Abstract
Targeted disruption of the histidine decarboxylase gene (HDC−/−), the only histamine-synthesizing enzyme, led to a histamine-deficient mice characterized by undetectable tissue histamine levels, impaired gastric acid secretion, impaired passive cutaneous anaphylaxis, and decreased mast cell degranulation. We used this model to study the role of histamine in bone physiology. Compared with WT mice, HDC−/− mice receiving a histamine-free diet had increased bone mineral density, increased cortical bone thickness, higher rate of bone formation, and a marked decrease in osteoclasts. After ovariectomy, cortical and trabecular bone loss was reduced by 50% in HDC−/− mice compared with WT. Histamine deficiency protected the skeleton from osteoporosis directly, by inhibiting osteoclastogenesis, and indirectly, by increasing calcitriol synthesis. Quantitative RT-PCR showed elevated 25-hydroxyvitamin D-1α-hydroxylase and markedly decreased 25-hydroxyvitamin D-24-hydroxylase mRNA levels. Serum parameters confirming this indirect effect included elevated calcitriol, phosphorus, alkaline phosphatase, and receptor activator of NF-κB ligand concentrations, and suppressed parathyroid hormone concentrations in HDC−/− mice compared with WT mice. After ovariectomy, histamine-deficient mice were protected from bone loss by the combination of increased bone formation and reduced bone resorption.
Bone remodeling maintains skeletal integrity in humans and higher vertebrates. This remodeling is mediated by the balanced activities of osteoclasts, which resorb existing bone, and osteoblasts, which form new bone (1). In many postmenopausal women, the extent of bone resorption exceeds that of formation, resulting in osteoporosis and increased fracture risk. Approximately 100 million people suffer from postmenopausal osteoporosis worldwide. Hormone replacement therapy, selective estrogen receptor modulators, calcitonin, and bisphosphonates are useful for prevention and/or treatment of postmenopausal osteoporosis (2). Improvement of bone mineral density has been documented in response to a diet high in calcium and vitamin D in elderly osteoporotic patients (2). In addition, several therapies may increase bone formation in osteoporotic patients, such as the lipid-lowering drugs “statins” (3), fibroblast growth factor-1 (4), and parathyroid hormone (PTH) (5). Recently, the use of histamine receptor antagonists for the prevention and treatment of osteoporosis has been suggested by studies on ovariectomized rats (6) and on postmenopausal women (7).
Significant advances have been made in our understanding of the factors that induce osteoporosis in select individuals, whereas others maintain sufficient bone mass even after menopause. Studies of knockout and transgenic mice demonstrated that one of the main mechanisms of osteoporosis caused by estrogen deficiency was related to the over-stimulation of osteoclast differentiation and activation by cytokines. Among these cytokines, the roles of tumor necrosis factor related receptor activator of NF-κB (RANK) (8), and its ligands RANK ligand (RANKL) (9, 10) are well documented. RANK is expressed in osteoclasts, whereas osteoprotegerin (11) is a soluble decoy receptor that binds to and neutralizes RANKL. RANKL secretion from osteoblasts is stimulated by calcitriol, affecting osteoclast development and function (12).
A few studies indicated that the cytokine histamine is another factor that regulates bone remodeling. Histamine influences many physiological functions through G protein-coupled receptors. Histamine modulates inflammatory and allergic responses via H1 and H4 receptors (13), gastric acid secretion via H2 receptors (14), and neurotransmitter release via H3 receptors (15). One study, in a synchronized resorption model, demonstrated that histamine increases osteoclast activity and number through H1 and H2 receptors, respectively (16), whereas other studies indicated that the histamine effects on bone resorption were indirect (17, 18). Consistent with the action of histamine on bone, high circulating levels of histamine were associated with osteoporosis and abnormal bone histomorphometry indices of resorption in patients with mastocytosis (19–21). Increased histamine levels were also associated with normal or decreased parameters of bone formation (21), although a direct effect of histamine on osteoblasts has not been reported. Because diseases that are associated with increased histamine serum levels, such as allergies, are common and increasing in frequency in developed countries, a better understanding of the impact of excess histamine on the development of postmenopausal osteoporosis is imperative.
Histamine serum levels are regulated mainly by the activity of the histamine-synthesizing enzyme, histidine decarboxylase (HDC), and to a lesser degree by dietary intake of histamine. Targeted deletion of HDC in mice combined with histamine-deficient diet provided us with a model of histamine deficiency (22). We used this model to explore roles of histamine in ovariectomy-induced osteoporosis.
Materials and Methods
Mice and Diet.
