Abstract
Oxidant-induced injury to the lung is associated with extensive damage to the lung epithelium. Instillation of keratinocyte growth factor (KGF) in the lungs of animals protects animals from oxidant-induced injury but the mechanism of protection is not well understood. An inherent problem in studying KGF function in vivo has been that constitutive overexpression of KGF in the lung causes embryonic lethality with extensive pulmonary malformation. Here we report the development of a stringently regulated, tetracycline-inducible, lung-specific transgenic system that allows regulated expression of KGF in the lung without causing developmental abnormalities from leaky KGF expression. By using this system, we show that exposure of KGF-expressing mice to hyperoxia protects the lung epithelium but not the endothelium from cell death in accordance with the selective expression of KGF receptor on epithelial and not on endothelial cells. Investigations of KGF-induced cell survival pathways revealed KGF-induced activation of the multifunctional pro-survival Akt signaling axis both in vitro and in vivo. Inhibition of KGF-induced Akt activation by a dominant-negative mutant of Akt blocked the KGF-mediated protection of epithelial cells exposed to hyperoxia. KGF-induced Akt activation may play an important role in inhibiting lung alveolar cell death thereby preserving the lung architecture and function during oxidative stress.
Keratinocyte growth factor (KGF) is a member of the fibroblast growth factor family and is produced by mesenchymal cells (1). The pattern of expression of KGF and its receptor suggests an important role for KGF in mediating mesenchymal–epithelial interactions (2). The expression of KGF mRNA has been detected in several stromal fibroblast lines derived from embryonic, neonatal, and adult tissues (3). The receptor for KGF (also called FGFR2-IIIb), which has intrinsic tyrosine kinase activity (4), is expressed specifically on epithelial cells and is diffusely expressed in day-11 lung epithelium (5). In keeping with the expression characteristics of KGF and its receptor in the lung, transgenic mice expressing a dominant-negative (DN) mutant of the KGF receptor under the control of the SP-C promoter exhibit embryonic lethality with grossly abnormal lung development with only two primordial epithelial tubes and no branching morphogenesis (6). Constitutive overexpression of KGF in transgenic mice, on the other hand, results in lethal papillary cystadenoma, with marked enlargement of bronchial air spaces (7).
Growth factors have been shown to stimulate different signal transduction pathways that promote cell survival. In in vitro studies, the serine-threonine kinase Akt has been shown to play an important role in prevention of cell death by growth factors (8–11). Also, phosphatidylinositol 3-kinase has been implicated in growth factor-dependent activation of Akt (8–15). In vitro, Akt has been shown to inhibit cell death triggered by various stimuli including growth factor withdrawal, cell-cycle discordance, DNA damage, and loss of cell adhesion (10, 11, 16–19). Interestingly, crosstalk between the Akt signaling axis and other pathways is also beginning to be appreciated in different studies (20–25).
Both in vitro and in vivo studies support a role for KGF in type II cell proliferation (26–28). KGF has been shown to prevent lung injury induced by different forms of oxidative stress including that induced by hyperoxia, radiation, and chemotherapy (26, 29–33). Because KGF mRNA expression has been shown to be induced by cytokines and growth factors such as IL-1 and transforming growth factor type α (TGF-α; ref. 34), increased production of these molecules from activated alveolar macrophages and other inflammatory cells during hyperoxic injury could potentially stimulate local KGF production, which in turn could act on type II cells, triggering repair of alveolar damage during the repair phase. In vitro studies reported to date suggest a role for KGF in type II cell proliferation (26), inhibition of increased oxidant-induced epithelial cell permeability (35), stimulation of transepithelial Na ion transport (36), and stimulation of DNA repair (37). To our knowledge, however, no study to date, either in vitro or in vivo, has explored the possibility that KGF might induce cell survival mechanisms in cells. Here we have developed an externally regulatable lung-specific transgene expression system that overcomes the problem of leakiness of transgene expression observed in our previous system (38). By using this improved system, we were able to express the KGF transgene without the detrimental consequences of leaky expression of the KGF transgene. We show that in the transgenic animals, KGF can inhibit lung epithelial cell death when the animals are subjected to hyperoxia. Also, KGF is able to activate the antiapoptotic Akt signaling axis both in vitro and in vivo. KGF-induced Akt activation seems to be critical for the protective functions of KGF.
Materials and Methods
Cells.
Human primary small-airway epithelial (SAE) cells were obtained from Clonetics (San Diego) and grown in supplied medium.
Generation of KGF Transgenic Animals.
