Abstract
Pioneer species are fast-growing, short-lived gap exploiters. They are prime candidates for neutral dynamics because they contain ecologically similar species whose low adult density is likely to cause widespread recruitment limitation, which slows competitive dynamics. However, many pioneer guilds appear to be differentiated according to seed size. In this paper, we compare predictions from a neutral model of community structure with three niche-based models in which trade-offs involving seed size form the basis of niche differentiation. We test these predictions using sowing experiments with a guild of seven pioneer species from chalk grassland. We find strong evidence for niche structure based on seed size: specifically large-seeded species produce fewer seeds but have a greater chance of establishing on a per-seed basis. Their advantage in establishment arises because there are more microsites suitable for their germination and early establishment and not directly through competition with other seedlings. In fact, seedling densities of all species were equally suppressed by the addition of competitors' seeds. By the adult stage, despite using very high sowing densities, there were no detectable effects of interspecific competition on any species. The lack of interspecific effects indicates that niche differentiation, rather than neutrality, prevails.
Keywords: competition/colonization trade-off, seed size, niche partitioning, seed limitation, neutral models, coexistence mechanisms
1. Introduction
Pioneers are expert gap-exploiters, whose hit-and-run strategy allows them to coexist with slower-growing, more competitive species (Levins & Culver 1971; Crawley & May 1987; Popma et al. 1988; Lavorel et al. 1994; Vandvik 2004). Pioneer guilds are typically diverse, comprising perhaps 6–10 species in calcareous grasslands (Rees 1995) while in tropical forests ten species can easily be found within a single pioneer genus (Davies 2001). Such diversity could have a neutral explanation, but only if all species have the same birth and death rates (Hubbell 2001). However, species within pioneer guilds often have widely different seed sizes (Gross 1984; Rees 1995; Dalling et al. 1998; Pearson et al. 2002) with large-seeded species paying a cost in terms of reduced seed output (Dalling et al. 1998; Turnbull et al. 1999). This suggests that there must be some corresponding benefit to large seeds which offsets their disadvantage in seed output (Rees 1995; Rees et al. 1996). The presence of such trade-offs indicates that a niche-based explanation is probably more likely than a neutral explanation.
Here, we consider four alternative models which attempt to explain how a range of seed sizes could coexist: three are niche-based and one is a neutral (or random drift) model. The niche-based models differ in ascribing either a direct competitive advantage to larger seeds or by assuming that larger seeds offer some advantage against environmental hazards. The models make different predictions about the expected correlation between seed size and abundance in natural communities and the results of seed sowing experiments (table 1). An experimental approach is necessary because relative abundance patterns are only weakly constrained by the underlying coexistence mechanism so they cannot be used to differentiate between mechanisms (Chave et al. 2002). The experiments involve both single-sown plots where each species is sown separately (e.g. 100 seeds of species i) and mixture-sown plots where seeds of all species are sown into the same plot (e.g. 100 seeds of each species). Equal numbers of seeds of each species are used so that we can directly compare species on a per-seed basis. Single-sown plots allow an evaluation of the density of suitable microsites available to each species when competitors' seeds are present at a low density. The density of each species in single-sown and mixture plots can then be compared to evaluate the impact of competitors. A range of densities is used, including a very high density to ensure that competition for suitable microsites ensues.
Table 1.
Comparison of predictions from the four models.
| measure | random drift | pure competition/colonization trade-off | included niche | establishment/colonization trade-off |
|---|---|---|---|---|
| background correlation between seed size and abundance | zero | positive | negative | zero |
| correlation between seed size and abundance in sown plots | zero | positive | zero | positive |
| change in correlation strength with increased sowing density | no change | increasingly positive | less negative | no change |
| extinctions in mixture-sown plots | no | yes, of small-seeded species | no | no |
| reduced population density in mixture-sown versus single-sown plots | yes, for all species | yes, for small-seeded species | yes, for small-seeded species | possible; but with no relationship to seed size |
| individual species seed-limiteda | yes, all species have lottery recruitment | yes, extent increases with seed size (essential for model) | yes, extent increases with seed size (essential for model) | probably, extent increases with seed size (not essential for model) |
A detailed analysis of the expected correlation between seed size and abundance under different model scenarios is presented in Levine & Rees (2002). Other predictions are discussed more fully in Pacala & Rees (1998) and Turnbull et al. (1999).
We use the term seed-limited to mean an increase in plant density with seed addition, as defined in Turnbull et al. (2000).
