting effects of conditions at birth on moose body mass. Ecography. 2004;27:677–687. doi:10.1111/j.0906-7590.2004.03864.x [Google Scholar]
- Stephens P.A, Frey-Roos F, Arnold W, Sutherland W.J. Model complexity and population predictions. The alpine marmot as a case study. J. Anim. Ecol. 2002;71:343–361. doi:10.1046/j.1365-2656.2002.00605.x [Google Scholar]
- Stillwell R.C, Fox C.W. Complex patterns of phenotypic plasticity: interactive effects of temperature during rearing and oviposition. Ecology. 2005;86:924–934. [Google Scholar]
- Stockwell C.A, Hendry A.P, Kinnison M.T. Contemporary evolution meets conservation biology. Trends Ecol. Evol. 2003;18:94–101. doi:10.1016/S0169-5347(02)00044-7 [Google Scholar]
- Sutherland W.J, Norris K. Behavioural models of population growth rates: implications for conservation and prediction. Phil. Trans. R. Soc. B. 2002;357:1273–1284. doi: 10.1098/rstb.2002.1127. doi:10.1098/rstb.2002.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis J.M.J. Climate change and habitat destruction: a deadly anthropogenic cocktail. Proc. R. Soc. B. 2003;270:467–473. doi: 10.1098/rspb.2002.2246. doi:10.1098/rspb.2002.2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuda M, Shimada M. Complexity, evolution, and persistence in host–parasitoid experimental systems with Callosobruchus beetles as the host. Adv. Ecol. Res. 2005;37:37–75. [Google Scholar]
- Turchin P, Hanski I. Contrasting alternative hypotheses about rodent cycles by translating them into parameterized models. Ecol. Lett. 2001;4:267–276. doi:10.1046/j.1461-0248.2001.00204.x [Google Scholar]
- Wearing H.J, Sait S.M, Cameron T.C, Rohani P. Stage-structured competition and the cyclic dynamics of host–parasitoid populations. J. Anim. Ecol. 2004;73:706–722. doi:10.1111/j.0021-8790.2004.00846.x [Google Scholar]
- Yearsley J.M, Fletcher D. Equivalence relationships between stage-structured population models. Math. Biosci. 2002;179:131–143. doi: 10.1016/s0025-5564(02)00119-0. doi:10.1016/S0025-5564(02)00119-0 [DOI] [PubMed] [Google Scholar]
- Yearsley J.M, Kyriazakis I, Gordon I.J. Delayed costs of growth and compensatory growth rates. Funct. Ecol. 2004;18:563–570. doi:10.1111/j.0269-8463.2004.00879.x [Google Scholar]