We generated transgenic animals by microinjecting three constructs into the pronuclei of C57BL/6xSJL F2 mouse embryos. One was a construct encoding the tetracycline (Tet) transrepressor tTR, a hybrid protein containing the class B DNA-binding domain and class E dimerization domain of TetR (39) and the Kruppel-associated box (KRAB) repressor domain of the mammalian Kox1 protein; the second was the reverse Tet transactivator rtTA (40); and the third was the TetO/P-KGF (O/P, operator/promoter) construct.
tTR is expressed under the control of the β-actin promoter (41, 42). rtTA is expressed under the control of the Clara cell-specific CC10 promoter, which is active in lung epithelial cells, whereas the KGF cDNA is linked to a minimal cytomegalovirus (CMV) promoter and Tet O/P sequences. All constructs were confirmed by sequencing. Linearized minigenes were separated from vector DNA and used for microinjection (detailed information of constructs is available from the authors). Transgenic mice were characterized by PCR amplification of DNA isolated from tail biopsies by using appropriate PCR primers (Table 1, which is published as supporting information on the PNAS web site, www.pnas.org). To detect RNA expression corresponding to the KGF transgene, transgenic mice and control nontransgenic littermates were supplied with doxycycline (Dox; 1 mg/ml) in water containing 5% sucrose for 3 days. Lungs of mice were harvested, 100 mg of lung tissue was homogenized in 1 ml of TRIzol (Life Technologies, Grand Island, NY), and RNA was isolated following the manufacturer's protocol. DNA contamination, if any, was removed by DNase I (GeneHunter, Frederick, MD) treatment followed by purification with RNAqueous-4PCR (Ambion, Austin, TX). The DNA-free RNA was subjected to RT-PCR. Reverse transcription was performed with 3 μg of DNA-free RNA (Superscript II, Life Technologies) according to manufacturer's protocol using the supplied random hexamers. PCR was performed by using Red Taq polymerase (Sigma). In addition to the KGF primers listed above, exon-specific primers for the HPRT- and the β-actin genes were used as positive controls. To confirm the absence of any PCR product generated from contaminating DNA, control RT-PCR was performed with reverse transcription without random primers followed by PCR.
Exposure of Mice to Hyperoxia.
Mice were housed in cages containing food and water. The cages were transferred to a hyperoxic chamber and exposed to 100% oxygen in a Plexiglas hyperoxia-exposure chamber as described (43). After exposure to hyperoxia, mice were killed when they appeared huddled and withdrawn from food and water.
Growth Factor Stimulation, Immunoprecipitation, and Western Blot Analysis.
Cells were incubated in serum-free medium for 24 h. For inhibition of phosphatidylinositol 3-kinase, cells were pretreated for 30 min with wortmannin and then stimulated with KGF (100 ng/ml, Roche Molecular Biochemicals). Cell extracts for protein analysis by immunoprecipitation, Western blotting, or kinase assay were prepared as described (44).
Akt Kinase Assay.
Akt kinase assay was performed by using an Akt Kinase Assay kit (Cell Signaling Technology, Beverly, MA) following the manufacturer's instructions as described (43).
Terminal Deoxynucleotidyltransferase-Mediated dUTP End Labeling (TUNEL) Assay.
Lungs were prepared for TUNEL assay (43) by perfusing the animal through the right ventricle with PBS to remove all blood as described (45).
Transmission Electron Microscopy.
Mouse lungs were perfusion-fixed through the heart with 2.5% glutaraldehyde in PBS. Lungs were removed and immersed in the same fixative overnight at 4°C. Lungs were processed for electron microscopy as described in Supporting Methods, which are published as supporting information on the PNAS web site.
Results
Generation of Transgenic Mice Overexpressing KGF in an Inducible Lung-Specific Fashion.
To investigate the mechanisms underlying the protective effects of KGF, we have established an externally regulatable system that can be stringently controlled for tissue-specific transgene expression. This system is based on our previously established Dox-inducible system (38), which has been adapted from the Tet-inducible system of Gossen et al. (40). As we described, there is a low degree of leaky expression of the transgene in our previous system (46). To minimize leaky transgene expression further, we have introduced a Tet repressor gene developed by Hillen and colleagues (39) in the inducible transgenic system. In our original Tet-inducible system, rtTA driven by the lung-specific CC10 promoter is constitutively expressed in lung epithelial cells (38). When Dox is introduced through drinking water, it induces the binding of the transactivator rtTA to Tet O/P sequences, resulting in induction of expression of the linked transgene from a second construct. In the modified system, tTR binds the target promoter in the absence of Dox. Addition of Dox results in removal of tTR from the target promoter and allows binding of the transactivator (rtTA) to the promoter, causing induction of gene transcription. Thus in this improved system, the binding of the transrepressor prevents leaky expression from the promoter.