The Animal Care and Use Committee of Semmelweis University, Faculty of Medicine approved all animal procedures. Mice homozygous for a target null allele of HDC were generated on a mixed genetic I29/Sv background, as described (22). Mice were genotyped by Southern blot analysis and PCR. Groups of adult female and male HDC−/− mice and their WT littermates were randomly selected from the transgenic colony. There were no differences in body weights between the groups. Mice were placed on a histamine-free diet (0.6 nmol histamine per g food, 500 units/kg vitamin D; Altormin, Lage, Germany) 14 days before surgery and maintained on this diet for 3 months. One group of mice was kept on a diet without vitamin D and reduced in calcium (0.4% calcium vs. 2% in normal diet) (D-deficient diet; Harlan Teklad, Madison, WI). The concentration of histamine in the skin of the HDC−/− mice kept for 7 days on the histamine-free diet was very low, 0.3 ± 0.1 nmol/g tissue (WT: 21.2 ± 4.3 nmol/g) (22, 23). Mice were sham-operated (SHAM) or ovariectomized (OVX) at the age of 4 months and killed either 45 or 90 days after surgery. Additional groups of intact (nonoperated) mice were killed at 4–6 months of age. To obtain dynamic parameters of bone formation, calcein (4 mg/kg; Sigma) was injected 9 days and 2 days before death. After death, femur, tibiae, and lumbar vertebrae were excised for measurements of bone density and histology. Uterus weight was determined to verify the successful removal of the ovaries. Length and two-dimensional subperiosteal diameter of excised femurs were measured with digital calipers to the nearest 0.01 mm.
Serum Parameters of Calcium Homeostasis.
Serum Ca, Pi, alkaline phosphatase activity (ALPase), blood urea nitrogen, albumin, intact PTH, 25-hydroxyvitamin D (25-OH-D), 1,25-dihydroxy-vitamin D, soluble RANKL (sRANKL), and estradiol concentrations were measured with standard techniques. For details see Supporting Materials and Methods, which is published as supporting information on the PNAS web site, www.pnas.org.
Bone Densitometry and X-Ray Analysis.
Bone mineral content (BMC) was measured from excised femurs by single photon absorptiometry (SPA). Bone mineral density and cortical thickness was measured from excised left femurs by quantitative computed tomography for control in one of the four experiments. Soft roentgenographs were taken from isolated femurs and whole body x-rays from anesthetized mice. From digitized roentgenographs, radioopacity values were obtained by using the metamorph software (Universal Imaging, West Chester, PA). Radioopacity values are expressed in arbitrary units as mean ± SE. Units were standardized to the opacity values of the aluminum filament on each picture. For more detail, see Supporting Materials and Methods.
Histological Examination.
Procedures for sample preparation, sectioning, fluorescence microscopy, staining with Goldner's-Masson-trichrome and von Kossa, tartarate-resistant acid phosphatase activity (TRAP) of osteoclasts, and histomorphometrical analysis are found in Supporting Materials and Methods.
Osteoclast Formation Assay.
Bone marrow cells were isolated from the tibiae and femurs of three WT and four HDC−/− female mice (7 months old, kept on histamine-free diet for 4 months). Nonadherent cells were separated by Ficoll gradient and plated at 1 × 105 cells per well in 96-well plates. Cells were cultured in phenol red-free MEMα (Life Technologies, Grand Island, NY) with 30 ng/liter RANKL and 10 mg/liter M-CSF (Sigma) as described (9). Media were changed on days 3 and 5, and cells were fixed on day 9. Osteoclast number was determined by counting the total number of multinucleated (more than three nuclei) TRAP-positive cells per well.
Real-Time Quantitative PCR Analysis.
Expression levels of 25-hydroxyvitamin D-1α-hydroxylase (1αOHase) and 25-hydroxyvitamin D-24-hydroxylase (24OHase) mRNA were determined from kidneys of WT and HDC−/− mice. Real-time RT-PCR was conducted as described in Supporting Materials and Methods.
Statistical Analysis.
Histomorphometry measurements were analyzed by a one-way ANOVA. Fisher's protected least significant difference further compared each group of data with other groups. All other group mean values were compared by Student's t test or one-way ANOVA, as appropriate. Data are mean ± SEM. Statistical significance was determined at P < 0.05.
Results
Phenotypic Features of Histamine-Deficient Mice.
The hallmarks of histamine deficiency have been established in HDC−/− mice. These mice, lacking histamine synthesizing activity and histamine in their tissues (22), exhibited a reduction in the secretory granule contents in the remaining mast cells (22), impaired cutaneous anaphylaxis (23), decreased basal gastric acid secretion and gastrin resistance (24), and decreased spontaneous locomotor activity during the dark period (25). HDC−/− mice exhibited normal body weight, growth, development, and fertility in comparison with the WT mice. There was no significant difference in femur length between the two groups. Femoral thickness, determined by caliper measurements, was significantly increased in HDC−/− mice compared with WT; thickness of femurs at mid-diaphysis from 7-month-old HDC−/− mice was 1.9 ± 0.1 mm and from WT litter mates 1.7 ± 0.1 mm (P < 0.01). Similarly, the width of the first thoracic vertebrae was also increased in HDC−/− mice by 5% compared with WT littermates (n = 15, P < 0.001).
Increased Bone Mineral Content in Histamine-Deficient Mice.
