Abstract
Neuropeptide Y (NPY), a 36-aa peptide, is widely distributed in the brain and peripheral tissues. Whereas physiological roles of NPY as a hormone/neurotransmitter have been well studied, little is known about its other peripheral functions. Here, we report that NPY acts as a potent angiogenic factor in vivo using the mouse corneal micropocket and the chick chorioallantoic membrane (CAM) assays. Unlike vascular endothelial growth factor (VEGF), microvessels induced by NPY had distinct vascular tree-like structures showing vasodilation. This angiogenic pattern was similar to that induced by fibroblast growth factor-2, and the angiogenic response was dose-dependent. In the developing chick embryo, NPY stimulated vascular sprouting from preexisting blood vessels. When [Leu31Pro34]NPY, a NPY-based analogue lacking high affinity for the NPY Y2 receptor but capable of stimulating both Y1 and Y5 receptors, was used in the corneal model, no angiogenic response could be detected. In addition, NPY failed to induce angiogenesis in Y2 receptor-null mice, suggesting that this NPY receptor subtype was mediating the angiogenic signal. In support of this finding, the Y2 receptor, but not Y1, Y4, or Y5 receptors, was found to be widely expressed in newly formed blood vessels. Further, a delay of skin wound healing with reduced neovascularization was found in Y2 receptor-null mice. These data demonstrate that NPY may play an important role in the regulation of angiogenesis and angiogenesis-dependent tissue repair.
Keywords: neovascularization‖G protein-coupled receptor‖7TM receptor
Neuropeptide Y (NPY), a 36-aa polypeptide, is one of the most abundant neurotransmitters in the mammalian central and peripheral nervous systems. Interest has mainly been focused on the central effects, and the NPY family of neuropeptides, including NPY, peptide YY (PYY), and pancreatic polypeptide (PP), have been shown to elicit diverse biological functions including hypothalamic control of food intake, anxiolysis, and sedation (1). These polypeptides activate members of the NPY receptor family of heptahelical G-protein coupled receptors. Four distinct human NPY Y receptor cDNAs have been cloned to date: Y1, Y2, Y4, and Y5. All known Y receptors mediate their responses through pertussis toxin-sensitive G proteins, resulting in inhibition of adenylate cyclase activity and increase in intracellular Ca2+ levels (2). Although the neuropeptides were isolated and characterized several decades ago and the NPY receptor cDNAs have been cloned for 5–10 yr, surprisingly little is known about the molecular mechanisms that regulate NPY receptor activity and the biological significance of NPY in the periphery.
It has been found that NPY regulates the vascular tone by inducing contractions of blood vessels (3). NPY also stimulates growth of vascular smooth muscle cell and hypertrophy of ventricular cardiomyocytes (4, 5). The trophic effect of NPY on blood vessels does not seem to be limited to vascular smooth muscle cells. More recently, NPY has been reported to directly stimulate endothelial cell proliferation and migration (6–8). These in vitro studies suggest that NPY may act as an angiogenic factor (9). However, it is not clear which receptor subtypes are involved in transducing angiogenic signals. There also remains a need to investigate the physiological role of NPY-induced angiogenesis during development and tissue repair. Angiogenesis is involved in many physiological and pathological conditions, including female reproductive cycles, embryonic development, wound repair, tumor growth, metastasis, chronic inflammations, and retinopathy (10–14). The angiogenic process is complex, and includes local degradation of basement membrane, endothelial cell proliferation and migration, tube and branch formation, and reconstitution of basement membrane (13, 14). The complexity of this process implies that it is highly regulated (15). It is generally accepted that a switch of angiogenesis in a tissue is operated by both positive and negative factors. It is believed that the trigger of an angiogenic response represents an imbalanced situation of increasing levels of angiogenic factors and decreasing levels of angiogenesis inhibitors (13, 16, 17). In this regard, NPY could be one of the positive contributors to angiogenesis.
Most angiogenic factors, including the families of fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF), stimulate angiogenesis through activation of their specific tyrosine kinase receptors. These tyrosine kinase-mediated signals have been extensively studied. For example, activation of mitogen-activated protein (MAP) kinase and protein kinase C (PKC) pathways are involved in the induction of angiogenesis (18). In contrast to the angiogenic factors mentioned above, biological functions of NPY are mediated by at least four G-protein-coupled receptors: Y1, Y2, Y4, and Y5. Interestingly, NPY Y1 and NPY Y2 receptors have been shown to activate intracellular PKC and MAP kinase (19–21). Thus, this G-protein-coupled receptor-mediated signaling pathway is, at least in part, overlapping those transduced by FGF-2 and VEGF receptors.
In the present study, we provide compelling evidence that NPY is a potent angiogenic factor in vivo and that the NPY-induced angiogenic response is mediated by the NPY Y2 receptor subtype. In knock-out mice lacking the Y2 receptor, skin wound repair was significantly delayed. This study demonstrates that NPY may play an important role in regulation of angiogenesis and angiogenesis-dependent physiological and pathological processes.
Materials and Methods
Reagents, Cells, and Animals.
NPY, NPY3–36, and [Leu31Pro34]NPY was purchased from Sigma. Scrambled NPY (N-RDGNMIYALPYQRARATHAPNKEPDDYYSSEYIPLR-C) was synthesized by Innovagen (Lund, Sweden). Recombinant human VEGF165 was obtained from R & D Systems. Recombinant human FGF-2 was obtained from PeproTech (Rocky Hill, NJ). C57BL/6 mice were obtained from the breeding unit of the Microbiology and Tumor Biology Center at the Karolinska Institute. The Y2 receptor-deficient mice were generated as described (22). Male and female 5- to 8-wk-old C57BL/6 or BALB/c wt and Y2−/− mice were acclimated and caged in groups of six or fewer. Animals were anesthetized by injection of a mixture of dormicum and hypnorm (1:1) before all procedures and killed with a lethal dose of CO2. All animal studies were reviewed and approved by the animal care and use committee of the Stockholm Animal Board.