(a) The models
The simplest null model assumes all species have the same demographic rates, the environment is homogeneous and recruitment is a lottery in which the fraction of sites captured is proportional to relative seed production (Chesson & Warner 1981). This is a neutral model of community structure and has no stable equilibrium involving more than one species (Chesson & Huntly 1997; Hubbell 2001). As many pioneer species are short-lived we would expect the time-scales of extinction to be short. However, pioneer species are often chronically rare due to a lack of suitable sites (Grubb 1986) and this could lead to low adult density and, in turn, low seed production. Recruitment limitation of the component species (failure to disperse seeds into suitable sites) would lead to large stochastic variation in the outcome of competition across sites, increasing times to extinction (Hurtt & Pacala 1995). Under this model, we would not expect plant traits to be correlated with abundance because trait variation does not lead to variation in demographic rates. Also, sowing mixtures containing equal numbers of seeds of each species should increase the relative abundance of rare species, while that of common species should decrease. For example, consider a two species community with abundances of 1 and 100: the rare species has a relative abundance of approximately 1% (1/101) and if we add 10 individuals of each species its abundance will increase to approximately 9% (11/121), whereas the relative abundance of the common species declines from 99% (100/101) to approximately 90% (110/121). This effect should increase with total sowing density and all species should capture many more sites in single-sown plots than in mixture-sown plots because there is less competition.
The pure competition/colonization trade-off model assumes a homogeneous environment where all microsites are considered identical (Skellam 1951; Tilman 1994). However, in this case we need to allow for variation in seed production per capita (small-seeded species produce more seeds than large-seeded species) and competitive abilities (large-seeded species are more competitive than small-seeded ones). The proportion of sites captured by species i is now
where Ni is the number of adults, λi is the intrinsic rate of increase (or the per capita seed production), and ci is the relative competitive ability of the ith species. The model allows multiple species to coexist as long as competitive asymmetries are extreme (Geritz 1995; Rees & Westoby 1997). The model also predicts a positive correlation between seed mass and abundance in the absence of seed sowing (Levine & Rees 2002) and this correlation would be enhanced with additional seed inputs (Turnbull et al. 1999). In high-density, mixture-sown plots we expect that species with the smallest seeds will be excluded as they are squeezed out by the superior competitors (Pacala & Rees 1998; Turnbull et al. 1999). For the same reason, we expect to see dramatic differences between the abundance of small-seeded species in single-sown versus mixture-sown plots.
The remaining hypotheses rely on microsite heterogeneity (for example, suitable microsites might differ in the availability of essential limiting nutrients or in their exposure to environmental hazards such as drought). The included niche model was proposed by Levine & Rees (2002) as an extension of the competition/colonization trade-off model. In this model, large-seeded species are still better competitors, but small-seeded species tolerate a broader range of environmental conditions (Maranon & Grubb 1993). This effectively gives small-seeded species a refuge from competition and makes the conditions for coexistence less restrictive. This model has the desirable property of reproducing the negative correlation between seed mass and abundance commonly seen in annual communities (Grubb et al. 1982; Maranon & Grubb 1993; Rees 1995; Pake & Venable 1996; Guo et al. 2000), although this correlation should become increasingly less negative with seed sowing as the recruitment limitation of the large-seeded species is overcome. We would predict a lack of competitive exclusion because each species has a refuge within which it is the best competitor. A comparison of seedling densities in single-sown plots, where interspecific competition is weak, should reveal that small-seeded species are more abundant, reflecting their tolerance of a broader range of environmental conditions.
Finally, the establishment/colonization trade-off (Dalling & Hubbell 2002; Coomes & Grubb 2003) proposes that the advantage to large seeds comes not through competition but through increased tolerance of environmental hazards. For example, species with large seed reserves may be better able to tolerate shade, herbivory and burial (Leishman & Westoby 1994; Westoby et al. 1996). Large-seeded species can therefore afford to produce fewer seeds because each has a greater chance of falling into a suitable site. Small-seeded species, by contrast, must produce a large number of seeds because the opportunities available to them are limited. Species with different seed sizes could still compete for those establishment sites which all species tolerate, although the outcome need not be seed-size dependent. Alternatively, competition between species would be weak if microsite specialization generates non-overlapping niches. If this were the case, we would predict similar numbers of plants in single-sown versus mixture plots for all species, irrespective of seed mass. Across single-sown plots we expect a positive correlation between seed mass and abundance (reflecting the higher density of microsites available to large-seeded species). The correlation in unsown plots would probably be close to zero because, although small-seeded species produce more seed, they have fewer opportunities to establish and vice versa.