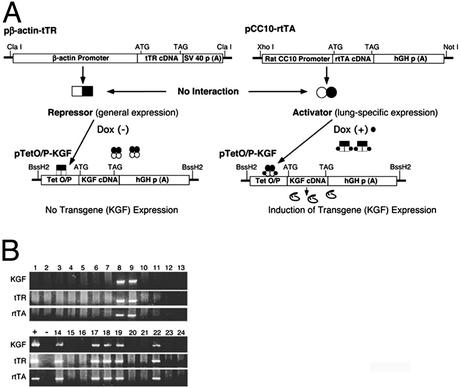
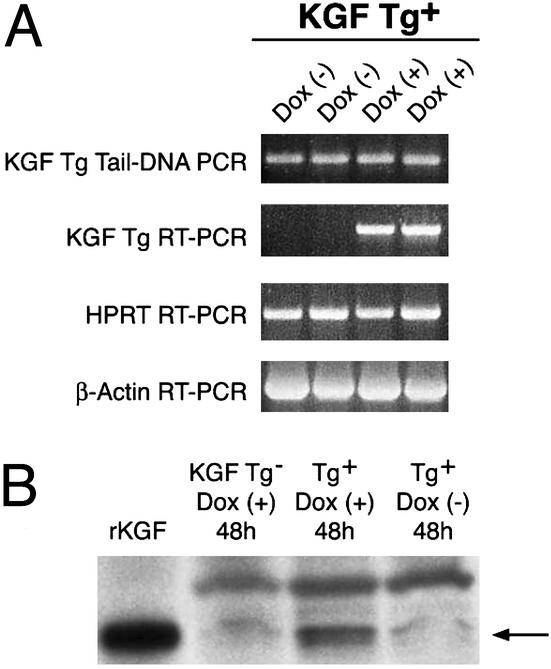
Fig. 1A is a schematic of the DNA constructs used to generate the transgenic mice. We obtained seven founders harboring all of the three transgenes: the KGF gene, the TetR (tTR) gene, and the rtTA gene as determined by PCR of tail-tip DNA (Fig. 1B). The different primer pairs used in the PCRs are shown in Table 1. Of the seven founders, five were female and two were male. We used the two male founders to establish two transgenic lines expressing KGF. Animals harboring all three transgenes were analyzed for transgene expression at the RNA and protein levels. As shown in Fig. 2A, we did not detect any leaky transgene expression in the absence of Dox in either line after 30 cycles of RT-PCR. KGF protein expression was also detected only after Dox induction in transgenic mice and was in the range of 5–10 ng per mouse lung (Fig. 2B). The level of expression was higher than observed levels of cytokine transgene expression using the CC10 promoter (≈0.5 ng/ml of BAL fluid; ref. 38).
Figure 1.
Generation of transgenic animals expressing KGF. (A) An inducible dual repressor-activator transgene expression system was developed by injecting the following three transgenes. One minigene for expression of the Tet Dox-inducible transrepressor (tTR) under the control of β-actin promoter (pβactin-tTR); a second minigene to express the Tet-inducible rtTA under the control of lung specific CC10 promoter (pCC10-rtTA); and a third construct consisted of a cytomegalovirus minimal promoter and binding sequence of tTR and rtTA (Tet O/P) linked to the KGF coding sequence containing a start codon after a Kozak sequence and its own stop codon (pTet O/P KGF). In the absence of Dox, tTR can bind and repress transcription whereas rtTA is unable to bind DNA. In the presence of Dox, tTR comes off DNA whereas rtTA binds DNA and activates transcription of the linked KGF gene. (B) Transgenic animals were identified by PCR of genomic DNA isolated from tail biopsies of individual animals. The primers and PCR conditions used are described in Materials and Methods and as supporting information on the PNAS web site. Transgene-positive founder mice were used to establish lines.
Figure 2.
Analysis of externally regulated expression of transgenic KGF RNA and protein in the lung. Two lines of transgene (Tg)-positive animals were treated with Dox or control solution (5% sucrose) for 2 days. Mice were killed and a part of the lung was used for RNA isolation and another part for protein isolation. (A) DNA-free RNA was used for RT-PCR with appropriate primers. In the absence of the external inducer (Dox−), animals from neither line expressed RNA corresponding to the KGF Tg but expressed transgenic RNA after induction (Dox+). (B) To detect KGF protein expression derived from the Tg, lungs were homogenized in Triton X-100 containing buffer, and the pelleted fraction was solubilized in hot Laemmli buffer and subjected to Western blot analysis. We were unable to detect KGF protein in the Triton X-100-soluble extract, which is in keeping with the propensity of KGF and other members of the fibroblast growth factor family to attach to matrix components. In the Laemmli buffer extract, KGF protein from the Tg could be identified within 48 h of Tg induction. Arrow indicates the KGF protein derived from the Tg that migrates similarly with the standard (recombinant KGF). The faint band in the lane containing extract prepared from the lungs of uninduced animals and non-Tg animals probably corresponds to low levels of endogenous KGF. The level of KGF expression per mouse was estimated to be between 5 and 10 ng. Tg expression in the two lines was confirmed at both RNA and protein levels in two independent experiments using five animals per line per group (±Dox). Shown are data representative of five animals in each group. We have not performed long-term experiments to determine effects of very low, undetectable leaky expression of Tg, if any.
KGF Expression Inhibits Epithelial Cell Death in Vivo.
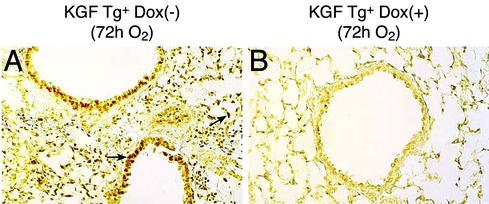
We investigated whether expression of KGF inhibits cell death induced by hyperoxia. In these experiments, lung sections from mice were subjected to TUNEL assay for nuclear DNA fragmentation, an indicator of cell death. As shown in Fig. 3A, in the absence of Dox, when KGF is not expressed, transgenic mice subjected to hyperoxia displayed extensive TUNEL+ nuclei in the airway epithelium as well as in alveolar epithelial cells at 72 h after O2 exposure. In contrast, in the presence of Dox, when KGF was overexpressed, cell death in the airway epithelium as well as in the alveolar epithelium was appreciably inhibited (Fig. 3B). Fig. 3B also reflects normal lung architecture in the KGF transgenic mice, demonstrating the absence of any leaky expression of KGF during development that would have adversely affected lung formation.