Mouse Corneal Micropocket Assay.
The mouse corneal assay was performed according to procedures previously described (23, 24). Briefly, corneal micropockets were created with a modified von Graefe cataract knife in both eyes of each male 5- to 6-wk-old C57BL/6 wt or Y2−/− mouse. A micropellet (0.35 × 0.35 mm) of sucrose aluminum sulfate coated with hydron polymer obtained from Interferon Sciences (New Brunswick, NJ) containing 80 ng of FGF-2, 160 ng of VEGF, or NPY 80, 160, 320 or 640 ng was implanted into each corneal pocket. In other experiments, 160 ng of NPY3–36, or [Leu31Pro34]NPY was used for corneal implantation. The pellet was positioned 1.0–1.2 mm from the corneal limbus. After implantation, erythromycin/ophthalmic ointment was applied to each eye. Eyes were examined by a slit-lamp biomicroscope on day 5 after pellet implantation. Vessel length and clock hours of circumferential neovascularization of six mice in each group were measured according to a previously described method (23).
Chick Embryonic Chorioallantoic Membrane (CAM) Assay.
The CAM assay was performed according to previously published methods (23, 25). Briefly, 3-day-old fertilized white Leghorn eggs (OVA Production, Sorgarden, Sweden) were cracked, and chick embryos with intact yolks were carefully placed in 20 × 100-mm plastic Petri dishes. After 6 days of incubation in 4% CO2 at 37°C, a disk of methylcellulose containing 1, 5, 10, 20, or 30 μg of NPY or BSA alone dried on a nylon mesh (3 × 3 mm) was implanted on the CAM of individual embryos. The nylon mesh disks were made by desiccation of 20 μl of 0.45% methylcellulose in H2O. After 4–5 days of incubation, embryos and CAMs were examined by a stereomicroscope for the formation of new blood vessels in the field of the implanted disks. Disks of methylcellulose containing the same amounts of BSA were used as negative controls. Neovascularization was quantified as described, and the number of microvessels within a define area of 25 mm2 surrounding the implanted mesh (9.3 mm2) were counted (23). Three independent CAM assay experiments were carried out, and, for each experiment, 5–10 chick embryos were used in each dose group.
Immunohistochemistry.
The NPY and growth factor-implanted mouse eyes were enucleated at day 6 after implantation and fixed with 4% paraformaldehyde in phosphate buffer for 12 h. Eyes were embedded in paraffin according to standard histological procedures. Tissue sections of 5 μm were deparaffinized and washed with PBS, blocked with 0.3% H2O2 in PBS for 15 min followed by 30% rabbit serum in PBS for 30 min, and immunostained by using an anti-Y1, anti-Y2, anti-Y4, or anti-Y5 receptor antibody (22). Peroxidase activity was developed by using diaminobenzidine (Vector Laboratories) or 3-amino-9-ethylcarbazole (DAKO), and sections were counterstained by using Mayer's hematoxylin. The tyramide signal amplification kit (NEN) was used to enhance staining.
For staining of blood vessels in the wound beds, mice were killed at day 7 after creation of wounds. Wounds and their surrounding tissues were fixed in 3% PFA, dehydrated, and embedded in paraffin. Embedded samples were sectioned, and 5-μm sections were immunostained by using a biotinylated monoclonal antibody against CD31 (PharMingen) or a rat anti-mouse monoclonal antibody, Mac 3, against macrophages. Peroxidase activity was developed with diaminobenzidine (DAB, Vector Laboratories), and sections were counterstained by using Mayer's hematoxylin. The tyramide signal amplification kit (NEN) was used to enhance staining. The vascular density was quantified by counting the total number of microvessels in six random fields under ×40 magnification, and the mean determinants were presented.
Wound-Healing Assay.
Female 5- to 8-wk-old C57BL/6 or BALB/c wt and Y2−/− mice were used for wound-healing studies. Animals were shaved, and full thickness skin wounds (6 mm in diameter and one wound per mouse) were created with a fine pair of scissors in a genotype blind manner at the center along the midline of the dorsum by using a round template made in our laboratory. The same method has been described elsewhere (26). Before measurement of the wound size, animals were anesthetized with 3% isofluran in O2 gas. Each wound site was digitally photographed daily, and wound diameters were determined on digital photographs by using photoshop v. 6.0 (Adobe Systems, Mountain View, CA). In another additional experiment, the wound sizes were measured daily by using a fine caliber. The wound-healing experiments were carried out twice, and six to seven animals were used in each group. For NPY and growth factor treatment, slow release polymers made of sucrose aluminum sulfate and hydron containing 400 ng of FGF-2 or 1,500 ng of NPY were implanted into each wound bed. Slow-release polymer without growth factor was used as a negative control. No inflammation was observed during the experiment. Wound pictures were taken with a Fuji digital camera.
Statistical Analysis.
Statistical analysis was carried out by using Student's two-tailed t test in Microsoft excel. P values below 0.05 and 0.001 were deemed significant and highly significant, respectively.
Results
Stimulation of Neovascularization by NPY.
