Abstract
In Neurospora, the flavin adenine dinucleotide-containing protein WHITE COLLAR-1 is the blue-light photoreceptor for the circadian clock and other light responses. The putative chromophore-binding domain of WC-1, its light, oxygen, or voltage (LOV) domain, is similar to the LOV domains found in the plant phototropins, the Neurospora VIVID (VVD) protein, and the Arabidopsis FKF1 and its related proteins. Studies of the plant phototropins have identified 11 flavin-contacting residues that are also conserved in the LOV domains of WC-1, VVD, and FKF1. In this study, by mutating the putative WC-1 flavin-binding sites, we show that these sites are important for the light function of the protein, suggesting that the WC-1 LOV domain adapts a structure similar to that of the phototropin LOV domains. By creating a Neurospora strain in which the LOV domain of WC-1 is swapped with that of VVD, we show that the LOV domain of VVD partially replaces the function of the WC-1 LOV domain, suggesting that VVD is a wc-dependent photoreceptor in Neurospora. Furthermore, we show that the Neurosporastrains containing a chimeric WC-1 protein with the LOV domain from FKF1 or phot1 can also sense light, suggesting that FKF1 and its related proteins are light sensors in Arabidopsis. Taken together, our data suggest that these LOV domains are structurally similar protein modules involved in blue-light sensing.
Blue light regulates a wide variety of physiological processes in many organisms (1–4). Studies in eukaryotic systems have led to the identification of several types of blue-light photoreceptors, all of which are flavin-containing proteins. Cryptochromes, which are similar to photolyases, use flavin adenine dinucleotide (FAD) as a chromophore and function as the blue-light photoreceptors for circadian clocks and other light-associated responses in plants, insects, and possibly mammals (2, 3, 5). A FAD-containing adenylyl cyclase was recently identified as the blue-light photoreceptor for the photoavoidance response in Euglena (6).
Plant phototropins, which are light-regulated protein kinases, mediate phototropism and other processes in plants (1, 7). The phototropins contain two light, oxygen, or voltage (LOV) domains upstream of a C-terminal serine/threonine kinase domain. The LOV domains belong to a special class of PER-ARNT-SIM (PAS) domain, which is found in proteins involved in sensing a wide range of environmental stimuli (8, 9). Biochemical analyses of the phototropin LOV domains found that each LOV domain noncovalently binds a flavin mononucleotide (FMN) molecule, which undergoes a fully reversible photocycle (10–12). Crystal structures of the LOV2 domain of the Adiantum phototropin PHY3 have identified 11 residues of the LOV domain that are in contact with the FMN molecule (13, 14). These data demonstrate that the mechanism for light sensing in phototropins is mediated by the formation of a covalent C(4a) flavin-cysteinyl adduct in the LOV domain.
We recently identified the FAD-containing Neurospora protein WHITE COLLAR-1 (WC-1) as another type of blue-light photoreceptor regulating the circadian clock and other light responses in this filamentous fungus (15–17). Most fungal photoresponses are mediated by blue light (4, 18, 19). In Neurospora, all known light responses are mediated by blue light, and the protein products of wc-1 and wc-2 genes are essential components for these responses. These blue-light responses include entrainment of the circadian clock, induction of carotenoid synthesis, induction of protoperithecia, phototropism of perithecial peaks, and induction of gene expression and protein modification (4, 19). In true wc-1 or wc-2 null mutants, virtually all of these light responses are abolished (15, 20, 21).
WC-1 and WC-2 are two PAS domain-containing transcription factors with GATA type Zn-finger DNA-binding domains (22, 23). In the dark, WC-1 and WC-2 form a heterodimeric complex through their PAS domains and function as the positive elements in the Neurospora frequency (frq)-wc-based circadian feedback loop (16, 24–30). FRQ proteins serve as the negative elements in this loop (31–35). Three PAS domains occur in WC-1, and the first one is similar to the LOV domains of plant phototropins (Fig. 1). Previously, we showed that deletion of the entire WC-1 LOV domain abolished all light responses examined, including light entrainment of the circadian conidiation rhythm, light induction of frq and other genes, and light-induced hyperphosphorylation of WC-1 (15). In this mutant, however, the dark function of the WC-1 protein is near normal, and the circadian clock can be entrained by temperature treatment. Purification of the endogenous WC complex showed that the complex is associated with stoichiometric amounts of chromophore FAD. In vitro DNA binding assays showed that, upon irradiation with light, a large WC complex binds to two light-regulated elements of the frq promoter in a light- and FAD-dependent manner to mediate rapid induction of frq transcription (16), resulting in the entrainment of the Neurospora circadian clock (20, 36). WC proteins have also been shown to bind to the promoter of albino-3 (al-3), a gene involved in carotenoid biosynthesis (23). Together, these genetic and biochemical evidence indicate that WC-1 is the blue-light photoreceptor for circadian clock and other light responses in Neurospora.
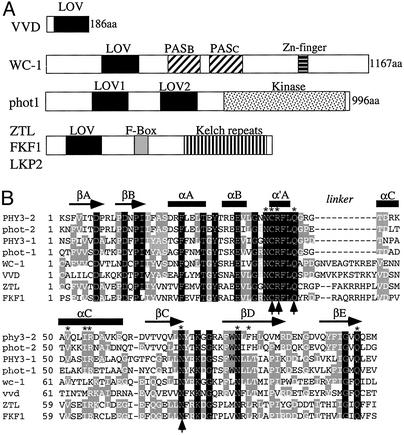
Figure 1.