Figure 3.
Inhibition of oxidant-induced lung cell death in KGF transgenic (Tg) animal. Expression of KGF was induced in KGF Tg-positive (Tg+) mice by supplying Dox to animals (Dox+). The mice were then exposed to 100% oxygen, lungs were harvested, and the TUNEL assay was performed. (A) Lung cell death after exposure of animals to 100% O2 for 72 h in the absence of KGF (brown-stained nuclei). (B) Inhibition of lung cell death in the presence of KGF after 72 h of 100% O2 exposure. Arrows indicate dark-brown TUNEL staining representing cell death. Three animals were included in each group, and the experiment was repeated with similar results.
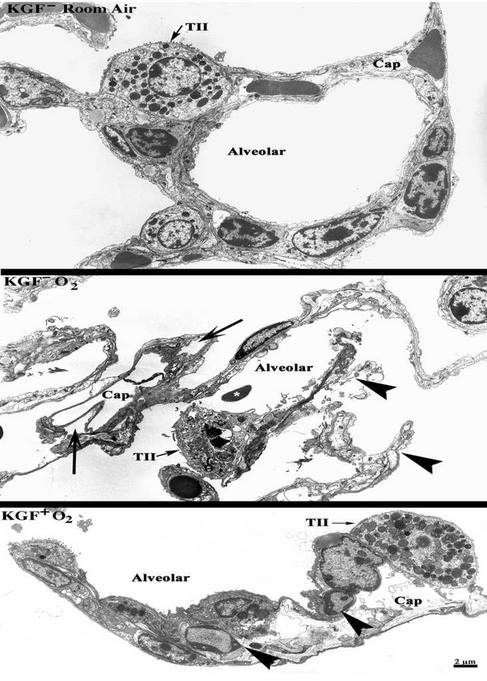
To determine the effect of KGF expression on specific cell types in the lung in mice exposed to hyperoxia, we carried out ultrastructural studies. The KGF transgenic mice were either left without Dox (KGF−) or were treated with Dox (KGF+) and exposed to room air or 100% O2 for 72 h. KGF expression was induced at 24 h before initiation of hyperoxic exposure. As shown in Fig. 4, a typical architecture and cellular morphology of lung cells lining the alveolar space and capillaries was observed in KGF transgene positive animals without Dox (KGF−) and exposed to room air for 72 h. Similar observations were made with KGF transgene negative animals (data not shown). Hyperoxic exposure in the absence of induction of KGF expression (Fig. 4 Middle, KGF− O2) showed incomplete alveolar lining (arrowheads) resulting from the death of the epithelium under hyperoxic conditions. Capillaries also showed aberrant morphology with endothelium peeling away from the basement membranes (Fig. 4, arrows). Other signs of injury including hemorrhage (∗) and infiltration of neutrophils were also detected in different sections. Hyperoxic conditions in the presence of KGF (Fig. 4 Bottom, KGF+ O2) preserved the ultrastructure of the cells lining the alveolar space, as a complete cell lining was apparent in all sections analyzed. However, the endothelium and accessory cells lining the capillaries showed features of apoptosis (Fig. 4 Bottom, arrowheads; nuclear condensation is evident), indicating that the amount of KGF expressed in these mice may not be enough to protect the microvasculature during hyperoxia. Several sections were analyzed from the mice and similar effects were seen in different parts of the lung (see Fig. 8, which is published as supporting information on the PNAS web site).
Figure 4.
KGF overexpression in the lung protects the lung alveolar epithelium from hyperoxia-induced cell death. KGF transgene-positive mice were maintained without Dox (KGF−) or were supplied with Dox (KGF+) in drinking water 24 h before exposure to room air or 100% O2 for up to 72 h. Lungs were fixed in glutaraldehyde and ultrathin sections were obtained and analyzed by electron microscopy. (Top) Normal lung architecture in KGF transgene-positive animals without Dox (KGF−) exposed to room air for 72 h. Similar observations were made with KGF transgene-negative animals (data not shown). (Middle) KGF− O2: Hyperoxic exposure in the absence of induction of KGF shows alveolar disintegration. Capillaries also show aberrant morphology with endothelium peeling away from the basement membranes (arrows). Blood cells (*) in the alveolar space indicate hemorrhage. (Bottom) KGF+ O2: Hyperoxic exposure in the presence of KGF preserved the ultrastructure of the cells lining the alveolar space. However, the endothelium and accessory cells lining the capillaries have undergone apoptosis (arrowheads; nuclear condensation is evident). Five animals were included per group and multiple areas, i.e., samples from various parts of each lung, were evaluated. (Bar = 2 μm, representative of all images.) TII, type II cell; Cap, capillary.
We investigated the effect of KGF on survival of the animals after hyperoxic exposure. Animals were killed when they appeared huddled and withdrawn from food and extremely sick, showing evidence of respiratory distress. We did not detect any appreciable difference in survival time regardless of the presence of KGF (see Figs. 9 and 10, which are published as supporting information on the PNAS web site). The inability of KGF to cause a significant increase in survival of the mice in response to hyperoxia can be explained by the fact that the level at which KGF was expressed in the lungs of the transgenic mice (5–10 ng) protected the lung epithelium but not the endothelium. The epithelial-specific protection by KGF was not surprising, because the KGF receptor is expressed on epithelial and not on endothelial cells.