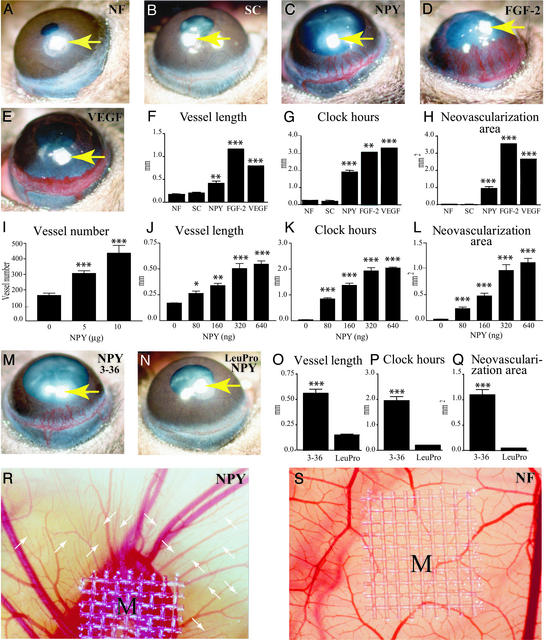
The corneal angiogenesis model is one of the most rigorous mammalian angiogenesis models that require a putative compound to be sufficiently potent to induce neovascularization in the corneal avascular tissue. Potent angiogenic factors including FGF-2 and VEGF have profound effects in this system (23, 24). Micropellets of aluminum sulfate coated with the slow release polymer-hydron containing NPY, FGF-2, or VEGF were surgically implanted into the corneas of C57BL/6 mice. Stimulation of new blood vessel growth was examined on day 5 after implantation. The angiogenic response of corneas stimulated by NPY was dramatic, with distinct and defined vascular tree structures (Fig. 1C). The newly formed vessels, as well as the limbal vessels, were dilated in the NPY-implanted corneas (Figs. 1C and 3A). The measured angiogenic responses induced by NPY were significantly greater than those induced by a scrambled NPY control peptide (Fig. 1 B and F–H) or by slow release polymer alone (Fig. 1 A and F–H). The vessel length, clock hours (the proportion of the circumference that is vascularized if the eye is viewed as a clock), and neovascularization area were all significantly greater than in the negative controls (P > 0.0001; Fig. 1 F–H). The overall angiogenic response induced by NPY was qualitatively similar to that induced by FGF-2, although less pronounced (Fig. 1 C and D). Both NPY- and FGF-2-induced microvessels were well organized and separated. In contrast, the VEGF-induced blood vessels seemed to be leaky, hemorrhagic, and fused into vascular sacks, and likely to rupture (Fig. 1E). At the front edge, VEGF-induced capillaries were fused into disorganized and sinusoidal structures (Fig. 1E). Thus, angiogenic responses induced by NPY and VEGF are markedly different, whereas NPY- and FGF-2-induced vessels seemed similar.
Figure 1.
In vivo angiogenic activity of NPY. Micropellets containing 160 ng of NPY (C), 80 ng of FGF-2 (D), or 160 ng of VEGF (E) were implanted into corneal micropockets of C57BL/6 mice as described in Materials and Methods. Slow-release polymers containing no factors (A, NF) or 160 ng of a scrambled peptide (B, SC) served as negative controls. Corneal neovascularization was measured and photographed with a slit-lamp stereomicroscope on day 5 after growth factor implantation. Arrows point to the implanted pellets. Photographs represent ×20 amplification of the mouse eye. Quantitation of corneal neovascularization is presented as maximal vessel length (F), clock hours of circumferential neovascularization (G), and area of neovascularization (H). Graphs represent mean values (±SEM) of 10 eyes (five to seven mice) in each group. Various amounts (80, 160, 320, and 640 ng) of NPY (J–L) were implanted into the micropockets of mouse corneas. Corneal neovascularization was measured as vessel length (J), clock-hours (K), and area (L) with a slit-lamp stereomicroscope on day 5 after growth factor implantation. Graphs represent mean values (±SEM) of 10 eyes (five to seven mice) in each group. Micropellets containing an equal amount (160 ng per pellet) of NPY3–36 (M) or [Leu31Pro34]NPY (N) were implanted into the corneas of C57BL/6 mice. The corneal neovascularization was examined and photographed on day 5 after pellet implantation. Arrows in M and N indicate the implanted pellet. Angiogenic responses were measured as vessel length (O), clock-hours of neovascularization (P), and vascular area (Q). Graphs represent mean values (±SEM) of 11–16 eyes (six to eight mice) in each group. Nylon meshes, M (9.3 mm2), coated with 0.45% methylcellulose containing various amounts of NPY or BSA were implanted on CAMs of 9-day-old chick embryos. After 5-day implantation, the formation of new blood vessels was examined under a stereomicroscope. A CAM with a methylcellulose mesh containing BSA (NF) alone served as a negative control (S). R shows an example of 5 μg of NPY-implanted CAM. New blood vessels are marked with arrows in R. The average of total numbers of microvessels within a defined area of 25 mm2 surrounding the implanted mesh is presented (I). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
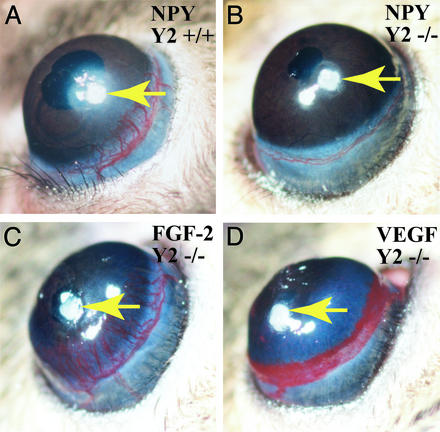
Figure 3.