(A) Schematic depictions of the domain structures of WC-1, VVD, phot1, and the ZTL family member proteins. (B) Amino acid sequence alignment of LOV domains from phototropins, WC-1, VVD, ZTL, and FKF1. This alignment was modified from ref. 13 and uses the same nomenclature for structural elements. Both LOV domains of the Adiantum capillus-veneris PHY3 and Arabidopsis thaliana phot1 were used in the alignment. Black residues are identical in all LOV domains, and gray residues are identical in most LOV domains. Asterisks mark the 11 FMN-interacting residues found in the LOV2 domain of Adiantum PHY3. Arrows mark the four residues that are essential for the light function of WC-1. For simplicity, the first residue of each LOV domain was numbered as the first residue.
FAD differs from FMN only by the presence of an adenosine monophosphate that is linked to FMN by a pyrophosphate linkage. Sequence comparison of the WC-1 LOV domain to those of the phototropins showed reasonably high sequence conservation, especially among the group of 11 FMN-interacting residues identified in the crystal structure of a LOV domain from PHY3 of Adiantum (Fig. 1) (13, 14). This finding suggests that the WC-1 LOV domain will adopt the same general PAS domain fold as PHY3 and bind the FMN moiety of FAD in a comparable manner. This arrangement is essential to the mechanism of the proper photocycling of the phototropins, which proceeds through a covalent adduct between the C(4a) position on the flavin and a highly conserved cysteine residue located in the α′A helix. Comparison of the LOV domains of WC-1 and phototropins revealed a significant extension in the loop connecting the α′A and αC helices in the WC-1 LOV domain (Fig. 1B). This extension may be important for accommodating the larger FAD molecule compared with FMN.
Several other proteins known to regulate light and circadian clock responses were also found to contain LOV domains. One of these is VIVID (VVD), a small (186-aa) Neurospora protein that is essentially composed of a single LOV domain (Fig. 1A) (37). Although it is not required for light responses and clock function, VVD functions as a repressor of light-regulated processes that regulates photoadaptation and clock resetting in Neurospora (37–39). The high degree of similarity of the sequences of VVD and WC-1 throughout the LOV domain suggests that VVD also binds FAD and functions as another blue-light photoreceptor in Neurospora.
ZEITLUPE (ZTL), FKF1, and LKP2 (LOV kelch repeat protein2) are three related Arabidopsis proteins, and they each contain a LOV domain, an F-box, and six kelch repeats (Fig. 1) (40–42). These proteins have been shown to be important for the photocontrol of the timing of day-length-dependent floral development and circadian clock periods. The sequence similarity of their LOV domains to those of WC-1 and the phototropins suggests that they may also be flavin-containing proteins that can sense light in Arabipdopsis.
In this study, we show that the putative flavin-contacting sites in WC-1 are important for the light function of the protein, suggesting that the basic structure of the WC-1 LOV domain is similar to those of the phototropins. By swapping the LOV domains of VVD and Arabidopsis phototropin 1 (phot1) and FKF1 with that of the WC-1, we show that these LOV domains can sense light in Neurospora, suggesting that VVD and FKF1 are blue-light photoreceptors.
Materials and Methods
Strains and Culture Conditions.
The wc-1RIP strain was described previously (15), and a wc-1RIP strain that carries a wild-type copy of the wc-1 gene at the his-3 locus (WC1-2) was used as the control in this study (30). Light responses in the WC1-2 strain are very similar to those of the wild-type Neurospora strain. In the WC1.LOV strain, described elsewhere (15), the WC-1 protein lacks the entire LOV domain. Liquid culture and race tube assay conditions were same as those previously described (30, 31, 33, 43).
Plasmids and Transformation.
The pwc1-3 plasmid containing the entire WC-1 ORF was used as the template for all mutagenesis constructs. All mutations of the WC-1 ORF were made by using the transformer site-directed mutagenesis kit (CLONTECH). Afterward, the mutated WC-1 LOV domains were subcloned into the pwc1-2 construct (29) and transformed into the wc-1RIP strain at the his-3 locus as previously described (44).
The pwc1-3.LOV construct has a deletion of amino acids 391–507 of wc-1 and a unique XbaI site at the junction of the deletion that was generated by site-specific mutagenesis (15). To make the chimeric wc-1 constructs, PCR fragments containing the LOV domain of VVD (aa71–186), Arabidopsis FKF1 (aa54–168) or Arabidopsis phot1 (aa475–580) were inserted into the XbaI site of the pwc1-3.LOV. cDNA clones for FKF1 and phot1 were obtained from The Arabidopsis Information Resource (TAIR). Because of the introduction of the XbaI sites in these constructs, additional residues were added to flank the LOV domain. To eliminate these additional residues, site-directed mutagenesis was conducted so that, in the resulting chimeric constructs, perfect fusions occur between the WC-1 sequence and the “foreign” LOV domains. Afterward, all constructs were confirmed by DNA sequencing and subcloned into the pwc1-2 construct. The final constructs were transformed into the wc-1RIP strain at the his-3 locus.
Protein and RNA Analyses.