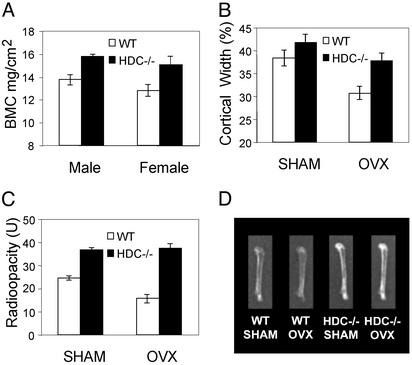
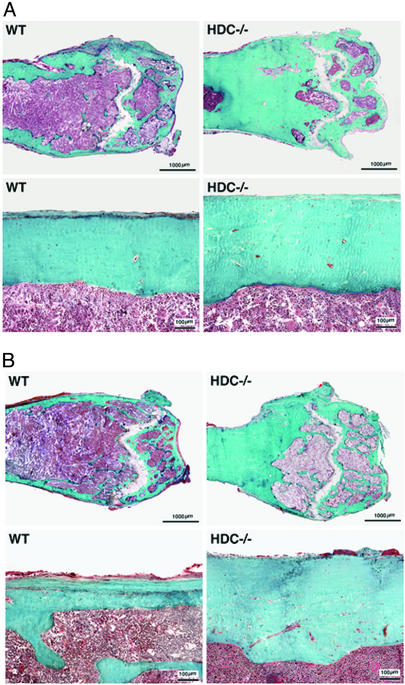
The bone phenotype was further characterized by comparing bone mineral content in HDC−/− and WT mice. Densitometry (Fig. 1A) and radioopacity measurements (Fig. 1C) of excised femurs revealed a small increase in bone mineral density in both male and female adult HDC−/− compared with WT littermates. Differences in bone density were even more pronounced 90 days after ovariectomy as shown by radiograms of representative femurs from each group (Fig. 1D) and by radioopacity measurements of excised femurs (Fig. 1C). Both radiographs and quantitative computed tomography measurements (not shown) demonstrated increased cortical thickness of femurs in HDC−/− mice compared with WT mice. Analysis of roentgenograms at the mid-diaphyseal cross-sectional areas of femurs showed an increase of cortical thickness in SHAM and a greater increase in the OVX groups (Fig. 1B). Quantitative computed tomography also showed increased periosteal and decreased endosteal circumference (not shown). Histological analysis confirmed the increase of mineralized bone and cortical thickness in femurs of HDC−/− mice (Fig. 2). This difference was apparent in both SHAM and OVX groups.
Figure 1.
(A) Bone mineral content of excised femurs from 4- to 5-month-old mice on histamine-free diet (17 HDC−/− and 16 WT males, 14 HDC−/− and 10 WT females) were measured by SPA. Differences between data from HDC−/− and WT mice were significant in both males (P < 0.01) and females (P < 0.05). (B) Measurements of cortical bone width and total width at the mid-diaphysis. Radiographs were taken from excised femurs of HDC−/− and WT mice. Femurs were obtained 45 days after sham operation (SHAM) or ovariectomy (OVX). Differences between data from HDC−/− and WT mice were significant in SHAM (P < 0.05) and OVX (P < 0.01) mice. (C) Radioopacity measurements from radiographic images taken 90 days after SHAM or OVX from HDC−/− and WT mice. Differences between data from HDC−/− and WT mice were significant in SHAM (P < 0.05) and OVX (P < 0.001) animals. (D) Representative roentgenogram of femurs isolated from WT and HDC−/− mice 90 days after OVX or SHAM.
Figure 2.
Histological appearance of distal epiphysis (Upper) and mid-diaphysis (Lower) of femurs from SHAM (A) and OVX (B) mice 90 days after surgery. Longitudinal undecalcified tissue sections were subjected to the Goldner's-Masson-trichrome stain to discriminate mineralized bone tissues (blue/green) from unmineralized tissues (pink/red). Increases in the amount of mineralized tissue and cortical width is apparent in sections from HDC−/− mice compared with WT.
In addition to the loss in cortical bone, estrogen-deficiency also causes loss in trabecular bone. To assess trabecular bones, first we compared radioopacity of trabecular regions from the vertebrae. Trabecular densities were similar in HDC−/− (134.2 ± 5.4 units) and WT mice (127.9 ± 6.9 units) after SHAM (P = 0.2), whereas densities were higher in HDC−/− mice (129 ± 5.4 units) than in WT mice (106.1 ± 4.9 units) after OVX (P < 0.001). Histomorphometrical analysis of trabecular bone confirmed the results of radioopacity measurements. Trabecular bone volumes were similar in HDC−/− (19.3 ± 3.2) and WT mice (21.7 ± 4.1) after SHAM (P = 0.16), whereas they were higher in HDC−/− OVX mice (14.3 ± 5.8%) than in WT OVX mice (9.5 ± 4.6%) (P < 0.001). This difference in bone loss (51% in WT compared with 26% in HDC−/−) indicates that histamine deficiency protects not only the cortical bone, but also the trabecular bone from the estrogen-deficiency induced changes.