KGF-Induced Activation of Akt in Vivo.
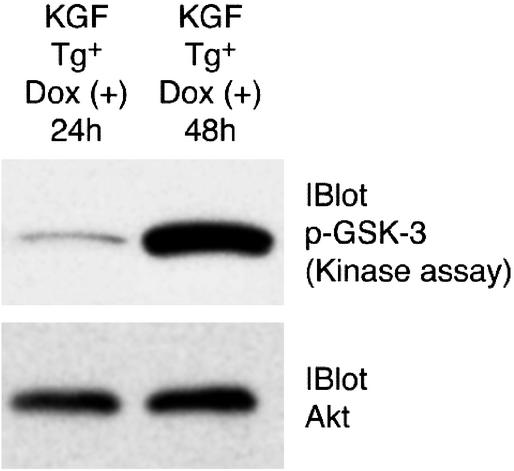
Because multiple studies have shown that KGF protects mice from oxidative lung injury (26, 29–33) and little is known about the mechanisms underlying the protective effects of KGF in vitro or in vivo, we have investigated whether KGF can induce an antiapoptotic axis through the activation of Akt in vivo in the lungs of mice. As shown in Fig. 5, KGF expression after 48 h of Dox treatment resulted in a dramatic increase in Akt activation as evaluated by Akt kinase assay. In these experiments, after Dox induction, lungs were homogenized in a buffer and used for immunoprecipitation of Akt with anti-Akt Ab and the immunoprecipitated Akt was incubated with the substrate glycogen synthase kinase-3 (GSK-3). The phosphorylation of GSK-3 was evident by immunoblotting with anti-phospho-GSK-3α/β(Ser-21/9) Ab (Fig. 5). Uniform Akt immunoprecipitation in both samples was confirmed by releasing the Akt bound to the agarose beads and analysis by immunoblotting by using anti-Akt Ab (Fig. 5B). We have shown that expression of constitutively active Akt in the lungs prevents lung cell death from hyperoxic injury (43). Thus, one possible explanation for growth factor-mediated protection of the lung from hyperoxic injury is activation of the Akt signaling axis to protect from cell death.
Figure 5.
Activation of Akt kinase by KGF in the murine lung. KGF transgene (Tg)-positive animals were supplied with Dox for 24 and 48 h to induce Tg expression. Soluble extract of lung tissue was prepared by using Triton X-100-containing extraction buffer. Equal amounts of soluble extracts were immunoprecipitated with anti-Akt Ab, and the Akt kinase assay was performed by using GSK-3 as substrate. Expression of KGF for 48 h induces Akt kinase activity. In animals with KGF expression for 24 h only basal level of Akt activation was detected as in uninduced controls (data not shown). (Lower) The blot was stripped and reprobed with anti-Akt Ab and shows equal amounts of Akt in both immunoprecipitates. Three animals were included per condition and data shown are representative of two independent experiments.
KGF Activates Akt-Mediated Antiapoptotic Signaling.
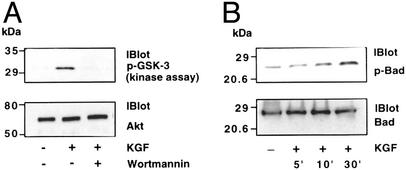
We next investigated activation of the antiapoptotic signaling axis by KGF in lung epithelial cells. In the cell culture studies, we have used both A549 cells, a cell line considered to be the closest representative of human lung alveolar type II epithelial cells (although they do not manifest all of the properties of type II cells) and primary SAE cells to determine the generality of KGF effects on lung epithelial cell types. Both cells undergo hyperoxic cell death, and we have not observed any difference between the two cell types with regard to Akt activation and cellular protection afforded by KGF. Cells were treated with KGF and cell extracts were used for the Akt kinase assay. The Akt kinase assay was performed by immunoprecipitating Akt from cell extracts and using GSK-3 as the substrate. As shown in Fig. 6A with SAE cells, KGF treatment of cells induced Akt kinase activity as revealed by phosphorylation of GSK-3. Furthermore, the KGF-induced Akt activation was inhibited by pretreatment with wortmannin. One of the major downstream substrates of Akt that promotes cell survival is the Bcl-family member Bad (47). Bad phosphorylation has been shown to be associated with multiple pathways that promote cell survival (48–53). Therefore, we investigated whether Akt activation regulated Bad phosphorylation in the lung cells. Cell extracts were immunoprecipitated with anti-Bad Ab and the immunoprecipitates were subjected to Western blot analysis by using Ab against phosphorylated Bad. As shown in Fig. 6B, stimulation by KGF resulted in increased phosphorylation of Bad at Ser-136. Similar results were also obtained by using A549 cells.
Figure 6.