Neurospora proteins were extracted as previously described (33, 43) except that 50 mM sodium fluoride, 10 mM sodium pyrophosphate, and 2 mM sodium orthovanadate were included in the extraction buffer. Equal amounts of total protein (40 μg) were loaded in each protein lane. Western blot analysis was performed as described elsewhere (33, 43), and afterward, the blots were stained by amido black to verify equal loading of proteins (32).
RNA extraction and Northern blot analysis were performed as described previously (31). Equal amounts of total RNA (20 μg) were loaded onto agarose gels for electrophoresis, and the gels were blotted and probed with an RNA probe specific for frq, albino-3 (al-3), or vvd.
Results
The Putative Flavin-Binding Sites of WC-1 Are Important for Light Function.
In the crystal structure of a LOV domain from PHY3 of Adiantum, 11 residues were shown to be in contact with FMN (13, 14). These residues are conserved in the WC-1 LOV domain (Fig. 1B), suggesting that these residues may compose the FAD-binding sites in the WC-1 LOV domain. Of these residues, the highly conserved cysteine in the α′A helix was shown to be critical for the formation of a covalent adduct with the C(4a) position of the flavin after light exposure, a step that is critical for the photocycle of the phototropin LOV domains (12–14, 45). To examine whether this cysteine and other putative flavin-binding sites are important for the light function of WC-1, they (10 of 11 residues) were individually mutated to other amino acids so that their interactions with flavin would be disrupted. These mutation constructs were introduced into a wc-1 null strain (wc-1RIP) (15), and a wc-1 null strain that carries a wild-type wc-1 construct (WC1-2) (28) was used as the control for light responses. The conserved cysteine at aa428 was mutated to serine (C428S). Such a mutation is predicted not to affect the flavin-binding capability of the LOV domain, but it would block the formation of the light-induced adduct between the protein and the flavin, thus abolishing the photocycle (12). As previously shown, a brief 15 min of light pulse resulted in rapid induction of the frq and albino-3 (al-3) genes and hyperphosphorylation of WC-1 in the WC1-2 strain (15) (Fig. 2 A and B). In addition, the level of FRQ protein was significantly higher in constant light (LL) than in the dark (DD). In the WC1.LOV strain (a strain with the entire WC-1 LOV domain deleted) (15) and in the C428S strain, all of these light responses were abolished presumably by blocking the photocycle of the protein, suggesting that this cysteine is critical for the light function of the WC-1 LOV domain.
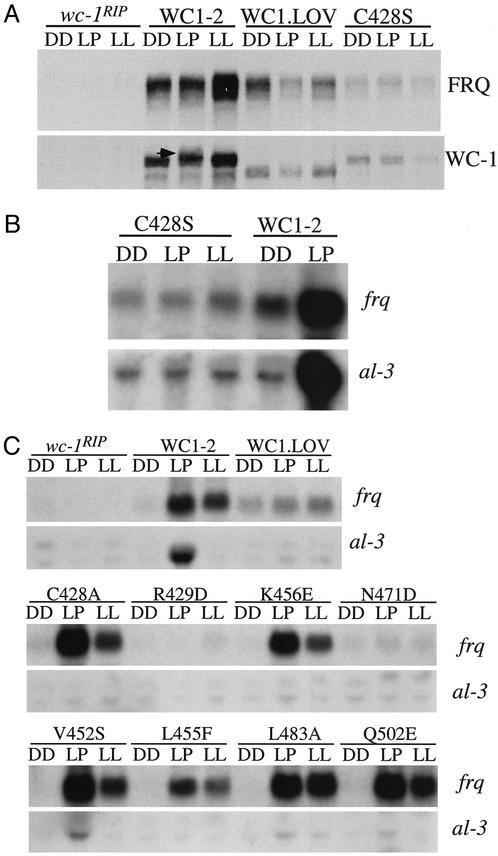
Figure 2.
The putative flavin-binding sites of the WC-1 LOV domain are important for its light function. (A and B) Western blot (A) and Northern (B) analyses showing that the C428S mutant is not light-sensitive. WC1-2 is a wc-1RIP strain that carries the wild-type wc-1 construct. In the WC1.LOV strain, the entire WC-1 LOV domain is deleted (15). DD, cultures were harvested after incubation for 24 h in constant darkness (DD24). Light pulse (LP), cultures were exposed to a 15-min light pulse (light intensity, ≈2,000 lux) at DD24. LL, cultures were grown in constant light for >1 day. In A, the arrow indicates the hyperphosphorylated WC-1 of the WC1-2 strain after a light pulse. (C) Northern analyses showing the light induction of frq and al-3 in various wc-1 mutants that contain a single mutation of the putative flavin-binding sites.
Mutations of the other putative flavin-binding sites all resulted in complete or severe reduction of the light induction of al-3, suggesting that these residues are also important for the light function of the WC-1 LOV domain (Fig. 2C and data not shown). However, the severity of the light defects in these mutants differs and they can be grouped into three classes based on their light deficiencies. First, like the C428S strain in which all light responses were abolished, mutations of the Arg-429 (R429D), Gln-432 (Q432E), and Asn-471 (N471D) completely eliminated the light induction of frq and al-3 (Fig. 2C and data not shown). These data suggest that these residues are essential for the light function of WC-1 and that the mutation of these amino acids probably abolished flavin-binding or blocked the photocycle (12). Three of these four residues are located in the NCRFLQ motif that makes the α′A helix (Fig. 1A), the most conserved region among these LOV domains. These data further highlight the importance of this helix in accomplishing the light function of the proteins.