We describe here a test of these models with seven pioneer chalk-grassland plants whose seed size varies over more than two orders of magnitude. All the species occur at low abundance within a matrix of perennials. The population size of these species had been monitored for 20 years prior to the experiment and their long-term abundance was therefore known. We found that one hypothesis, the establishment/colonization trade-off, was most consistent with the evidence.
2. Materials and methods
(a) Study site and species
The study site is at Castle Hill National Nature Reserve, a chalk grassland in East Sussex, UK. Long-term monitoring of nine short-lived species began in 1978 and continued until 1997. The species (with their seed masses) are all short-lived and monocarpic: Blackstonia perfoliata (0.011 g), Centaurium erythraea (0.016 g), Gentianella amarella (0.155 g), Euphrasia nemorosa (0.18 g), Linum catharticum (0.18 g), Medicago lupulina (1.68 g), Carlina vulgaris (1.69 g), Picris hieracioides (1.27 g) and Rhinanthus minor (1.73 g). Rhinanthus and Euphrasia are also hemiparasites. The number of flowering individuals was recorded in August each year along a 50×0.5 m transect which had been divided into 2500 10×10 cm2 (the East-facing transect described in Grubb (1986)). The sowing experiment was undertaken with seven of the nine species (C. vulgaris and P. hieracioides had to be excluded because we could not collect sufficient numbers of seeds). All nomenclature follows Stace (1997).
(b) Experimental design
In August 1997, we selected twenty 0.5×0.5 m quadrats at 2.5 m intervals along the original transect. Within each quadrat we classified each 10×10 cm2 into one of four categories, reflecting increasing turf height and percentage cover. Each species was then sown at four densities (0, 10, 50 and 250 seeds) across the four turf height categories, with each species sown alone and in an additive full-species mixture (containing 0, 10, 50 and 250 seeds of each species, respectively). Each experimental treatment had three replicates giving 48 single-sown plots (4×4×3) for each species and 48 plots sown with the full-species mixture (a total of 384 plots). All seeds were sown in October 1997, shortly after the time of natural seed-fall, and plots were monitored for 2 years in order to follow cohorts of all species during their lifetime. The exception was Medicago, which was unlikely to flower after only 2 years under these field conditions. Therefore, the analyses for this species only include counts of non-flowering individuals. We present analyses from three census dates: peak seedling emergence (April 1998), first-year adults (August 1998) and second-year adults (August 1999). A small second cohort of seedlings emerged in the spring of 1999 for some species which we analysed where possible. All analyses were carried out with the statistical package R (R Development Core Team, 2004).
3. Results
(a) Correlations between seed mass and abundance
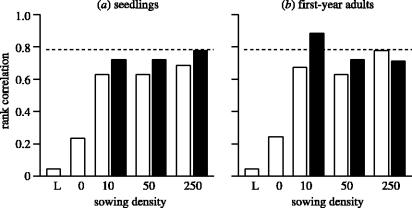
Seed mass and abundance are uncorrelated in the long-term data and in unsown plots but positively correlated in sown plots. This argues against the included niche and pure competition/colonization trade-off models (which respectively predict negative and positive correlations in the unmanipulated community). For both seedlings and first-year adults the correlation between seed size and abundance was consistently positive across seed addition plots of differing sowing density (figure 1). Both the random drift and included niche models fail to predict these positive correlations. This result implies that large-seeded species would be more abundant if all species produced the same number of seeds. This result is not driven simply by higher viability of large seeds: as part of a pot experiment we sowed equal numbers of each species' seeds onto bare soil separately. In this case, the relationship between seed mass and the number of plants was actually negative (rs=−0.649, p>0.1, n=7). It appears that conditions in the natural community provide more germination opportunities for large-seeded species. Correlations were assessed using Spearman ranks (long-term data: rs=0.04, p>0.9, n=9; unsown plots: seedlings: rs=0.24, p>0.4, n=7; first-year adults: rs=0.24, p>0.4, n=7). We used Fisher's (1954) test to combine the separate correlations in sown plots to confirm an overall positive correlation between seed size and abundance for both seedlings (χ2=29.38, d.f.=12, p=0.0035) and first year adults (χ2=32.38, d.f.=12, p=0.0012).
Figure 1.
Correlations (Spearman rank) between seed size and abundance for (a) seedling and (b) first-year adult communities at each of four sowing densities in both single-sown plots (open bars) and mixture-sown plots (filled bars). For comparison, the correlation in the long-term data (L) is also shown. The dashed line shows the critical value for a 2-tailed test (n=7, p=0.05). Values were obtained by summing abundances across all plots of the same sowing density.