Absence of corneal neovascularization in Y2 receptor null mice. NPY at the amount of 160 ng per pellet was implanted into each cornea of NPY Y2 +/+ (A) and NPY Y2−/− (B) mice. As controls, 80 ng per pellet of FGF-2 (C) or 160 ng per pellet of VEGF (D) was implanted into each of NPY Y2−/− mice. After 5-day implantation, corneal neovascularization was detected and pictured. Six mice and 12 corneas were used in each group of mice.
To quantify whether NPY-induced corneal angiogenesis was dose-dependent, increasing amounts of NPY were implanted into mouse corneas. As shown in Fig. 1, increasing doses of NPY produced escalating angiogenic responses in this assay. The dosages used in this study were 80, 160, 320, and 640 ng per pellet. This dose-dependent effect became saturated after 320 ng per pellet (Fig. 1 J–L) because NPY at the dose of 640 ng did not result in a significant increase of corneal neovascularization. Thus, 320 ng or more of NPY produce the maximal angiogenic effect in this assay system.
Several NPY receptor subtypes have been found to be expressed on cell types associated with blood vessels (9, 27), and it is unclear which one of these receptor subtypes is involved in transducing the angiogenic signals of NPY. To study the biological effect mediated by the individual receptor subtypes, we tried selective receptor agonists in the mouse corneal system. NPY3–36, a C-terminal fragment of NPY and a selective ligand for the Y2 receptor, but not for the Y1 receptor (28, 29), induced a similar angiogenic pattern as unprocessed NPY in mouse corneas (Fig. 1M). In contrast, LeuProNPY, a NPY agonist lacking high affinity for the Y2 receptor but a potent activator of Y1 and Y5 receptors, was unable to stimulate angiogenesis in the mouse cornea (Fig. 1N). The measured vessel length (Fig. 1O), clock-hours (Fig. 1P) and areas of neovascularization (Fig. 3Q) of NPY3–36-induced blood vessels were significantly greater than that induced by LeuProNPY (P < 0.0001). These data suggest that the Y2 receptor subtype is responsible for mediation of NPY-stimulated angiogenesis.
To further evaluate the in vivo angiogenic activity of NPY, we performed the chick embryonic CAM assay. The CAM assay is widely used as an in vivo angiogenesis assay that detects angiogenic activity of compounds during embryonic development (25). The early embryos in this angiogenesis assay avoid immune reactions and inflammatory influences on growing vessels. To study whether NPY could induce angiogenesis in vivo, various doses of NPY were implanted onto the chick CAM in the developing embryo. NPY at the dose of 5 μg per disk was able to stimulate microvessel growth in each implanted chicken embryo (Fig. 1R). A significant increase of neovascularization with a high vessel density was observed in the areas surrounding the NPY implant. Notably, NPY induced the formation of new branches (small arrows in Fig. 1R) from the existing vessels that grew toward the implanted disks. In contrast, disks without growth factors did not seem to stimulate neovascularization in chick embryos (Fig. 1S). To quantify the angiogenic response induced by NPY in the CAM assay, the area was defined within 25 mm2 surrounding the implants (9.3 mm2). The total vessel numbers within this area were scored in each sample (Fig. 1I). The total vessel numbers in both 5- and 10-μg and 10-μg NPY-implanted were significantly higher than that of the control group. These CAM data were reproducible because similar results were obtained in three independent experiments with 5–10 embryos per sample. The dosages were chosen because our pilot experiments using a broad range of NPY doses indicated that, at these quantities, NPY could elicit potent angiogenic responses.
Expression of the Y2 Receptor on Newly Formed Microvessels.
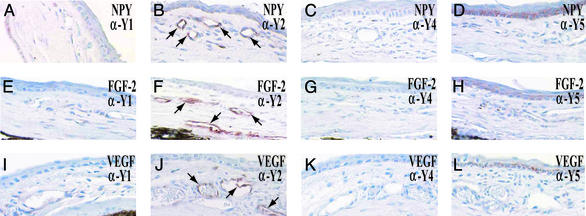
To further validate the involvement of the Y2 receptor in transducing angiogenic signals, tissues from NPY-implanted corneas containing newly formed blood vessels were subjected to immunohistological analysis using specific antibodies. As shown in Fig. 2, Y2 receptor protein was present in virtually all newly formed blood vessels (arrows) as detected by a specific antibody against the Y2 receptor. Y2 receptor was found to be widely distributed on the newly formed blood vessels induced by NPY, FGF-2, and VEGF (Fig. 2 B, F, and J). In contrast, no immunoreactivity for Y1, Y4, and Y5 receptors was detected in corneal capillaries induced by NPY, FGF-2 and VEGF (Fig. 2 A, C–E, G–I, K, and L). Thus, the Y2 receptor seems to be the only NPY receptor that is expressed on various angiogenic factor-induced blood vessels and likely to directly transduce NPY-induced angiogenic signals in endothelial cells in vivo.
Figure 2.
Localization of NPY Y2 receptor on the newly formed blood vessels. Histological sections of NPY-implanted (A–D), FGF-2-implanted (E–H), and VEGF-implanted (I–L) corneas were incubated with an anti-Y1 (A, E, and I), an anti-Y2 (B, F, and J), an anti-Y4 (C, G, and K), or an anti-Y5 (D, H, and L) receptor antibody and stained with a peroxidase-conjugated secondary antibody. Endothelial cells of corneal microvessels were immunoreactive as indicated by arrows (B, F, and J).