Although the light induction of al-3 was completely eliminated in the second group of mutants (L455F and K456E), light induction of frq was near normal. In the third group of mutants (V452S, N481D, L483A, and Q502E), small amounts of light induction of al-3 could still be observed. These data suggest that light induction of frq does not require a fully functional WC-1 LOV domain. Consistent with this, several lines of previous evidence have indicated that light induction of frq and light induction of other genes differ significantly in their requirements for the WC proteins (17, 20, 21, 28–30). The light induction of frq does not require a fully functional WC-1 protein and requires only very small amounts of the WC proteins.
Surprisingly, however, we found that the C428A mutant belongs to the second group of mutants with near-normal light induction of frq, whereas light induction of al-3 was completely eliminated. Previously, it was shown that the same mutation in a phototropin LOV domain blocked the photocycle and light-induced adduct formation (12, 45). However, the recombinant proteins with such a mutation in phototropin LOV domains are not light-insensitive, and they can still generate the early photoproduct without formation of the adduct and the early photoproduct can decay back to the ground state (45, 46). Thus, it is possible that light generates the early photoproduct of WC-1 in the C428A mutant, leading to conformational changes of WC-1 and activation of frq.
Together, these mutagenesis data suggest that these putative flavin-binding sites of WC-1 are important for the light-sensing function of its LOV domain. These data also suggest that the LOV domain of WC-1 should adopt a PAS structure similar to those of the phototropin LOV domains and bind the FMN moiety of FAD in a comparable manner.
VVD Is a wc-Dependent Neurospora Photoreceptor.
Although VVD is not essential for light responses and the function of the circadian clock, all aspects of light responses examined are affected in vvd mutants (37–39). First, light-induced gene expression examined was elevated in the mutants, including that of frq. The high sensitivity of frq transcription to light in the mutants leads to change of circadian phase and altered light-induced phase-shifting of the clock. Second, photoadaptation of gene expression is impaired in the vvd mutants. In addition, circadian gating of light responses is partially lost in the vvd mutants. Therefore, VVD is an important negative regulator of light responses in Neurospora.
vvd is strongly induced by light, and its light induction requires both WC proteins (37, 47). Although the expression of vvd is clock-controlled, its expression drops to an undetectable level after prolonged incubation in the dark (>2 days) in the wild-type strain. In addition, expression of VVD protein and vvd RNA was not detectable in the wc null mutants (data not shown). Therefore, VVD appears to require the presence of both WC proteins to be expressed and to function as a regulator of light responses.
VVD is a small protein (186 aa) that essentially is composed of one LOV domain (Fig. 1). Its LOV domain is very similar to that of WC-1 with 42% identity and 69% similarity. Like the WC-1 LOV domain, the LOV domain of VVD also contains the extended linker region between the α′A and αC helices. Such similarities between the LOV domains of WC-1 and VVD and the roles of VVD in light responses suggest that VVD, like WC-1, may bind FAD and function as a blue light photoreceptor in Neurospora.
To demonstrate that the VVD LOV domain is a light-sensory domain in Neurospora, we swapped the LOV domain of WC-1 with that of VVD by inserting the VVD LOV domain (aa71–aa186) into the WC1.LOV construct (15) (Fig. 3A). The resulting construct was introduced into the wc-1 null strain (WC1.LOV.VVD). Because the mutant lacking the WC-1 LOV domain is not sensitive to light (15) (Fig. 2), the rescue of any light responses in the WC1.LOV.VVD strain would indicate the light-sensing role of the VVD LOV domain. Four light responses were examined in the WC1.LOV.VVD strain, including the light induction of genes (frq, al-3, and vvd), light-induced FRQ protein expression, light-induced WC-1 hyperphosphorylation, and light entrainment of the circadian conidiation rhythm.
Figure 3.
The LOV domain of VVD can partially replace the function of the WC-1 LOV domain. (A) Schematic depiction of the domain structure of the WC1.LOV.VVD chimeric protein. (B–D) Western blot (B and D) and Northern (C) analyses showing the light responses of the expression of different genes and the phosphorylation states of WC-1 in the WC1.LOV.VVD strain. In B and D, two different exposures of the WC-1 Western blot results are shown, and arrows indicate the light-induced hyperphosphorylated WC-1 species. For D, cultures were first grown in DD for 18 h before transferred into LL and harvested at the indicated times.
Although the WC-1 level in the mutant strain was significantly lower than in the control strain (WC1-2) (Fig. 3 B and D), all four of these light responses were partially rescued in the WC1.LOV.VVD strain (Figs. 3 and 4). First, even though the levels of FRQ protein in DD were lower in the mutant than in the control strain (owing to the low WC-1 level in the mutant), its level in LL was similar to that in the control strain (Fig. 3B). frq mRNA was also clearly light-induced after a brief light pulse and in LL, but the amount of frq induction by light pulse was lower in the mutant than in the control strain (Fig. 3C). In addition, the induction kinetics of FRQ protein expression after a dark-to-light transition was similar in both the mutant and the control strains (Fig. 3D). Small amounts of induction of vvd were also observed for the light pulse and LL mutant samples, but light induction of al-3 was not seen in the mutant. The weak induction of vvd and the lack of al-3 induction may be due to the low level of the WC-1 protein in the WC1.LOV.VVD strain. It is known that the light induction of these two genes is sensitive to changes in WC-1 level (21, 30).