(b) Seed size/number trade-offs
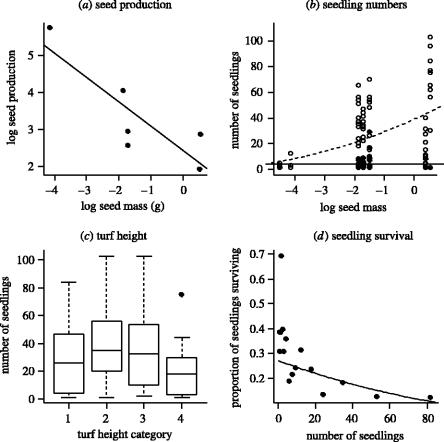
If there is no correlation between seed size and abundance in the unmanipulated community but large-seeded species are more abundant when seed inputs are equalized, large-seeded species must normally produce fewer seeds. We obtained data from the literature on average per capita seed production for four species growing at Castle Hill: Linum, Gentianella, Rhinanthus and Euphrasia (Kelly 1989a) and used fecundity data for Carlina and Centaurium from the long-term dataset. There was a significant negative relationship between seed mass and per capita seed production for these six species (F1,4=10.71, p<0.04, r2=0.66; figure 2a), confirming that large-seeded species suffer reduced fecundity.
Figure 2.
Single-sown plots: (a) The per capita seed production declines with increasing seed mass (data from previous work at Castle Hill). (b) The number of seedlings in occupied plots as a function of seed mass in both unsown plots (closed circles, solid line) and those sown with 250 seeds (open circles, dashed line), fitted lines from linear regression on square-root transformed data; (c) The mean number of seedlings emerging in the different turf height categories. (d) Mean seedling survival as a function of initial seedling density. The fitted line is from a logistic regression of the proportion of seedlings surviving. In (a) and (b), species with similar seed masses have been slightly shifted for clarity.
(c) The effect of seed mass on germination and survival
In single-sown plots, seedling densities increased from a total of 150 seedlings to 2324 at the highest sowing density. This implies widespread recruitment limitation, which in turn implies that the level of interspecific competition in single-sown plots is relatively weak. Despite this, species with large seed mass achieved much higher abundances than species with smaller seeds (figure 2b and Electronic Appendix part 1). This might occur because large-seeded species have a greater tolerance of tall or dense turf. However, although seedling densities were reduced in tall turf (figure 2c), there was no interaction between seed size and turf height (F3,158=1.0, p>0.4) or species identity and turf height (F15,158=0.4, p>0.9).
Seedling survival was analysed using generalized linear models assuming binomial errors and a logit link function. Significance was assessed using F-tests (McCullagh & Nelder 1989). The probability of a seedling surviving to the end of year 1 in single-sown plots was strongly density-dependent (initial seedling density: F1,158=26.1, p<0.0001; figure 2d). Although there were significant differences between species in the proportion of seedlings surviving (F5,158=6.9, p<0.0001), seed mass had no effect (F1,158=1.1, p>0.3). This implies that the advantage to large seeds lies firmly at the germination and early establishment stage and that the factors influencing survival are different for different species. As survival is strongly density-dependent, we can be certain that our sowing densities in mixture-sown plots are high enough to result in strong competition for any shared microsites.
(d) The effect of competition on individual species
We analysed species presence/absence using logistic regression to determine the effects of the mixture treatment on individual species. At the seedling stage, the probability of occurrence (defined as the probability of finding at least one seedling in a plot) increased with sowing density for all species but there were no significant effects of the mixture treatment or an interaction with sowing density on any species (Electronic Appendix part 2a). Therefore, we did not see any evidence of local exclusion due to the input of other species' seeds. For first-year adults, the effect of sowing density had disappeared for all species except Rhinanthus and Medicago (the two species with the largest seeds). Medicago was slightly less likely to occur in mixture-sown plots and Rhinanthus was slightly more likely to occur (perhaps because it could parasitize other seedlings). The negative effect of the mixture treatment on Medicago persisted until the second year. By the end of the second year, presence/absence patterns among the small second cohort of Rhinanthus plants still showed a significant effect of the previous year's sowing treatments, while the second cohort of Euphrasia did not. There were too few flowering plants to analyse for Blackstonia, Centaurium, Gentianella and Linum, which suggests they are microsite rather than seed-limited. By contrast, sowing density continued to have significant effects on the longer-lived Medicago. The lack of local exclusion repudiates the competition/colonization trade-off model, especially as the only species locally excluded by competition was Medicago (the species with the second largest seeds).