The bone protective effect of histamine deficiency was not caused by differences in serum estradiol levels. Serum concentrations of estradiol were 31.7 ± 6.9 pg/ml in HDC−/− and 30.1 ± 4.9 pg/ml in WT mice (P = 0.42). The dramatic decrease of uterine weight in OVX mice compared with SHAM animals demonstrated that OVX animals became estrogen-deficient (uterus weight SHAM: WT, 0.19 ± 0.03 g; HDC−/−, 0.21 ± 0.05 g; uterus weight OVX: WT, 0.053 ± 0.01 g; HDC−/−, 0.053 ± 0.03 g).
Increased Bone Formation and Diminished Bone Resorption in Histamine-Deficient Mice.
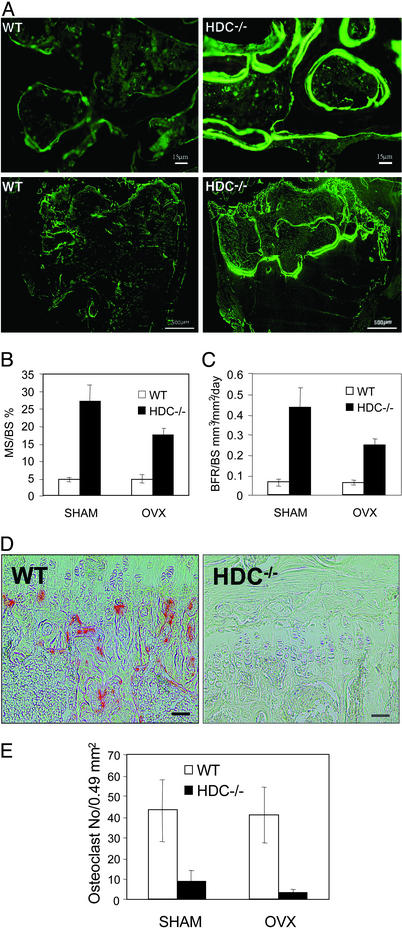
Histomorphometrical analysis was carried out to gain insight into the mechanism of the bone anabolic effects. Calcein labeling allowed us to assess dynamic and static parameters of bone formation. Fig. 3A shows that both in SHAM (Fig. 3A Upper) and in OVX (Fig. 3A Lower) animals the amount of calcein labeling and the extent of double labeled surfaces were increased in HDC−/− mice compared with WT. Morphometric analysis of femurs revealed that histamine deficiency caused a marked increase in both surface- and volume-based parameters of bone formation. Histamine deficiency increased ratios of mineralized surfaces vs. bone surfaces (Fig. 3B; SHAM P < 0.003, OVX P < 0.005, by genotype) and increased ratios of bone formation rate vs. bone surfaces (Fig. 3C; SHAM P < 0.001, OVX P < 0.001, by genotype). The ratios of bone formation rate vs. bone volume showed similar differences (BFR/BV: SHAM WT, 0.16 ± 0.018%/day; SHAM HDC−/−, 0.795 ± 0.16%/day P < 0.002; OVX WT, 0.22 ± 0.04, OVX HDC−/−, 0.56 ± 0.07 P < 0.001).
Figure 3.
(A) Calcein fluorescence in longitudinal undemineralized bone sections of distal femoral growth plate and metaphysis 90 days after SHAM (Upper, ×50 magnification) and OVX (Lower, ×6 magnification) from WT and HDC−/− mice. Fluorescence is more intense and more labeling is present in HDC−/− than in WT bones. (B) Histomorphometrical parameters of bone formation. The ratio of mineralized surface vs. bone surface was higher in HDC−/− than in WT group (P < 0.005). (C) Histomorphometrical parameters of bone formation. The ratio of bone formation rate vs. bone surface was higher in HDC−/− than in WT group (P < 0.001). (D) TRAP staining of osteoclasts in the primary spongiosa. Complete absence of TRAP-positive osteoclasts in bone section from HDC−/− mice. (Bars = 5 μm.) (E) Measurements of TRAP-positive cells from WT and HDC−/− mice 90 days after SHAM operation or OVX show differences in osteoclast number.
Histomorphometry indicated decreased bone resorption in HDC−/− mice compared with WT. Osteoclast number was significantly reduced in HDC−/− mice, as assessed by TRAP staining (Fig. 3D) and morphometrical analysis (Fig. 3E). Reduced osteoclast number was found both in SHAM and OVX HDC−/− mice. In vitro cultures of osteoclast-like cells from bone marrow suggested that histamine deficiency directly inhibits osteoclast development. TRAP-positive multinucleated cell number was reduced in HDC−/− cultures (WT, 19.3 ± 4.4 cells per well and HDC−/−, 7.5 ± 3.3; P < 0.05).
Histamine Deficiency Increases Bone Mineral Content by Increasing Serum Calcitriol Concentrations.