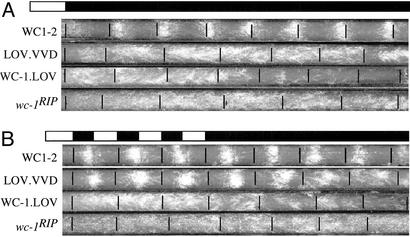
Figure 4.
Conidiation rhythms of the WC1.LOV.VVD strain can be entrained by LD cycles and persist in DD. Race-tube assays show the conidiation rhythms of various strains in DD after a single LD entrainment (A) or after multiple LD cycles (B). Light intensity used was ≈1,200 lux. WC1-2 is a wc-1RIP strain that carries the wild-type wc-1 construct. In the WC1.LOV strain, the entire WC-1 LOV domain is deleted. The LOV.VVD strain is WC1.LOV.VVD strain.
As in the control strain, WC-1 in the WC1.LOV.VVD strain became hyperphosphorylated after the 15-min light pulse (Fig. 3 B and D). However, unlike in the control strain, in which the hyperphosphorylated WC-1 species quickly disappeared after 2 h in LL (26, 48), WC-1 stayed hyperphosphorylated after 8 h in LL in the WC1.LOV.VVD strain (Fig. 3D), and its hyperphosphorylation became more pronounced after incubation for 1 day in LL (Fig. 3B). Similar kinetics of WC-1 hyperphosphorylation was also observed previously in vvd mutants (37, 38).
Race-tube assays were used to examine the conidiation rhythms of the WC1.LOV.VVD strain in LD cycles and in DD (Fig. 4). After a single LD transition, a weak circadian conidiation rhythm could be observed for the first 2–3 days for the WC1.LOV.VVD strain, but the rhythm was not robust (Fig. 4A). This finding was probably due to the reduced light induction of FRQ in the mutant strain (Fig. 3). Under 12/12 LD cycles, however, a robust conidiation rhythm was seen in the WC1.LOV.VVD strain (Fig. 4B). After three to four LD entrainment cycles, a robust circadian conidiation rhythm was observed in constant darkness, which contrasts with the WC-1.LOV and wc-1RIP strains, in which no conidiation rhythms were seen under either LD or DD conditions. Taken together, these molecular and physiological experiments strongly suggest that the LOV domain of VVD is a light-sensing domain in Neurospora and that VVD is another Neurospora blue-light photoreceptor. Furthermore, these data demonstrate that, by using the domain-swapping strategy we used for VVD, we can examine the light-sensing role of other putative photoreceptors in Neurospora.
The LOV Domains of phot1 and FKF1 Are Light-Sensitive in Neurospora.
ZTL, FKF1, and LKP2 are three closely related Arabidopsis proteins that have been shown to be involved in the photoperiodic control of flowering (40–42). Mutants of ZTL show altered period length of the circadian clock, whereas overexpression of LKP2 leads to arrhythmic phenotypes. Because all three proteins contain a LOV domain similar to those of WC-1 and phototropins in addition to their F-boxes and kelch repeats, it was proposed that they may function as light switches in Arabidopsis to regulate degradation of proteins involved in controlling flowering time and circadian clock (42). No experimental evidence is currently available, however, to demonstrate the light-sensing function of their LOV domains.
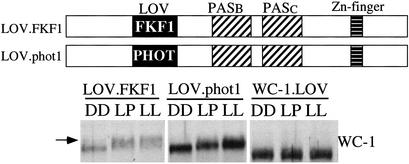
The success of our domain-swapping experiments with VVD prompted us to ask whether we could demonstrate the light-sensing function of the LOV domains of the ZTL family member proteins in Neurospora. In addition, we also wondered whether the LOV domains of the phototropins could sense light in Neurospora. Constructs were made in which the entire LOV domain of the Arabidopsis FKF1 (aa54–168, 39% identity and 55% similarity with the LOV domain of WC-1) or the LOV2 domain of the Arabidopsis phot1 (aa475–580) was swapped for the LOV domain of WC-1; and these constructs were then introduced into the wc-1 null strain (Fig. 5). Although no light responses were observed in these strains for light induction of gene expression and light entrainment of conidiation rhythms, light-induced hyperphosphorylation of WC-1 was seen consistently in these two strains (Fig. 5). The extent of WC-1 hyperphosphorylation induced by light pulse or LL treatment in these two strains, however, was not as extensive as that in the wild-type strain (Fig. 3). In contrast, no light-induced hyperphosphorylation of WC-1 was seen in the strain lacking the entire LOV domain (WC1.LOV) (Fig. 5) or in strains with mutated WC-1 LOV domain (Fig. 2A). These data indicate that the LOV domains of FKF1 and PHOT1 can sense light in Neurospora, suggesting that the function of the FKF1 protein is regulated by light in Arabidopsis.
Figure 5.
The FKF1/WC-1 and phot1/WC-1 chimeric proteins can sense light in Neurospora. (Upper) Schematic depictions of the domain structures of the WC1.LOV.FKF1 and WC1.LOV.PHOT1 chimeric proteins. (Lower) Western blot analysis results showing the light-induced WC-1 hyperphosphorylation in the WC1.LOV.FKF1 and WC1.LOV.phot1 strains. The arrow indicates the hyperphosphorylated WC-1 species.