(A) Induction of Akt kinase activity by KGF. Primary SAE cells were incubated for 20 min with KGF (100 ng/ml) in the presence or absence of wortmannin (0.1 μM). Cells were lysed, cell extracts containing equal amounts of protein were immunoprecipitated with anti-Akt Ab, and the kinase activity of Akt in the immunoprecipitates was determined by using GSK-3 as substrate as described in Materials and Methods. (B) Induction of Bad phosphorylation by KGF. Cells were treated with KGF (100 ng/ml) for different lengths of time. Cell extracts were prepared and subjected to immunoprecipitation with anti-Bad Ab. Immunoprecipitates were subjected to Western blot analysis by using anti-phospho-Bad Ab. The blot was stripped and reprobed with anti-Bad Ab. Results are representative of three independent experiments.
Inhibition of Akt Activation by DN Mutant of Akt Blocks the Protective Effect of KGF in Lung Epithelial Cells Subjected to Oxidative Injury.
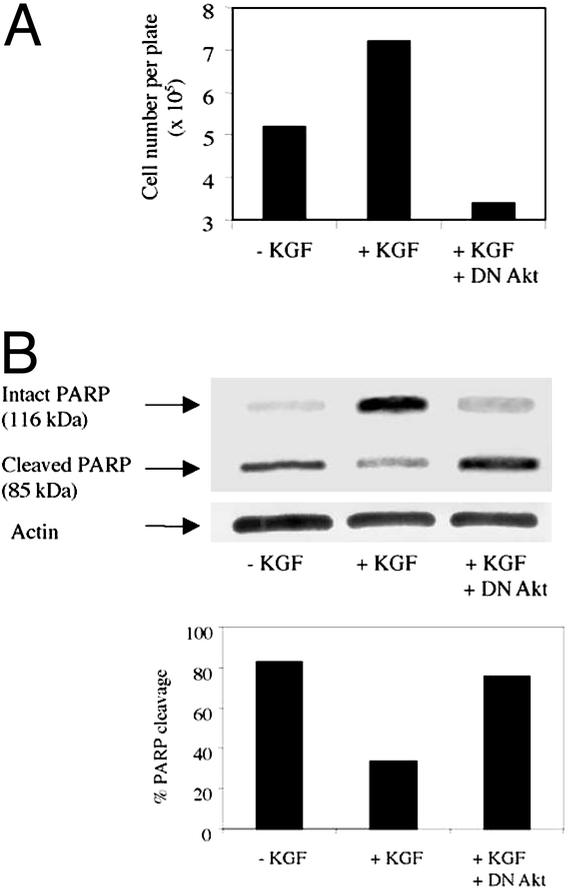
We next investigated whether KGF-induced Akt activation is required for protection from oxidative injury by using SAE cells. SAE cells were grown to subconfluence, and a fraction of the cells were infected at a multiplicity of infection of 1 with either control recombinant adenovirus expressing GFP (middle lane in Fig. 7A) or with recombinant adenovirus expressing a DN mutant of Akt for 24 h before being exposed to hyperoxia. Next, plates containing uninfected cells were placed in normoxia (data not shown) or were exposed to hyperoxia (95% O2/5% CO2) for 72 h. The control virus- and DN Akt-expressing virus-infected cells were exposed to hyperoxia and were also treated with KGF (100 ng/ml). Adherent viable cell number was counted at 72 h after hyperoxic exposure. The culture medium was changed once in between. As shown in Fig. 7A, more adherent cells were recovered when cells exposed to hyperoxia were left in the presence of KGF. The number of viable adherent cells recovered from the KGF-treated plates exposed to hyperoxia matched that obtained from control plates maintained under normoxia (without KGF). The net change in adherent cell numbers between cells exposed to normoxia vs. hyperoxia at this time point, averaging 50% in two independent experiments, corresponded with data obtained in previous studies showing a 25–30% decrease in cell recovery after 4 days of hyperoxic exposure of SAE cells (54). The increased cell number in the presence of KGF was, however, not evident when the KGF-treated cells expressed DN Akt. This result shows that Akt activation is critical to cellular protection conferred by KGF. To substantiate these results further, we investigated the state of the protein PARP, a substrate of caspase-3, in the cells with or without KGF. As shown in Fig. 7B, in the absence of KGF, 70–80% of the PARP was cleaved in cells exposed to hyperoxia. In the presence of KGF, only 25–30% of the PARP was cleaved. However, expression of DN Akt reversed the effect of KGF. Collectively, our data show that epithelial protection from oxidative injury by KGF requires Akt activation.
Figure 7.
Expression of DN Akt in SAE cells blocks KGF-induced protective effects. SAE cells were grown in supplied medium and were infected at a multiplicity of infection of 1 with control adenovirus expressing GFP or with adenovirus expressing DN Akt for 24 h. Cells were starved for 3 h in serum-free medium supplemented with 0.5 g/l BSA and 25 mM Hepes. Uninfected cells were left in the absence of KGF, and control virus- and DN Akt-expressing virus-infected cells were treated with KGF (100 ng/ml). All cells shown were exposed for 72 h to hyperoxic conditions (95% O2/5% CO2). (A) Adherent cell number and cell viability were assessed by using trypan blue. (B) PARP cleavage was assessed as indicator of cell apoptosis by Western blotting techniques. Membranes were probed with anti-PARP Ab, stripped, and reprobed with anti-actin Ab to demonstrate equivalent loading of protein in all lanes. Densitometric analysis of the PARP bands was performed, and the ratio of intensity of cleaved band to the total intensity (cleaved + uncleaved bands) × 100 was estimated as percentage of PARP cleavage. Similar results were obtained in two independent experiments.