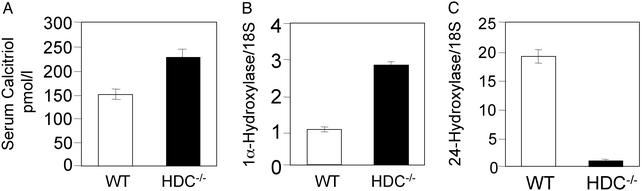
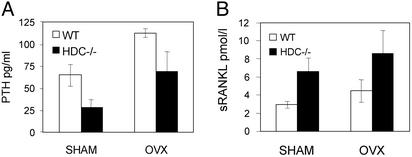
Serum concentrations of calcitriol were higher in the HDC−/− mice than in the WT (Fig. 4A). In addition, real-time RT-PCR measurements indicated that the 1αOHase mRNA expression was much higher in kidneys from HDC−/− mice than in kidneys from WT mice (Fig. 4B). Moreover, 24OHase expression level was much lower in kidneys from HDC−/− mice that in kidneys from WT mice (Fig. 4C). The up-regulation of the calcitriol synthesizing enzyme and down-regulation of the calcitriol catabolizing enzyme thus explain the increased calcitriol serum concentration in histamine-deficient mice. In accordance, we found changes in serum parameters that are usually under the influence of calcitriol, such as Pi, ALPase, PTH, and sRANKL concentrations in HDC−/− mice. Histamine-deficient mice had higher Pi concentrations than serum from WT mice (12.6 ± 0.4 vs. 11.2 ± 0.5 mg/dl; P < 0.05, n = 11), higher ALPase concentrations (181.8 ± 19.9 vs. 68.5 ± 12.3 units/liter; P < 0.001, n = 20), suppressed PTH concentrations (Fig. 5A), and increased sRANKL concentrations (Fig. 5B). Serum total calcium concentrations were similar in HDC−/− and in WT mice (8.9 ± 0.1 vs. 9.0 ± 0.1 mg/dl; P = 0.5, n = 14) despite the higher calcitriol concentrations. The suppression of PTH release by the high calcitriol levels in the HDC−/− mice likely compensated for the effect of high calcitriol on serum calcium levels. Serum calcidiol (25-OH-D3) levels were similar in HDC−/− and WT mice (11.6 ± 1.7 vs. 10.9 ± 1.2 ng/ml, P = 0.4, n = 5). Thus, the increased calcitriol concentrations in HDC−/− mice are likely the result of an increased calcitriol synthesis and decreased calcitriol catabolism.
Figure 4.
(A) Serum calcitriol concentrations were measured in WT and HDC−/− mice (n = 6). Values were significantly higher in the HDC−/− group than in the WT group (P < 0.01). (B) Detection of kidney 1αOHase mRNA levels by using real-time quantitative RT-PCR. Results are presented as fractions of 1αOHase/18S mRNA levels in comparison with levels in WT (1). (C) Detection of kidney 24OHase mRNA levels by using real-time quantitative RT-PCR. Results are presented as fractions of 24OHase/18S mRNA levels in comparison with levels in HDC−/−.
Figure 5.
(A) Serum PTH levels were measured from WT and HDC−/− mice 90 days after SHAM operation (WT, n = 11, HDC−/−, n = 12) or OVX (WT, n = 6 HDC−/−, n = 10). Values were significantly lower in the HDC−/− group than in WT group both after SHAM (P < 0.05) and after OVX (P < 0.01). (B) Serum sRANKL concentrations were measured from WT and HDC−/− mice 90 days after SHAM operation (n = 5 each) or OVX (n = 4 each). Values were significantly higher in the HDC−/− groups than in the WT groups (both SHAM and OVX, P < 0.05).
To evaluate the role of calcitriol in mediating the bone-protective effect of histamine deficiency, in a separate experiment mice were fed a vitamin D-deficient and reduced calcium diet for 3 months. Both SPA and radioopacity measurements of excised femurs showed similar values in HDC−/− and WT mice fed a vitamin D-deficient reduced calcium diet (SPA: HDC−/−, 12.1 ± 0.8; WT, 12.5 ± 0.6 g/cm2, P = 0.3, radioopacity: HDC−/−, 55.4 ± 6.6 units, WT, 62.0 ± 8.7 units; P = 0.09). These results show that vitamin D deficiency prevents the increase in bone mineral density in HDC−/− mice, and suggest that increased calcitriol synthesis plays a role in the bone-protective effect of histamine deficiency.
Discussion
In this study, we have demonstrated a bone phenotype in mice lacking the capacity to synthesize histamine. These mice showed all of the expected abnormalities of histamine deficiency in immune function, neuronal function, and gastrointestinal function. The bone phenotype of histamine deficiency is characterized by increased cortical bone thickness and mineral content, which develops due to increased bone formation and reduced bone resorption. Studies elucidating the mechanisms of this bone phenotype revealed the role of histamine in renal calcitriol synthesis and catabolism.
The appearance of bone phenotype in HDC−/− mice was not unexpected. Previous studies demonstrated the abundance of histamine synthesizing and catabolizing enzymes in bone and bone marrow (26, 27). Moreover, the bone abnormalities in patients with mastocytosis and high circulating levels of histamine are well documented (28). These patients often develop osteoporosis, because of increased bone resorption primarily at the ends of the long bones (20). Histamine increased osteoclast number in rats (16, 29) induced bone resorption in laying hens (17), and increased osteoclastogenesis in mouse bone marrow cell culture by stimulating RANKL expression by osteoblasts (30). Conversely, histamine receptor inhibition prevented rapid periosteal and trabecular bone resorption in ovariectomized rats (6). Taken together, these previous studies with our findings of markedly decreased osteoclast number in HDC−/− mice suggest that histamine has a direct effect on osteoclast development and function. This direct effect could decrease bone resorption and decrease ovariectomy-induced bone loss, similar to the effects of other antiresorptive agents. However, osteoclast inhibition could lead to a low turnover osteopenia, unless bone formation is also stimulated.