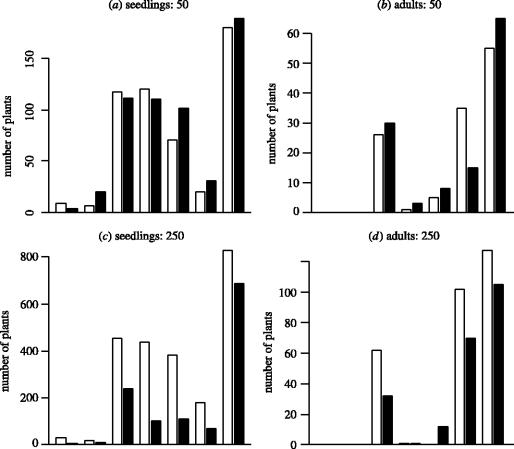
Plant numbers in occupied plots were square-root transformed to meet the assumptions of ANOVA. Species-by-species analysis of seedling numbers revealed that all species responded to seed addition except Blackstonia and Centaurium where there were very low numbers of seedlings for both species (Electronic Appendix part 2b). Gentianella, Euphrasia and Linum all responded more strongly to sowing in single-sown than mixture plots, as did Medicago, although it was only marginally significant in this case (F1,21=3.7, p<0.07). The negative effect of the mixture treatment on seedling numbers is only apparent at the very highest sowing density (figure 3).
Figure 3.
The total number of (a, c) seedlings and (b, d) first-year adults for each of seven species, in (a, b) plots sown with 50 seeds and (c, d) plots sown with 250 seeds and in both sown-alone (open bars) and mixture-sown plots (filled bars). Bars are arranged in order of increasing seed size. There was significant but equal suppression of all species' seedling numbers in mixture plots, regardless of seed mass. There was no significant suppression of adult plants.
By the end of the first year there were more plants in sown plots for all species except Blackstonia and Centaurium; however, there were no detectable effects of the mixture treatment on any species (Electronic Appendix part 2b). By the end of the second year, the second generations of Rhinanthus and Euphrasia did not show any effects from the previous year's sowing treatments; Blackstonia, Centaurium, Gentianella and Linum suffered such high rates of mortality that there were insufficient numbers to analyse. There were even more Medicago plants in sown plots, although the effect of the mixture treatment was no longer apparent.
(e) Competitive suppression and seed mass
To compare the extent of competitive suppression on species with different seed mass it was necessary to overcome the lack of statistical independence in mixture plots. Therefore, we summed plant numbers for each species over turf height treatments and replicates at each density. There were no interactions between either seed mass or species identity and the mixture treatment at the seedling stage (seed mass×mixture: F1,33=0.05, p>0.8; species×mixture: F5,33=1.1, p>0.3), so that all species were more or less equally suppressed (figure 3c). By the end of the first year, despite the persistence of an effect of sowing density (F1,33=41.1, p<0.000 01), seed mass (F1,33=44.190, p<0.000 01) and their interaction (F1,33=10.4, p<0.003), there were no longer any main effects from the mixture treatment (F1,33=1.2, p>0.3) or any significant interactions involving the mixture treatment. This confirms that the number of plants in mixture-sown plots at the end of the first year was indistinguishable from numbers of plants in their respective single-sown plots. The lack of any significant difference in the number of plants in single-sown and mixture plots is strong evidence that refutes the random drift, the pure competition/colonization trade-off and included niche models.
4. Discussion
The species studied here form a typical pioneer guild in which species pursue broadly similar strategies but where each has found a different solution to the seed size problem (Smith & Fretwell 1974). Although several alternative explanations have been advanced to explain this phenomenon, the establishment/colonization trade-off (Dalling & Hubbell 2002) received the strongest support from our data. In this model, large-seeded species have more recruitment opportunities and this compensates for their reduced seed production. In contrast, small-seeded species specialize on rare microsites and must produce many seeds in order to survive. Within-guild competition was weak, implying that species' microsite requirements are not usually overlapping, although all species suffered some suppression at the seedling stage when sowing densities were very high. The model correctly predicts correlations between seed size and abundance, the stronger response of large-seeded species to sowing and the weak interspecific effects.