Discussion
We described the establishment of an inducible lung-specific transgenic system, adapted from the Tet system of Gossen et al. (40), which allows us to express transgenes in an externally regulatable and tissue-specific fashion (38). The versatility of this system is evident in our ability to express cytokines (38), chemokines (55), and transcription factors (46) in an inducible fashion in different tissues such as the lung by using the CC10 promoter or in the thymus and peripheral T cells by using the lck promoter (46). Although in our original system (38, 55) the transgene could be efficiently induced by supplying Dox to the animals, some basal expression of the transgene was detected in most transgenic lines, which entailed screening of a large number of animals to obtain lines that displayed low levels of basal transgene expression (i.e., without Dox). Because our objective in this study was to study the effects of KGF, it was important to develop a system that was more stringently regulated, because inappropriate expression of KGF during development was shown to cause embryonic lethality with extensive lung malformation (7). The modified dual repressor-activator Tet system described in this study shows tight control of transgene expression as evidenced by the lack of any RNA expression corresponding to the transgene even after 30 cycles of RT-PCR. We show that transgene expression is efficiently induced in the presence of Dox and that the induction of KGF expression inhibits oxidant-induced lung cell death. Furthermore, KGF induces the activation of the prosurvival Akt signaling axis both in vivo and in vitro.
Administration of KGF has been shown to protect the lung from a variety of insults including oxygen (26, 29, 33), radiation and chemotherapy (bleomycin; refs. 31, 56, and 57), and acid (30). In bleomycin-induced lung injury, KGF was shown to decrease lung edema (57). Matthay and Wiener-Kronish (58) showed that intact epithelial barrier function is critical for resolution of alveolar edema in humans. It has been shown that pretreatment with KGF prevents H2O2-induced increases in airway epithelial cell permeability (35). Because oxidant-induced cell death would compromise epithelial barrier function, inhibition of cell death by KGF, as evident in this study, may play an important role in maintaining epithelial barrier function during the initial phase of oxidative lung injury.
Although KGF can induce type II cell proliferation (26–28), this proliferative effect of KGF was abolished in oxygen-breathing animals but KGF was still able to inhibit alveolar damage (33). This finding demonstrates that the protective effect of KGF within the lung cannot simply be explained by the ability of KGF to stimulate type II cell proliferation. Along the same lines, it was shown recently that deficiency of FGFR2-IIIb, the receptor for KGF (FGF7) and FGF10, leads to embryonic lethality associated with lack of limb bud growth, which is mainly due to extensive epithelial cell apoptosis with an apparent small reduction in cell proliferation (59). Also, although in vitro studies have shown increased surfactant protein synthesis in the presence of KGF, only modest effects were noted in in vivo studies (33). Collectively, although multiple studies clearly demonstrate a protective role of KGF in hyperoxic alveolar damage, it seems that the protective function of KGF can be attributed to different mechanisms primary or secondary to KGF action. Our studies invoke the participation of the multifunctional phosphatidylinositol 3-kinase-activated Akt signaling axis that may be upstream of many of these effects of KGF. KGF-induced Akt activation may be the common underlying mechanism of cytoprotection conferred by KGF on epithelial cells in different tissues, including the skin, where it also plays an important role in the reepithelialization of wounds (60) and in the thymus (61).
Although previous studies have shown that large doses of KGF protect both the alveolar epithelium and endothelium from oxygen-induced injury (33), we found preservation of the alveolar epithelium but not the endothelium lining the blood vessels in the lung at the level of KGF expression that was attained in the transgenic animals (5–10 ng per mouse, which is equivalent to 0.2–0.4 μg/kg). This finding is in accordance with expression of the KGF receptor on epithelial but not on endothelial cells. The endothelial protection by KGF that was observed in the previous study at significantly higher doses of KGF (1–5 mg/kg) was likely due to secondary effects of KGF such as stimulation of secretion of vascular endothelial growth factor (VEGF), which is cytoprotective for endothelial cells. Interestingly, the cytoprotective effect of VEGF was also reported to involve Akt activation (23, 62).
We have shown recently that expression of a constitutively active derivative of Akt in the mouse lung by adenovirus gene transfer methods inhibits lung cell death and protects lungs from oxidant-induced injury (43). It is possible that in addition to direct effects on cellular apoptosis, alternative mechanisms that promote survival through influencing cellular metabolism also contribute to the protective function of Akt. Interestingly, it has been shown that activated Akt protects against oxidative stress-induced impairment of glucose transport by insulin (63). This function of activated Akt could be an important player in the protective function of KGF in oxidative stress. Thus, both in in vitro and in in vivo studies using an improved inducible transgenic system, we have identified a downstream target of KGF action, Akt, which may play an important role in the protective function of KGF in oxidative injury.