The most interesting feature of HDC−/− mice phenotype was the increased bone mineral density and bone formation indexes. Our results indicate that one of the mechanisms that increases bone mineral density and bone formation rate in histamine-deficient mice is indirect, acting through stimulation of calcitriol synthesis. Excess calcitriol increases intestinal calcium absorption and stimulates osteoblast activity, as indicated by the increased sRANKL concentrations.
The connection between serum calcitriol concentration and histamine-induced bone loss has been indicated by a study on patients with mastocytosis (21). Before treatment, serum and urinary histamine levels were elevated and bone histomorphometry showed impaired bone formation in these patients. Serum calcitriol levels were low despite normal 25-OH-D levels, suggestive of impaired 1α-hydroxylase activity. After treatment with histamine release inhibitor ketotifen, serum and urinary histamine levels normalized, together with a reversal of bone loss, an increase in bone formation and a decrease in bone resorption. Serum calcitriol levels also increased from 12 to 21.5 pg/ml, whereas serum 25-OH-D levels remained unchanged. Calcitriol levels were also decreased in another study on patients with mastocytosis (31). Here we found that histamine deficiency increases serum calcitriol levels by regulating expression of calcitriol synthesizing and catabolizing enzymes in the kidney. Increases in serum calcitriol levels were accompanied by increased serum Pi and ALPase, and sRANKL concentrations, and decreased PTH concentrations, as expected. Our studies with vitamin D-deficient diet also supported the connection between the increased calcitriol and the bone phenotype in this model of histamine deficiency.
Although calcitriol is a well known stimulator of osteoclast development in vitro (32), and osteoclastic bone resorption in vivo, antiresorptive effect of low dose calcitriol has also been described in murine models of osteoporosis (33, 34). This raised the possibility that the antiresorptive effect observed in histamine-deficient animals is indirect, deriving from the increased calcitriol levels. Our results on osteoclast development from cultured bone marrow, however, show a direct effect of histamine deficiency. These experiments demonstrated that significantly less TRAP-positive multinucleated cells developed from the marrow of HDC−/− than from WT mice even in the absence of calcitriol. Combined, these results argue against the possibility that calcitriol prevents bone loss by inhibiting bone resorption in HDC−/− mice.
It is well established that calcitriol directly stimulates in vitro bone formation (35) and mineralized matrix formation by osteoblasts (36). This effect was recently confirmed in transgenic mice overexpressing the vitamin D receptor, which featured increased cortical bone thickness and mineral density (37). A recent report pointed out that calcitriol also induces osteoclast differentiation by increasing RANKL secretion by osteoblasts (38). Part of the reason that increased RANKL secretion did not lead to an increase in osteoclast differentiation in the HDC−/− mice is that the RANKL-induced increase in osteoclast H2 receptor expression (39) is ineffective to stimulate osteoclastogenesis in the absence of the ligand, histamine. This effect of histamine deficiency on osteoclasts is similar to the effect of estrogen that inhibits osteoclast development and bone resorption (40) by stimulating osteoprotegerin secretion, which in turn blocks RANK activation (9). Indeed, our histology showed that the bone histology patterns in femurs from HDC−/− mice (Fig. 2) resemble the bone histology patterns reported in mice treated with high doses of estrogen (40, 41).
In summary, our studies on a mouse model of histamine deficiency have provided insights into the direct and indirect effects of histamine deficiency on bone remodeling and vitamin D metabolism. These studies established that histamine deficiency increases bone formation, at least in part, through increasing serum calcitriol levels. Our results are consistent with the few reports proposing bone-protective effects of histamine antagonists in OVX rats (6, 42) and in postmenopausal women (7). Combined, these results lead to the hypothesis that people with allergies and other illnesses associated with increased histamine synthesis may have increased susceptibility to age-related and estrogen deficiency induced bone loss. Furthermore, the well-controlled and large-scale testing of antihistamines and HDC inhibitors alone or in combination with high calcium diet and other bone formation inducing drugs could lead to an easily accessible treatment option for osteoporosis.
Supplementary Material
Acknowledgments
We thank Dr. Szilvia Meszaros (Semmelweis University) for assistance with SPA measurements; Mr. Oscar Garcia (Clinical Center, National Institutes of Health, Bethesda) for assistance with x-rays; Jessica Scott (Laboratory of Cell Biochemistry and Biology, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health), Bruce Hissong, and Dr. John J. O'Shea (Laboratory of Cell Biochemistry and Biology, National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health) for technical assistance with real-time RT-PCR analysis; AniLytics (Gaithersburg, MD) for laboratory tests; Dr. Jean Sibonga (Mayo Clinic, Rochester, MN) for assistance with bone histomorphometry; and Drs. William Prinz, Gilbert Ashwell (National Institutes of Health), and Marie Demay (Harvard Medical School, Cambridge, MA) for critical reading of the manuscript.