Support for this model suggests that larger-seeded species have a broader, or less restrictive, regeneration niche (Grubb 1977). For example, seedling densities in single-sown plots were positively related to seed size with combined densities of Medicago and Rhinanthus (the two species with the largest seeds) up to twenty times higher than combined densities of Blackstonia and Centaurium (the two species with the smallest seeds). Small-seeded species could have more restrictive germination conditions. For example, two studies of pioneers found that small-seeded species only germinated under high light conditions while large-seeded species were much less particular (Milberg et al. 2000; Pearson et al. 2002). However, small-seeded species could have an advantage under certain rare conditions; for example, they might perform better in large gaps which are highly prone to desiccation (Maranon & Grubb 1993). The differential survival of species in single-sown plots shows that species are affected differently by the same environmental conditions, again implying microsite differentiation. For example, a detailed study of factors affecting survival in Gentianella, Linum and Euphrasia revealed that the species responded differently to snail and rabbit herbivory, soil moisture, soil nitrogen level and the presence or absence of particular perennials (Kelly 1989b).
The simple lottery model (displaying neutral dynamics) would only allow long-term persistence of our short-lived guild, albeit transiently, if there were widespread recruitment limitation to slow competitive dynamics (Hurtt & Pacala 1995). However, seed additions did not enhance adult densities of four of the seven species (not even in single-sown plots) despite the fact that seedling densities of all species could be enhanced by sowing and the chronic rarity of adults (on average around 16 adult plants per species m−2). This implies that they produce sufficient seed (perhaps with the help of a seed-bank) to fill the microsites available to them and that, for these species at least, recruitment is limited by the availability of suitable microsites. Large-seeded species responded much more strongly to seed additions: for example, Medicago and Rhinanthus achieved densities of up to almost 7000 seedlings m−2 in sown plots (compared with around 60 m−2 in unsown plots) and remained seed-limited at the adult stage. Therefore, the rarity of large-seeded species in unsown plots probably has a different cause: large seeds may unlock more opportunities but low seed production leaves many potential sites unexploited.
The competition/colonization trade-off model couched in terms of seed size seems to provide a convincing explanation for the seed size variation in pioneer guilds. There has been a great deal of attention focused on this model recently, particularly on the importance of strongly asymmetric competition if the model is to work in its pure form (Kinzig et al. 1999; Adler & Mosquera 2000; Yu & Wilson 2001; Levine & Rees 2002). In general, measured competitive effects are not deemed sufficient to offset the colonizing disadvantage of species producing large seeds (Freckleton & Watkinson 2001; Turnbull et al. 2004). In this study, we found little direct evidence for competitive suppression of small-seeded species: there was no local exclusion due to competition and no significant effect on plant numbers beyond the seedling stage. Perhaps more importantly, small-seeded species were no more suppressed than large-seeded ones, even at the seedling stage. The inclusion of both single-sown and mixture-sown plots proved a vital element in testing this hypothesis. Previously, we used an observed positive correlation between seed size and abundance in plots sown with seed mixtures as evidence of a competition/colonization trade-off (Turnbull et al. 1999). This is not, in itself, sufficient and direct measurement of competitive effects are also necessary (Turnbull et al. 2004). A lack of interspecific effects at the population level was also noted for a guild of sand-dune annuals which vary substantially in their seed sizes (Rees et al. 1996).
The included niche model (Levine & Rees 2002) which assumes small-seeded species have the greatest environmental tolerance does not seem to apply in this case. We found no evidence of a negative correlation between seed size and abundance in the long-term data, although this is commonly observed in other annual communities (Grubb et al. 1982; Maranon & Grubb 1993; Pake & Venable 1996; Guo et al. 2000). In addition, small-seeded species were always rare; for example, the two species with the smallest seeds (Blackstonia and Centaurium) were rare even in high density, single-sown plots. This was not due to poor seed viability or enforced dormancy: 30% of seeds of both species germinated and survived their first year in a pot experiment compared with less than 1% in the field. The lack of seedlings in natural grassland must therefore be due to restrictive germination conditions and indicates narrow environmental tolerance. In contrast, large-seeded species were able to greatly increase their population size with sowing.
This study confirms the benefit of setting up a number of competing alternative hypotheses (an approach advocated by Hilborn & Mangel (1997)) instead of confirming or refuting a single null hypothesis. Although the models were simple models of ideas, a set of different testable predictions could be generated from each of the models considered. In this case, we were able to determine that a lottery model, the pure competition/colonization trade-off and the included niche model made substantially poorer predictions than the establishment/colonization trade-off, leading us to accept this as the best current description of this particular pioneer community's dynamics.