Discussion
Although the Neurospora WC complex was found to be associated with FAD in vivo and WC-1 is responsible for light sensing (15, 16), the direct demonstration that the WC-1 LOV domain is responsible for the FAD binding is still lacking because of the difficulty of expressing the WC-1 LOV domain in a heterologous expression system. In this study, by mutating the putative flavin-binding sites in the WC-1 LOV domain, we showed that these residues are important for the light function of WC-1. These data are consistent with the WC-1 LOV domain being the FAD-binding domain and suggest that the structure of the WC-1 LOV domain is similar to those of the phototropin LOV domains. Without the biochemical and photochemical evidence, however, it is unclear whether the light defects of these WC-1 mutants are due to the blockage of the photocycle or to their inability to bind FAD. Consistent with the structural similarity between the LOV domain of WC-1 and those of the phototropins, we showed that the phot1 LOV2 domain could sense light in Neurospora.
A surprising finding from the mutagenesis study is that, in the C428Amutant, light induction of frq is normal. The complete elimination of al-3 light induction indicates that the WC-1 in this mutant is only partially functional. In contrast, no light response was seen in the C428S mutant. On the basis of the previous studies of the plant phototropin LOV domains, both mutations should blocked the normal photocycle and the formation of the light-induced flavin-cysteinyl adduct without affecting the flavin-binding ability of the proteins (12, 45). However, such mutations of the phototropin LOV domains do not yield light-insensitive proteins, and early photoproducts can still be formed without the formation of the flavin-cysteinyl adduct (45, 46, 49). Thus, it is likely that an abnormal photocycle of the WC-1 protein is responsible for the frq light induction seen in the C428A mutant. Unlike most other light-inducible genes in Neurospora, the light induction of frq requires only a very low level of WC-1 and does not need a fully functional WC-1 (17, 20, 21, 28–30). But it is unclear why the C428S mutant is light blind. It is possible that these two mutations affect the photocycle and flavin binding of the WC-1 LOV domain differently, a possibility that can only be addressed by future photochemical studies.
By swapping the LOV domain of WC-1 with that of the VVD, we showed that the WC1.LOV.VVD protein could rescue several light responses in Neurospora, including the light induction of frq and entrainment of the circadian clock. Because of the important roles VVD has in light induction of gene expression and photoadaptation (37–39), we propose that VVD is a blue-light photoreceptor that regulates these processes. Because the sequence similarity between the LOV domains of VVD and WC-1 is high and they both share the extended linker region between the α′A and αC helices, we suggest that VVD is also a FAD-binding protein in Neurospora. Recently, Schwerdtfeger et al. have also proposed that VVD is Neurospora photoreceptor for photoadaptation.† In their study, VVD protein expressed in Escherichia coli. was found to be noncovalently associated with a flavin chromophore, and it undergoes a photocycle in vitro. Their biochemical evidence together with the data presented here indicates that VVD is another blue-light photoreceptor in Neurospora. Because the expression of VVD is wc-dependent, the action of VVD should also be dependent on the presence of functional WC proteins.
In Neurospora, photoadaptation is a process that the expression of light-induced genes adapts to a given light intensity in constant light (37–39, 50). After the initial rapid induction by light, their expression quickly decrease after a period of light exposure and stay at low levels in LL. If Neurospora cultures are shifted from a low-light intensity to a high-light intensity, a second light induction can be observed. In vvd mutants, both of these responses are partially impaired. Photoadaptation has been well studied in vertebrates, and photoadaption of the visual systems in vertebrates is achieved by the desensitization of the light-input pathways (51). In Neurospora, photoadaptation may use a similar strategy. Although how VVD functions is unclear, it is likely that after its expression following light exposure, it either directly or indirectly down-regulates the activity of the WC complex.
Replacement of the LOV domain of WC-1 with that of FKF1 showed that the LOV domain of FKF1 can sense light in Neurospora, suggesting that it is a photosensor in Arabidopsis. Because the LOV domains of ZTL and LKP2 are highly similar to that of FKF1, we suggest that they are also light sensors in Arabidopsis. Therefore, light may regulate the abilities of these proteins to mediate ubiquitination and degradation of proteins involved in photoperiodic control and circadian clock in Arabidopsis (40–42). In addition, our data demonstrate that, by using Neurospora as a system, the domain-swapping strategy can be used as a method to identify other putative photoreceptors from Neurospora and other organisms.
Although the results of our domain-swapping experiments suggest that these LOV domains should adapt similar PAS and flavin-binding folds, they also indicate that these “foreign” LOV domains cannot fully replace the function of the WC-1 LOV domain. This partial replacement is especially true for the WC1.LOV.FKF1 and WC1.LOV.phot1 strains in which only one weak light response was observed whereas other light responses examined were not seen. Even in the WC1.LOV.VVD strain, light responses are lower than those in the wild-type control. These data suggest that, despite the similarities of the LOV domains of WC-1, VVD, FKF1, and phot1, they are significantly different. Such differences may reflect the functional distinctions that each protein has in different systems, in particular, how to couple the output of LOV domains to a wide range of enzymatic and nonenzymatic targets.