Abbreviations
- ALPase
alkaline phosphatase
- 25-OH-D
25-hydroxyvitamin D
- HDC
histidine decarboxylase
- OVX
ovariectomized
- Pi
phosphate
- PTH
parathyroid hormone
- RANKL
receptor activator of NF-κB ligand
- SHAM
sham-operated
- TRAP
tartarate-resistant acid phosphatase
- 1αOHase
25-hydroxyvitamin D-1α-hydroxylase
- 24OHase
25-hydroxyvitamin d-24-hydroxylase
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Boyce B F, Hughes D E, Wright K R, Xing L, Dai A. Lab Invest. 1999;79:83–94. [PubMed] [Google Scholar]
- 2.Sherman S. Ann NY Acad Sci. 2001;949:188–197. [PubMed] [Google Scholar]
- 3.Wang P S, Solomon D H, Mogun H, Avorn J. J Am Med Assoc. 2000;283:3211–3216. doi: 10.1001/jama.283.24.3211. [DOI] [PubMed] [Google Scholar]
- 4.Dunstan C R, Boyce R, Boyce B F, Garrett I R, Izbicka E, Burgess W H, Mundy G R. J Bone Miner Res. 1999;14:953–959. doi: 10.1359/jbmr.1999.14.6.953. [DOI] [PubMed] [Google Scholar]
- 5.Reeve J. Br Med J. 2002;324:435–436. doi: 10.1136/bmj.324.7335.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lesclous P, Guez D, Saffar J L. Bone. 2002;30:131–136. doi: 10.1016/s8756-3282(01)00629-9. [DOI] [PubMed] [Google Scholar]
- 7.Tyan M L. J Intern Med. 1993;234:143–148. doi: 10.1111/j.1365-2796.1993.tb00723.x. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Sarosi I, Yan X Q, Morony S, Capparelli C, Tan H L, McCabe S, Elliott R, Scully S, Van G, et al. Proc Natl Acad Sci USA. 2000;97:1566–1571. doi: 10.1073/pnas.97.4.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shevde N K, Bendixen A C, Dienger K M, Pike J W. Proc Natl Acad Sci USA. 2000;97:7829–7834. doi: 10.1073/pnas.130200197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roggia C, Gao Y, Cenci S, Weitzmann M N, Toraldo G, Isaia G, Pacifici R. Proc Natl Acad Sci USA. 2001;98:13960–13965. doi: 10.1073/pnas.251534698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simonet W S, Lacey D L, Dunstan C R, Kelley M, Chang M S, Luthy R, Nguyen H Q, Wooden S, Bennett L, Boone T, et al. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 12.Thomas G P, Baker S U, Eisman J A, Gardiner E M. J Endocrinol. 2001;170:451–460. doi: 10.1677/joe.0.1700451. [DOI] [PubMed] [Google Scholar]
- 13.de Pollak C, Arnaud E, Renier D, Marie P J. J Cell Biochem. 1997;64:128–139. [PubMed] [Google Scholar]
- 14.Black J W, Duncan W A, Durant C J, Ganellin C R, Parsons E M. Nature. 1972;236:385–390. doi: 10.1038/236385a0. [DOI] [PubMed] [Google Scholar]
- 15.Arrang J M, Garbarg M, Schwartz J C. Nature. 1983;302:832–837. doi: 10.1038/302832a0. [DOI] [PubMed] [Google Scholar]
- 16.Dobigny C, Saffar J L. J Cell Physiol. 1997;173:10–18. doi: 10.1002/(SICI)1097-4652(199710)173:1<10::AID-JCP2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 17.Cabrera-Saadoun M C, Nys Y, Sauveur B, Gautron J. Comp Biochem Physiol C. 1987;86:395–398. doi: 10.1016/0742-8413(87)90102-2. [DOI] [PubMed] [Google Scholar]
- 18.Goldhaber P, Rabadjija L. Am J Physiol. 1983;244:E141–E144. doi: 10.1152/ajpendo.1983.244.2.E141. [DOI] [PubMed] [Google Scholar]
- 19. de Gennes, C., Kuntz, D. & de Vernejoul, M. C. (1992) Clin. Orthop., 281–291. [PubMed]
- 20.Delsignore J L, Dvoretsky P M, Hicks D G, O'Keefe R J, Rosier R N. Iowa Orthop J. 1996;16:126–134. [PMC free article] [PubMed] [Google Scholar]
- 21.Graves L, III, Stechschulte D J, Morris D C, Lukert B P. J Bone Miner Res. 1990;5:1113–1119. doi: 10.1002/jbmr.5650051104. [DOI] [PubMed] [Google Scholar]
- 22.Ohtsu H, Tanaka S, Terui T, Hori Y, Makabe-Kobayashi Y, Pejler G, Tchougounova E, Hellman L, Gertsenstein M, Hirasawa N, et al. FEBS Lett. 2001;502:53–56. doi: 10.1016/s0014-5793(01)02663-1. [DOI] [PubMed] [Google Scholar]