Acknowledgments
Thanks to Peter Grubb and Dave Kelly for access to the long-term data and to Andy Hector, Jasmin Joshi, Luca Wacker and Bernhard Schmid who gave helpful comments on the manuscript. Thanks also to English Nature who gave permission to work at the site. The work was supported by the Natural Environment Research Council of the UK.
Supplementary Material
References
- Adler F.R, Mosquera J. Is space necessary? Interference competition and limits to biodiversity. Ecology. 2000;81:3226–3232. [Google Scholar]
- Chave J, Muller-Landau H.C, Levin S.A. Comparing classical community models: theoretical consequences for patterns of diversity. Am. Nat. 2002;159:1–23. doi: 10.1086/324112. [DOI] [PubMed] [Google Scholar]
- Chesson P, Huntly N. The roles of harsh and fluctuating conditions in the dynamics of ecological communities. Am. Nat. 1997;150:519–553. doi: 10.1086/286080. [DOI] [PubMed] [Google Scholar]
- Chesson P.L, Warner R.R. Environmental variability promotes coexistence in lottery competitive systems. Am. Nat. 1981;117:923–943. [Google Scholar]
- Coomes D.A, Grubb P.J. Colonization, tolerance, competition and seed-size variation within functional groups. Trends Ecol. Evol. 2003;18:283–291. [Google Scholar]
- Crawley M.J, May R.M. Population-dynamics and plant community structure—competition between annuals and perennials. J. Theor. Biol. 1987;125:475–489. [Google Scholar]
- Dalling J.W, Hubbell S.P. Seed size, growth rate and gap microsite conditions as determinants of recruitment success for pioneer species. J. Ecol. 2002;90:557–568. [Google Scholar]
- Dalling J.W, Hubbell S.P, Silvera K. Seed dispersal, seedling establishment and gap partitioning among tropical pioneer trees. J. Ecol. 1998;86:674–689. [Google Scholar]
- Davies S.J. Tree mortality and growth in 11 sympatric Macaranga species in Borneo. Ecology. 2001;82:920–932. [Google Scholar]
- Fisher R.A. Oliver & Boyd; Edinburgh: 1954. Statistical methods for research workers. [Google Scholar]
- Freckleton R.P, Watkinson A.R. Predicting competition coefficients for plant mixtures: reciprocity, transitivity and correlations with life-history traits. Ecol. Lett. 2001;4:348–357. [Google Scholar]
- Geritz S.A.H. Evolutionarily stable seed polymorphism and small-scale spatial variation in seedling density. Am. Nat. 1995;146:685–707. [Google Scholar]
- Gross K.L. Effects of seed size and growth form on seedling establishment of 6 monocarpic perennial plants. J. Ecol. 1984;72:369–387. [Google Scholar]
- Grubb P.J. The maintenance of species-richness in plant communities: the importance of the regeneration niche. Biol. Rev. 1977;52:107–145. [Google Scholar]
- Grubb P.J. Problems posed by sparse and patchily distributed species in species-rich plant communities. In: Diamond J, Case T.J, editors. Community ecology. Harper and Row; New York: 1986. pp. 207–225. [Google Scholar]
- Grubb P.J, Kelly D, Mitchley J. The control of relative abundance in communities of herbaceous plants. In: Newman E.I, editor. The plant community as a working mechanism. Blackwell Scientific; Oxford: 1982. [Google Scholar]
- Guo Q.F, Brown J.H, Valone T.J, Kachman S.D. Constraints of seed size on plant distribution and abundance. Ecology. 2000;81:2149–2155. [Google Scholar]
- Hilborn R, Mangel M. Princeton University Press; Princeton: 1997. The ecological detective. [Google Scholar]
- Hubbell S.P. Princeton University Press; Princeton: 2001. The unified neutral theory of biodiversity and biogeography. [DOI] [PubMed] [Google Scholar]
- Hurtt G.C, Pacala S.W. The consequences of recruitment limitation—reconciling chance, history and competitive differences between plants. J. Theor. Biol. 1995;176:1–12. [Google Scholar]
- Kelly D. Demography of short-lived plants in Chalk Grassland. 1. Life-cycle variation in annuals and strict biennials. J. Ecol. 1989a;77:747–769. [Google Scholar]
- Kelly D. Demography of short-lived plants in chalk grassland. 2. Control of mortality and fecundity. J. Ecol. 1989b;77:770–784. [Google Scholar]
- Kinzig A.P, Levin S.A, Dushoff J, Pacala S. Limiting similarity, species packing, and system stability for hierarchical competition-colonization models. Am. Nat. 1999;153:371–383. doi: 10.1086/303182. [DOI] [PubMed] [Google Scholar]