Acknowledgments
We thank Dr. Kevin Gardner and Shannon Harper for advice and for critical readings of the manuscript, and Dr. Winslow Briggs for seminal discussions. We are also grateful for the comments made by three anonymous reviewers. This study is supported by a grant from the National Institutes of Health (to Y.L.). Y.L. is the Louise W. Kahn Scholar in Biomedical Research at University of Texas Southwestern Medical Center.
Abbreviations
- LOV
light, oxygen, voltage
- WC-1
WHITE COLLAR-1
- FRQ
FREQUENCY
- vvd, vivid
phot1, phototropin 1
- FKF1
flavin-binding, kelch repeat, F box
- FAD
flavin adenine dinucleotide
- PAS
PER-ARNT-SIM
- FMN
flavin mononucleotide
- LL
constant light
- DD
dark
Footnotes
Schwerdtfeger, C., Loros, J. J., Dunlap, J. C. & Linden, H. (2003) 22nd Fungal Genetics Conference, poster 236.
References
- 1.Briggs W R, Huala E. Annu Rev Cell Dev Biol. 1999;15:33–62. doi: 10.1146/annurev.cellbio.15.1.33. [DOI] [PubMed] [Google Scholar]
- 2.Devlin P F, Kay S A. Annu Rev Physiol. 2001;63:677–694. doi: 10.1146/annurev.physiol.63.1.677. [DOI] [PubMed] [Google Scholar]
- 3.Sancar A. Annu Rev Biochem. 2000;69:31–67. doi: 10.1146/annurev.biochem.69.1.31. [DOI] [PubMed] [Google Scholar]
- 4.Linden H, Ballario P, Macino G. Fungal Genet Biol. 1997;22:141–150. doi: 10.1006/fgbi.1997.1013. [DOI] [PubMed] [Google Scholar]
- 5.Cashmore A R, Jarillo J A, Wu Y J, Liu D. Science. 1999;284:760–765. doi: 10.1126/science.284.5415.760. [DOI] [PubMed] [Google Scholar]
- 6.Iseki M, Matsunaga S, Murakami A, Ohno K, Shiga K, Yoshida K, Sugai M, Takahashi T, Hori T, Watanabe M. Nature. 2002;415:1047–1051. doi: 10.1038/4151047a. [DOI] [PubMed] [Google Scholar]
- 7.Briggs W R, Christie J M. Trends Plant Sci. 2002;7:204–210. doi: 10.1016/s1360-1385(02)02245-8. [DOI] [PubMed] [Google Scholar]
- 8.Huala E, Oeller P W, Liscum E, Han I S, Larsen E, Briggs W R. Science. 1997;278:2120–2123. doi: 10.1126/science.278.5346.2120. [DOI] [PubMed] [Google Scholar]
- 9.Crosson S, Rajagopal S, Moffat K. Biochemistry. 2003;42:2–10. doi: 10.1021/bi026978l. [DOI] [PubMed] [Google Scholar]
- 10.Christie J M, Reymond P, Powell G K, Bernasconi P, Raibekas A A, Liscum E, Briggs W R. Science. 1998;282:1698–1701. doi: 10.1126/science.282.5394.1698. [DOI] [PubMed] [Google Scholar]
- 11.Christie J M, Salomon M, Nozue K, Wada M, Briggs W R. Proc Natl Acad Sci USA. 1999;96:8779–8783. doi: 10.1073/pnas.96.15.8779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salomon M, Christie J M, Knieb E, Lempert U, Briggs W R. Biochemistry. 2000;39:9401–9410. doi: 10.1021/bi000585+. [DOI] [PubMed] [Google Scholar]
- 13.Crosson S, Moffat K. Proc Natl Acad Sci USA. 2001;98:2995–3000. doi: 10.1073/pnas.051520298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crosson S, Moffat K. Plant Cell. 2002;14:1067–1075. doi: 10.1105/tpc.010475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He Q, Cheng P, Yang Y, Wang L, Gardner K H, Liu Y. Science. 2002;297:840–843. doi: 10.1126/science.1072795. [DOI] [PubMed] [Google Scholar]
- 16.Froehlich A C, Liu Y, Loros J J, Dunlap J C. Science. 2002;297:815–819. doi: 10.1126/science.1073681. [DOI] [PubMed] [Google Scholar]
- 17. Liu, Y. (2003) J. Biol. Rhythms18, in press. [DOI] [PubMed]
- 18.Carlile M J. Annu Rev Plant Physiol. 1965;16:175–202. [Google Scholar]
- 19.Lakin-Thomas P, Coté G, Brody S. Crit Rev Microbiol. 1990;17:365–416. doi: 10.3109/10408419009114762. [DOI] [PubMed] [Google Scholar]
- 20.Collett M A, Garceau N, Dunlap J C, Loros J J. Genetics. 2002;160:149–158. doi: 10.1093/genetics/160.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee K, Dunlap J C, Loros J J. Genetics. 2003;163:103–114. doi: 10.1093/genetics/163.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ballario P, Vittorioso P, Magrelli A, Talora C, Cabibbo A, Macino G. EMBO J. 1996;15:1650–1657. [PMC free article] [PubMed] [Google Scholar]
- 23.Linden H, Macino G. EMBO J. 1997;16:98–109. doi: 10.1093/emboj/16.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dunlap J C. Cell. 1999;96:271–290. doi: 10.1016/s0092-8674(00)80566-8. [DOI] [PubMed] [Google Scholar]