Lack of Corneal Angiogenesis in Y2-Deficient Mice.
If Y2 receptor was important for mediation of NPY-induced angiogenesis, deletion of Y2 receptor in mice might impair the angiogenic response in vivo. To test this hypothesis, we implanted pellets containing 160 ng of NPY in corneas of Y2−/− mice. These Y2 receptor-deficient mice have previously been reported to develop increased body weight, food intake, and fat deposition. They also show a reduced response to leptin but normal response to NPY-induced food intake and intact regulation of refeeding and body weight after starvation. In addition, absence of Y2 receptor also affects basal control of heart rate (22). In the present study, we have found that NPY completely failed to induce corneal blood vessel growth in these knock-out mice (Fig. 3B). In contrast, FGF-2- (Fig. 3C) and VEGF- (Fig. 3D) induced angiogenesis was not affected in Y2−/− mice, indicating that the Y2 receptor is specifically responsible for NPY-induced, but not for other angiogenic factor-induced, angiogenesis.
Impairment of Wound Healing in Y2 Receptor-Deficient Mice.
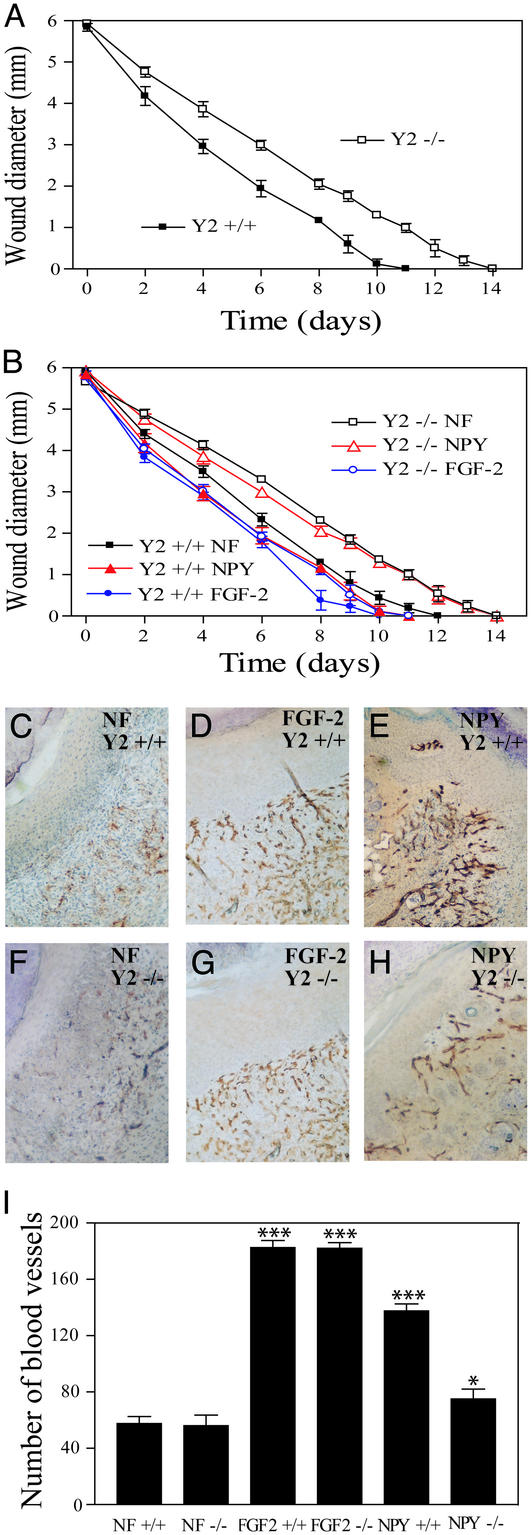
NPY is widely distributed in the brain and peripheral tissues (30–33). Its in vivo angiogenic activity promoted us to further examine the possible physiological role of NPY during wound healing, a process dependent on angiogenesis (6). Full thickness skin wounds were surgically created on the middle back of Y2−/− mice, and healing of wounds was measured daily. Skin wound healing was delayed in Y2−/− mice as measured by the diameters (Fig. 4 A and B). The measured sizes of wounds were significantly larger (P < 0.001) in Y2−/− mice throughout the experiment.
Figure 4.
Delay of skin wound healing in Y2 receptor-deficient mice. Full-thickness skin wounds (6 mm in diameter) were created on the back of shaved NPY Y2+/+ and NPY Y2−/− mice by using a template. In B, each group of animals (six to seven per group) was topically treated with slow-release polymers containing NPY, FGF-2, or PBS (NF) as indicated. Diameters of wounds were measured daily (A and B). Data are presented in A and B as mean determinants (±SEM) of wounds of six to seven mice in each group. At day 7 after implantation, some wounds in each group were removed for immunohistochemical analysis by using an anti-CD31 staining antibody. Positive immunostaining signals of blood vessels in wounds of NPY Y2+/+ mice (C–E) and of Y2−/− mice (F–H) treated with PBS (C and F), FGF-2 (D and G), and NPY (E and H) were revealed by a peroxidase reaction. NF, no factor (PBS). Vascular density was quantified by counting microvessel numbers in six random fields under ×40 magnification (I). The average numbers of vessels (±SEM) are presented.