- 23.Ohtsu H, Kuramasu A, Tanaka S, Terui T, Hirasawa N, Hara M, Makabe-Kobayashi Y, Yamada N, Yanai K, Sakurai E, et al. Eur J Immunol. 2002;32:1698–1708. doi: 10.1002/1521-4141(200206)32:6<1698::AID-IMMU1698>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka S, Hamada K, Yamada N, Sugita Y, Tonai S, Hunyady B, Palkovits M, Falus A, Watanabe T, Okabe S, et al. Gastroenterology. 2002;122:145–155. doi: 10.1053/gast.2002.30312. [DOI] [PubMed] [Google Scholar]
- 25.Kubota Y, Ito C, Sakurai E, Sakurai E, Watanabe T, Ohtsu H. J Neurochem. 2002;83:837–845. doi: 10.1046/j.1471-4159.2002.01189.x. [DOI] [PubMed] [Google Scholar]
- 26.Endo Y, Kikuchi T, Nakamura M, Shinoda H. Calcif Tissue Int. 1992;51:67–71. doi: 10.1007/BF00296220. [DOI] [PubMed] [Google Scholar]
- 27.Dy M, Arnould A, Lemoine F M, Machavoine F, Ziltener H, Schneider E. Blood. 1996;87:3161–3169. [PubMed] [Google Scholar]
- 28.Johansson C, Roupe G, Lindstedt G, Mellstrom D. Age Ageing. 1996;25:1–7. doi: 10.1093/ageing/25.1.1. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura M, Kuroda H, Narita K, Endo Y. Life Sci. 1996;58:1861–1868. doi: 10.1016/0024-3205(96)00170-1. [DOI] [PubMed] [Google Scholar]
- 30.Deyama Y, Kikuiri T, Ohnishi G, Feng Y G, Takeyama S, Hatta M, Yoshimura Y, Suzuki K. Biochem Biophys Res Commun. 2002;298:240–246. doi: 10.1016/s0006-291x(02)02440-3. [DOI] [PubMed] [Google Scholar]
- 31.Harvey J A, Anderson H C, Borek D, Morris D, Lukert B P. Bone. 1989;10:237–241. doi: 10.1016/8756-3282(89)90059-8. [DOI] [PubMed] [Google Scholar]
- 32.Raisz L G, Trummel C L, Holick M F, DeLuca H F. Science. 1972;175:768–769. doi: 10.1126/science.175.4023.768. [DOI] [PubMed] [Google Scholar]
- 33.Erben R G, Mosekilde L, Thomsen J S, Weber K, Stahr K, Leyshon A, Smith S Y, Phipps R. J Bone Miner Res. 2002;17:1498–1511. doi: 10.1359/jbmr.2002.17.8.1498. [DOI] [PubMed] [Google Scholar]
- 34.Uchiyama Y, HiguchI Y, Takeda S, Masaki T, Shira-Ishi A, Sato K, Kubodera N, Ikeda K, Ogata E. Bone. 2002;30:582–588. doi: 10.1016/s8756-3282(02)00682-8. [DOI] [PubMed] [Google Scholar]
- 35.Lempert U G, Scharla S H, Minne H W, Ziegler R. Bone Miner. 1991;13:103–109. doi: 10.1016/0169-6009(91)90077-d. [DOI] [PubMed] [Google Scholar]
- 36.Marie P J, Hott M, Garba M T. Metabolism. 1985;34:777–783. doi: 10.1016/0026-0495(85)90030-7. [DOI] [PubMed] [Google Scholar]
- 37.Gardiner E M, Baldock P A, Thomas G P, Sims N A, Henderson N K, Hollis B, White C P, Sunn K L, Morrison N A, Walsh W R, et al. FASEB J. 2000;14:1908–1916. doi: 10.1096/fj.99-1075com. [DOI] [PubMed] [Google Scholar]
- 38.Cenci S, Weitzmann M N, Roggia C, Namba N, Novack D, Woodring J, Pacifici R. J Clin Invest. 2000;106:1229–1237. doi: 10.1172/JCI11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cappellen D, Luong-Nguyen N H, Bongiovanni S, Grenet O, Wanke C, Susa M. J Biol Chem. 2002;277:21971–21982. doi: 10.1074/jbc.M200434200. [DOI] [PubMed] [Google Scholar]
- 40.Bain S D, Jensen E, Celino D L, Bailey M C, Lantry M M, Edwards M W. J Bone Miner Res. 1993;8:219–230. doi: 10.1002/jbmr.5650080213. [DOI] [PubMed] [Google Scholar]
- 41.Edwards M W, Bain S D, Bailey M C, Lantry M M, Howard G A. Bone. 1992;13:29–34. doi: 10.1016/8756-3282(92)90358-4. [DOI] [PubMed] [Google Scholar]
- 42.Rico H, Gomez M, Revilla M, Gonzalez-Riola J, Seco C, Hernandez E R, Villa L F, Gervas J J. Calcif Tissue Int. 1999;65:272–275. doi: 10.1007/s002239900697. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.