- 25.Loros J J, Dunlap J C. Annu Rev Physiol. 2001;63:757–794. doi: 10.1146/annurev.physiol.63.1.757. [DOI] [PubMed] [Google Scholar]
- 26.Talora C, Franchi L, Linden H, Ballario P, Macino G. EMBO J. 1999;18:4961–4968. doi: 10.1093/emboj/18.18.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crosthwaite S K, Dunlap J C, Loros J J. Science. 1997;276:763–769. doi: 10.1126/science.276.5313.763. [DOI] [PubMed] [Google Scholar]
- 28.Cheng P, Yang Y, Liu Y. Proc Natl Acad Sci USA. 2001;98:7408–7413. doi: 10.1073/pnas.121170298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng P, Yang Y, Gardner K H, Liu Y. Mol Cell Biol. 2002;22:517–524. doi: 10.1128/MCB.22.2.517-524.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng P, Yang Y, Wang L, He Q, Liu Y. J Biol Chem. 2003;278:3801–3808. doi: 10.1074/jbc.M209592200. [DOI] [PubMed] [Google Scholar]
- 31.Aronson B, Johnson K, Loros J J, Dunlap J C. Science. 1994;263:1578–1584. doi: 10.1126/science.8128244. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Garceau N, Loros J J, Dunlap J C. Cell. 1997;89:477–486. doi: 10.1016/s0092-8674(00)80228-7. [DOI] [PubMed] [Google Scholar]
- 33.Cheng P, Yang Y, Heintzen C, Liu Y. EMBO J. 2001;20:101–108. doi: 10.1093/emboj/20.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Denault D L, Loros J J, Dunlap J C. EMBO J. 2001;20:109–117. doi: 10.1093/emboj/20.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merrow M, Franchi L, Dragovic Z, Gorl M, Johnson J, Brunner M, Macino G, Roenneberg T. EMBO J. 2001;20:307–315. doi: 10.1093/emboj/20.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crosthwaite S K, Loros J J, Dunlap J C. Cell. 1995;81:1003–1012. doi: 10.1016/s0092-8674(05)80005-4. [DOI] [PubMed] [Google Scholar]
- 37.Heintzen C, Loros L L, Dunlap J C. Cell. 2001;104:453–464. doi: 10.1016/s0092-8674(01)00232-x. [DOI] [PubMed] [Google Scholar]
- 38.Schwerdtfeger C, Linden H. Mol Microbiol. 2001;39:1080–1087. doi: 10.1046/j.1365-2958.2001.02306.x. [DOI] [PubMed] [Google Scholar]
- 39.Shrode L B, Lewis Z A, White L D, Bell-Pedersen D, Ebbole D J. Fungal Genet Biol. 2001;32:169–181. doi: 10.1006/fgbi.2001.1264. [DOI] [PubMed] [Google Scholar]
- 40.Somers D E, Schultz T F, Milnamow M, Kay S A. Cell. 2000;101:319–328. doi: 10.1016/s0092-8674(00)80841-7. [DOI] [PubMed] [Google Scholar]
- 41.Nelson D C, Lasswell J, Rogg L E, Cohen M A, Bartel B. Cell. 2000;101:331–340. doi: 10.1016/s0092-8674(00)80842-9. [DOI] [PubMed] [Google Scholar]
- 42.Schultz T F, Kiyosue T, Yanovsky M, Wada M, Kay S A. Plant Cell. 2001;13:2659–2670. doi: 10.1105/tpc.010332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garceau N, Liu Y, Loros J J, Dunlap J C. Cell. 1997;89:469–476. doi: 10.1016/s0092-8674(00)80227-5. [DOI] [PubMed] [Google Scholar]
- 44.Bell-Pedersen D, Shinohara M, Loros J, Dunlap J C. Proc Natl Acad Sci USA. 1996;93:13096–13101. doi: 10.1073/pnas.93.23.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swartz T E, Corchnoy S B, Christie J M, Lewis J W, Szundi I, Briggs W R, Bogomolni R A. J Biol Chem. 2001;276:36493–36500. doi: 10.1074/jbc.M103114200. [DOI] [PubMed] [Google Scholar]
- 46.Kay C W, Schleicher E, Kuppig A, Hofner H, Rudiger W, Schleicher M, Fischer M, Bacher A, Weber S, Richter G. J Biol Chem. 2003;278:10973–10982. doi: 10.1074/jbc.M205509200. [DOI] [PubMed] [Google Scholar]
- 47.Dragovic Z, Tan Y, Gorl M, Roenneberg T, Merrow M. EMBO J. 2002;21:3643–3651. doi: 10.1093/emboj/cdf377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwerdtfeger C, Linden H. Eur J Biochem. 2000;267:414–422. doi: 10.1046/j.1432-1327.2000.01016.x. [DOI] [PubMed] [Google Scholar]
- 49.Kottke T, Heberle J, Hehn D, Dick B, Hegemann P. Biophys J. 2003;84:1192–1201. doi: 10.1016/S0006-3495(03)74933-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arpaia G, Cerri F, Baima S, Macino G. Mol Gen Genet. 1999;262:314–322. doi: 10.1007/s004380051089. [DOI] [PubMed] [Google Scholar]
- 51.Pugh E N J, Nikonov S, Lamb T D. Curr Opin Neurobiol. 1999;9:410–418. doi: 10.1016/S0959-4388(99)80062-2. [DOI] [PubMed] [Google Scholar]