To study whether exogenous NPY or FGF-2 could correct the impaired wound healing, we topically applied NPY and FGF-2 at the dosages of 1,500 and 400 ng, respectively, to the wound tissue. Consistent with the experiment reported in Fig. 4A, Y2−/− mice had delayed wound healing as compared with the Y2+/+ group. As expected, FGF-2 accelerated wound healing in both Y2+/+ and Y2−/− groups (Fig. 4B). NPY also promoted wound healing in Y2+/+ mice although this effect was less potent as compared with FGF-2. However, NPY was unable to correct the impaired wound healing phenotype in the Y2−/− mice. These data demonstrate an essential role of the Y2 receptor in mediating NPY-stimulated wound healing. Consistent with these findings, immunohistochemical analysis with an anti-CD31 antibody revealed that FGF-2-treated wounds in both Y2+/+ and Y2−/− mice contained increased numbers of blood vessels (Fig. 4 D and G) as compared with the buffer-treated wounds (Fig. 4 C and F). Similarly, the NPY-treated wounds in Y2+/+ mice were also highly vascularized (Fig. 4E). In contrast, NPY barely had any effect in acceleration of wound healing in the Y2−/− mice as compared with controls (Fig. 4 H, G, and F). Quantification analysis showed that FGF-2 potently stimulated nearly identical skin neovascularization in both Y2+/+ and Y2−/− mice (Fig. 4I). Similarly, NPY also significantly increased vessel density in the wounds of Y2+/+ mice (Fig. 4I). In contrast, NPY was unable to restore its angiogenic activity in Y2−/− mice. In addition to anti-CD31 staining, we performed immunohistochemistry on these wound tissues by using a monoclonal antibody, Mac 3, which specifically detects macrophages. The levels of macrophage infiltration into these wound tissues were nearly identical among all groups (data not shown). Thus, it is unlikely that inflammation is an important contributor for these wound-healing studies.
Discussion
Our present study provides evidence that NPY is a potent angiogenic factor in at least two in vivo systems, i.e., the mouse corneal angiogenesis model and the CAM assay, the latter of which measures neovascularization during embryonic development. The angiogenic signal is transmitted through the Y2 receptor subtype. Although various dosages of NPY have been found to induce angiogenesis, the observed angiogenic effect is unlikely because of histamine release, because concentrations of NPY in the range of 0.1 mM are required for histamine release from mast cells in vitro (34, 35). Furthermore, NPY and the C-terminal fragments seem equally potent in this respect, but not in stimulating angiogenesis. Because angiogenesis is a critical component in many physiological and pathological processes, our data suggest that NPY has even greater diverse biological functions than was previously thought. As an example of such a novel physiological function, we demonstrate that NPY acting through the Y2 receptor may facilitate wound repair, an angiogenesis-dependent process.
Our findings that NPY stimulates angiogenesis in vivo may shed further light on our understanding of the complexity of the cross-talk between the nervous system and vascular systems. The nervous system regulates the vascular system at several levels. First, during embryonic development, nerve growth factors originating from premature CNS tissues may control the growth of other organs and tissues and may subsequently remodel the growth of the vascular tree. Very recently, the finding that nerve growth guides blood vessel growth supports this idea (15). Secondly, endocrine hormones regulated by the CNS can govern both local and distal angiogenesis. For example, the GH family and corticotrophin-releasing hormone have been found to act as proangiogenic molecules (36, 37). Unlike the families of FGFs and VEGFs, which are in their most forms locally sequestered by binding to heparan sulfate proteoglycans in the extracellular matrix (38), these endocrine hormones are regulators of the vascular systems throughout the organism. Thus, they may control and remodel the entire vascular tree in the body. In this regard, these global angiogenic modulators are crucial for guiding and coordinating blood vessel growth because local angiogenic factors are frequently unevenly expressed in various tissues and growth of new vessels often crosses the boundaries between various tissues and organs. Whether NPY could act as a master player in controlling vascular development remains to be investigated. Third, several neurotrophic factors such as nerve growth factor (NGF) are proangiogenic (39), suggesting that many of these factors have diverse biological functions. In addition, the brain is one of the few tissues that are enriched in angiogenic factors. In fact, both FGF-2 and VEGF, the two most potent angiogenic factors, were isolated from brain tissue (38, 40). Like several other neuroregulators, NPY is found in many peripheral tissues although its primary targets are thought to reside in the brain (28). Both NPY and NGF proteins are expressed at a very high level in the salivary gland (41, 42), and the widely peripheral distribution of NPY suggests that it may have broad biological effects also on other tissues. The vascular system is apparently a target for NPY acting on both vascular smooth muscle cells and endothelial cells.
Among NPY receptors, the Y1 receptor has been found to be expressed on vascular smooth muscle cells (43), but to a lesser extent on endothelial cells, whereas our present study shows that endothelial cells of newly developed vessels in response to NPY, FGF-2, and VEGF stimulation express Y2, but not Y1, Y4, or Y5, receptors. These findings suggest that NPY induces angiogenesis in a coordinated manner. At the leading edge of growing capillaries, endothelial cells are the only cell type that forms the primary vascular plexus. The role of Y2 receptor may be guiding of the capillary outgrowth. As these microvessels become mature, other vascular cell types including pericytes and smooth muscle cells are literally recruited to the newly formed blood vessels by expression of Y1 receptor. Although this speculation needs to be further validated, NPY may activate both Y2 and Y1 receptors expressed on different types of cells. Deletion of Y2 receptor in mice does, however, not apparently affect reproduction of these mice (22). Because angiogenesis is an important part of embryogenesis, it is surprising that Y2-deficient mice produce offspring of equal litter size as do the wild-type mice. The Y2-deficient mice are characterized by an increased body weight in mature ages, increased food intake, and fat deposition (22). This phenotype is similar to, although not as severe as, ob/ob and db/db mice lacking the active leptin or receptor signals. Interestingly, leptin has been found to stimulate angiogenesis and possibly to increase energy expenditure. Thus, NPY could play a role in regulation of angiogenesis in the adipose tissue.