- Lavorel S, Lepart J, Debussche M, Lebreton J.D, Beffy J.L. Small-scale disturbances and the maintenance of species—diversity in mediterranean old fields. Oikos. 1994;70:455–473. [Google Scholar]
- Leishman M.R, Westoby M. The role of large seed size in shaded conditions: experimental evidence. Funct. Ecol. 1994;8:205–214. [Google Scholar]
- Levine J.M, Rees M. Coexistence and relative abundance in annual plant assemblages: the roles of competition and colonization. Am. Nat. 2002;160:452–467. doi: 10.1086/342073. [DOI] [PubMed] [Google Scholar]
- Levins R, Culver D. Regional coexistence of species and competition between rare species. Proc. Natl Acad. Sci. USA. 1971;68:1246–1248. doi: 10.1073/pnas.68.6.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maranon T, Grubb P.J. Physiological-basis and ecological significance of the seed size and relative growth-rate relationship in mediterranean annuals. Funct. Ecol. 1993;7:591–599. [Google Scholar]
- McCullagh P, Nelder J.A. Monographs on statistics and applied probability. Chapman & Hall; London: 1989. Generalized linear models. [Google Scholar]
- Milberg P, Andersson L, Thompson K. Large-seeded species are less dependent on light for germination than small-seeded ones. Seed Sci. Res. 2000;10:99–104. [Google Scholar]
- Pacala S.W, Rees M. Models suggesting field experiments to test two hypotheses explaining successional diversity. Am. Nat. 1998;152:729–737. doi: 10.1086/286203. [DOI] [PubMed] [Google Scholar]
- Pake C.E, Venable D.L. Seed banks in desert annuals—implications for persistence and coexistence in variable environments. Ecology. 1996;77:1427–1435. [Google Scholar]
- Pearson T.R.H, Burslem D, Mullins C.E, Dalling J.W. Germination ecology of neotropical pioneers: interacting effects of environmental conditions and seed size. Ecology. 2002;83:2798–2807. [Google Scholar]
- Popma J, Bongers F, Martinezramos M, Veneklaas E. Pioneer species distribution in treefall gaps in neotropical rain-forest—a gap definition and its consequences. J. Trop. Ecol. 1988;4:77–88. [Google Scholar]
- Rees M. Community structure in sand dune annuals. Is seed weight a key quantity? J. Ecol. 1995;83:857–863. [Google Scholar]
- Rees M, Westoby M. Game-theoretical evolution of seed mass in multi-species ecological models. Oikos. 1997;78:116–126. [Google Scholar]
- Rees M, Grubb P.J, Kelly D. Quantifying the impact of competition and spatial heterogeneity on the structure and dynamics of a four-species guild of winter annuals. Am. Nat. 1996;147:1–32. [Google Scholar]
- Skellam Random dispersal in theoretical populations. Biometrika. 1951;38:196–218. [PubMed] [Google Scholar]
- Smith C.C, Fretwell S.D. The optimal balance between size and number of offspring. Am. Nat. 1974;108:499–506. [Google Scholar]
- Stace C. Cambridge University Press; Cambridge: 1997. New flora of the British Isles. [Google Scholar]
- Tilman D. Competition and biodiversity in spatially structured habitats. Ecology. 1994;75:2–16. [Google Scholar]
- Turnbull L.A, Rees M, Crawley M.J. Seed mass and the competition/colonization trade-off: a sowing experiment. J. Ecol. 1999;87:899–912. [Google Scholar]
- Turnbull L.A, Crawley M.J, Rees M. Are plant populations seed-limited? A review of seed sowing experiments. Oikos. 2000;88:225–238. [Google Scholar]
- Turnbull L.A, Coomes D.A, Hector A, Rees M. Seed mass and the competition/colonization trade-off: competitive interactions and spatial patterns in a guild of annual plants. J. Ecol. 2004;92:97–109. [Google Scholar]
- Vandvik V. Gap dynamics in perennial subalpine grasslands: trends and processes change during secondary succession. J. Ecol. 2004;92:86–96. [Google Scholar]
- Westoby M, Leishman M.R, Lord J. Comparative ecology of seed size and dispersal. Phil. Trans. R. Soc. B. 1996;351:1309–1318. [Google Scholar]
- Yu D.W, Wilson H.B. The competition-colonization trade-off is dead; long live the competition-colonization trade-off. Am. Nat. 2001;158:49–63. doi: 10.1086/320865. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.