It seems that the Y2 receptor is expressed not only on the NPY-induced new blood vessels. High levels of Y2 receptor expression are also found in the FGF-2- and VEGF-induced vessels. These data suggest the general role of NPY in regulation of angiogenesis. As multiple angiogenic factor receptors are coexpressed on newly formed blood vessels, the growth and remodeling of blood vessels require a coordinated effort among these angiogenic factors. In this regard, NPY may produce a synergistic effect with FGF-2 and VEGF. Unlike the limited expression patterns of leptin, NPY is expressed in several tissues, including the skin (44). Impairment of wound healing in the skin of Y2 receptor-deficient mice supports the fact that NPY as a proangiogenic molecule plays an important role in physiological conditions. However, further work needs to be done to determine whether there is a direct cause between angiogenesis stimulation and vessel stability in Y2 receptor-deficient mice.
Acknowledgments
We thank Ulla Veicjer-Gustafsson for technical assistance, and Ebba Bråkenhielm and Niina Veitonmäki for critical reading of the manuscript. A.J.E. was partly supported from the Research Foundation of the Department of Oncology of Umeå University and the Konrad and Svea Ulins Donation Fund (Socialstyrelsen). This work was further supported by research grants in Yihai Cao's laboratory, including the Swedish Research Council, the Swedish Heart and Lung Foundation, the Swedish Cancer Foundation, the Human Frontier Science Program, the Karolinska Institute Foundation, and the Åke Wibergs Foundation. Y.C. is supported by the Swedish Research Council.
Abbreviations
- NPY
neuropeptide Y
- FGF
fibroblast growth factor
- VEGF
vascular endothelial growth factor
- CAM
chorioallantoic membrane
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Blomqvist A G, Herzog H. Trends Neurosci. 1997;20:294–298. doi: 10.1016/s0166-2236(96)01057-0. [DOI] [PubMed] [Google Scholar]
- 2.Larhammar D. Regul Pept. 1996;65:165–174. doi: 10.1016/0167-0115(96)00110-3. [DOI] [PubMed] [Google Scholar]
- 3.Franco-Cereceda A, Lundberg J M, Dahlof C. Acta Physiol Scand. 1985;124:361–369. doi: 10.1111/j.1748-1716.1985.tb07671.x. [DOI] [PubMed] [Google Scholar]
- 4.Erlinge D, Brunkwall J, Edvinsson L. Regul Pept. 1994;50:259–265. doi: 10.1016/0167-0115(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 5.Zukowska-Grojec Z, Pruszczyk P, Colton C, Yao J, Shen G H, Myers A K, Wahlestedt C. Peptides. 1993;14:263–268. doi: 10.1016/0196-9781(93)90040-n. [DOI] [PubMed] [Google Scholar]
- 6.Ghersi G, Chen W, Lee E W, Zukowska Z. Peptides. 2001;22:453–458. doi: 10.1016/s0196-9781(01)00340-0. [DOI] [PubMed] [Google Scholar]
- 7.Marion-Audibert A M, Nejjari M, Pourreyron C, Anderson W, Gouysse G, Jacquier M F, Dumortier J, Scoazec J Y. Gastroenterol Clin Biol. 2000;24:644–648. [PubMed] [Google Scholar]
- 8.Zukowska-Grojec Z, Karwatowska-Prokopczuk E, Fisher T A, Ji H. Regul Pept. 1998;75–76:231–238. doi: 10.1016/s0167-0115(98)00073-1. [DOI] [PubMed] [Google Scholar]
- 9.Zukowska-Grojec Z, Karwatowska-Prokopczuk E, Rose W, Rone J, Movafagh S, Ji H, Yeh Y, Chen W T, Kleinman H K, Grouzmann E, Grant D S. Circ Res. 1998;83:187–195. doi: 10.1161/01.res.83.2.187. [DOI] [PubMed] [Google Scholar]
- 10.Carmeliet P, Jain R K. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 11.Epstein S E, Kornowski R, Fuchs S, Dvorak H F. Circulation. 2001;104:115–119. doi: 10.1161/01.cir.104.1.115. [DOI] [PubMed] [Google Scholar]
- 12.Ferrara N, Alitalo K. Nat Med. 1999;5:1359–1364. doi: 10.1038/70928. [DOI] [PubMed] [Google Scholar]
- 13.Folkman J. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 14.Hanahan D, Folkman J. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 15.Mukouyama Y S, Shin D, Britsch S, Taniguchi M, Anderson D J. Cell. 2002;109:693–705. doi: 10.1016/s0092-8674(02)00757-2. [DOI] [PubMed] [Google Scholar]
- 16.Folkman J. N Engl J Med. 1995;333:1757–1763. doi: 10.1056/NEJM199512283332608. [DOI] [PubMed] [Google Scholar]
- 17.Grundemar L, Krstenansky J L, Hakanson R. Eur J Pharmacol. 1993;232:271–278. doi: 10.1016/0014-2999(93)90784-f. [DOI] [PubMed] [Google Scholar]
- 18.Lee M J, Thangada S, Paik J H, Sapkota G P, Ancellin N, Chae S S, Wu M, Morales-Ruiz M, Sessa W C, Alessi D R, Hla T. Mol Cell. 2001;8:693–704. doi: 10.1016/s1097-2765(01)00324-0. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg Y, Taimor G, Piper H M, Schluter K D. Am J Physiol. 1998;275:C1207–C1215. doi: 10.1152/ajpcell.1998.275.5.C1207. [DOI] [PubMed] [Google Scholar]
- 20.Nie M, Selbie L A. Regul Pept. 1998;75–76:207–213. doi: 10.1016/s0167-0115(98)00070-6. [DOI] [PubMed] [Google Scholar]
- 21.Pellieux C, Sauthier T, Domenighetti A, Marsh D J, Palmiter R D, Brunner H R, Pedrazzini T. Proc Natl Acad Sci USA. 2000;97:1595–1600. doi: 10.1073/pnas.030533197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naveilhan P, Hassani H, Canals J M, Ekstrand A J, Larefalk A, Chhajlani V, Arenas E, Gedda K, Svensson L, Thoren P, Ernfors P. Nat Med. 1999;5:1188–1193. doi: 10.1038/13514. [DOI] [PubMed] [Google Scholar]
- 23.Cao Y, Linden P, Farnebo J, Cao R, Eriksson A, Kumar V, Qi J H, Claesson-Welsh L, Alitalo K. Proc Natl Acad Sci USA. 1998;95:14389–14394. doi: 10.1073/pnas.95.24.14389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao Y, Cao R. Nature. 1999;398:381. doi: 10.1038/18793. [DOI] [PubMed] [Google Scholar]
- 25.Jain R K, Schlenger K, Hockel M, Yuan F. Nat Med. 1997;3:1203–1208. doi: 10.1038/nm1197-1203. [DOI] [PubMed] [Google Scholar]
- 26.Brakenhielm E, Cao R, Cao Y. FASEB J. 2001;15:1798–1800. doi: 10.1096/fj.01-0028fje. [DOI] [PubMed] [Google Scholar]
- 27.You J, Edvinsson L, Bryan R M., Jr J Cereb Blood Flow Metab. 2001;21:77–84. doi: 10.1097/00004647-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Blomqvist A, Mackerlova L. NeuroReport. 1995;6:605–608. doi: 10.1097/00001756-199503000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Smith-White M A, Potter E K. Neuropeptides. 1999;33:526–533. doi: 10.1054/npep.1999.0774. [DOI] [PubMed] [Google Scholar]
- 30.Jackson D, Volpert O V, Bouck N, Linzer D I. Science. 1994;266:1581–1584. doi: 10.1126/science.7527157. [DOI] [PubMed] [Google Scholar]
- 31.Larhammar D, Ericsson A, Persson H. Proc Natl Acad Sci USA. 1987;84:2068–2072. doi: 10.1073/pnas.84.7.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lundberg J M, Terenius L, Hokfelt T, Martling C R, Tatemoto K, Mutt V, Polak J, Bloom S, Goldstein M. Acta Physiol Scand. 1982;116:477–480. doi: 10.1111/j.1748-1716.1982.tb07171.x. [DOI] [PubMed] [Google Scholar]
- 33.Tatemoto K, Carlquist M, Mutt V. Nature. 1982;296:659–660. doi: 10.1038/296659a0. [DOI] [PubMed] [Google Scholar]
- 34.Grundemar L, Hakanson R. Br J Pharmacol. 1991;104:776–778. doi: 10.1111/j.1476-5381.1991.tb12505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hiraoka N, Allen E, Apel I J, Gyetko M R, Weiss S J. Cell. 1998;95:365–377. doi: 10.1016/s0092-8674(00)81768-7. [DOI] [PubMed] [Google Scholar]
- 36.Arbiser J L, Karalis K, Viswanathan A, Koike C, Anand-Apte B, Flynn E, Zetter B, Majzoub J A. J Invest Dermatol. 1999;113:838–842. doi: 10.1046/j.1523-1747.1999.00760.x. [DOI] [PubMed] [Google Scholar]
- 37.Ferrara N. Recent Prog Horm Res. 2000;55:15–35. ; discussion 35–36. [PubMed] [Google Scholar]
- 38.Santos P M, Winterowd J G, Allen G G, Bothwell M A, Rubel E W. Otolaryngol Head Neck Surg. 1991;105:12–25. doi: 10.1177/019459989110500103. [DOI] [PubMed] [Google Scholar]
- 39.Gospodarowicz D, Cheng J, Lirette M. J Cell Biol. 1983;97:1677–1685. doi: 10.1083/jcb.97.6.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hauser-Kronberger C, Albegger K, Saria A, Hacker G W. Acta Otolaryngol. 1992;112:343–348. doi: 10.1080/00016489.1992.11665430. [DOI] [PubMed] [Google Scholar]
- 41.Liuzzi A, Angeletti P U, Levi-Montalcini R. J Neurochem. 1965;12:705–708. doi: 10.1111/j.1471-4159.1965.tb06784.x. [DOI] [PubMed] [Google Scholar]
- 42.Mihara S, Shigeri Y, Fujimoto M. Biochem Int. 1990;22:205–212. [PubMed] [Google Scholar]
- 43.Wallengren J, Moller H, Ekman R. Arch Dermatol Res. 1987;279:512–515. doi: 10.1007/BF00413281. [DOI] [PubMed] [Google Scholar]
- 44.Zetter B R. J Cell Biol. 2001;152:35–36. doi: 10.1083/jcb.152.6.f35. [DOI] [PMC free article] [PubMed] [Google Scholar]