Supplementary Material
Acknowledgments
We thank Drs. M. Gossen and H. Bujard (University of Heidelberg, Heidelberg) for the plasmid pUHD172-neo containing the rtTR, Dr. W. Hillen (University of Erlangen, Erlangen, Germany) for the plasmid pCMV-TetR(B/E)-KRAB encoding TetR, Dr. J. Whitsett (University of Cincinnati, Cincinnati) for the plasmid pCC10CAT-2300 containing the CC10 promoter and for helpful suggestions, L. Parkyn for excellent assistance with breeding and maintenance of transgenic mice, Drs. K. Walsh and Y. Kureishi (Boston University, Boston) for the DN mutant of Akt, Dr. J. S. Rubin (National Cancer Institute, Bethesda) for KGF cDNA, and Dr. R. Mason for helpful suggestions. This work was supported by National Institutes of Health Grants HL 69810 and HL 60207 (to P.R.) and CA 76541 (to D.B.S.).
Abbreviations
- KGF
keratinocyte growth factor
- Tet
tetracycline
- Dox
doxycycline
- tTR
Tet transrepressor
- rtTA
reverse Tet transactivator
- TUNEL
terminal deoxynucleotidyltransferase-mediated dUTP end labeling
- O/P
operator/promoter
- GSK-3
glycogen synthase kinase 3
- SAE
small-airway epithelial
- DN
dominant-negative
References
- 1.Rubin J S, Osada H, Finch P W, Taylor W G, Rudikoff S, Aaronson S A. Proc Natl Acad Sci USA. 1989;86:802–806. doi: 10.1073/pnas.86.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finch P W, Cunha G R, Rubin J S, Wong J, Ron D. Dev Dyn. 1995;203:223–240. doi: 10.1002/aja.1002030210. [DOI] [PubMed] [Google Scholar]
- 3.Finch P W, Rubin J S, Miki T, Ron D, Aaronson S A. Science. 1989;245:752–755. doi: 10.1126/science.2475908. [DOI] [PubMed] [Google Scholar]
- 4.Miki T, Fleming T P, Bottaro D P, Rubin J S, Ron D, Aaronson S A. Science. 1991;251:72–75. doi: 10.1126/science.1846048. [DOI] [PubMed] [Google Scholar]
- 5.Cardoso W V, Itoh A, Nogawa H, Mason I, Brody J S. Dev Dyn. 1997;208:398–405. doi: 10.1002/(SICI)1097-0177(199703)208:3<398::AID-AJA10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 6.Peters K, Werner S, Liao X, Wert S, Whitsett J, Williams L. EMBO J. 1994;13:3296–3301. doi: 10.1002/j.1460-2075.1994.tb06631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simonet W S, DeRose M L, Bucay N, Nguyen H Q, Wert S E, Zhou L, Ulich T R, Thomason A, Danilenko D M, Whitsett J A. Proc Natl Acad Sci USA. 1995;92:12461–12465. doi: 10.1073/pnas.92.26.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellacosa A, Chan T O, Ahmed N N, Datta K, Malstrom S, Stokoe D, McCormick F, Feng J, Tsichlis P. Oncogene. 1998;17:313–325. doi: 10.1038/sj.onc.1201947. [DOI] [PubMed] [Google Scholar]
- 9.Franke T F, Yang S I, Chan T O, Datta K, Kazlauskas A, Morrison D K, Kaplan D R, Tsichlis P N. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 10.Kulik G, Klippel A, Weber M J. Mol Cell Biol. 1997;17:1595–1606. doi: 10.1128/mcb.17.3.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dudek H, Datta S R, Franke T F, Birnbaum M J, Yao R, Cooper G M, Segal R A, Kaplan D R, Greenberg M E. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 12.Franke T F, Kaplan D R, Cantley L C. Cell. 1997;88:435–437. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- 13.Burgering B M, Coffer P J. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 14.Vanhaesebroeck B, Alessi D R. Biochem J. 2000;346:561–576. [PMC free article] [PubMed] [Google Scholar]
- 15.Kohn A D, Takeuchi F, Roth R A. J Biol Chem. 1996;271:21920–21926. doi: 10.1074/jbc.271.36.21920. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed N N, Grimes H L, Bellacosa A, Chan T O, Tsichlis P N. Proc Natl Acad Sci USA. 1997;94:3627–3632. doi: 10.1073/pnas.94.8.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kennedy S G, Wagner A J, Conzen S D, Jordan J, Bellacosa A, Tsichlis P N, Hay N. Genes Dev. 1997;11:701–713. doi: 10.1101/gad.11.6.701. [DOI] [PubMed] [Google Scholar]
- 18.Kauffmann-Zeh A, Rodriguez-Viciana P, Ulrich E, Gilbert C, Coffer P, Downward J, Evan G. Nature. 1997;385:544–548. doi: 10.1038/385544a0. [DOI] [PubMed] [Google Scholar]
- 19.Hemmings B A. Science. 1997;275:628–630. doi: 10.1126/science.275.5300.628. [DOI] [PubMed] [Google Scholar]
- 20.Moelling K, Schad K, Bosse M, Zimmermann S, Schweneker M. J Biol Chem. 2002;277:31099–31106. doi: 10.1074/jbc.M111974200. [DOI] [PubMed] [Google Scholar]
- 21.Ingram D A, Hiatt K, King A J, Fisher L, Shivakumar R, Derstine C, Wenning M J, Diaz B, Travers J B, Hood A, et al. J Exp Med. 2001;194:57–69. doi: 10.1084/jem.194.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin H K, Yeh S, Kang H Y, Chang C. Proc Natl Acad Sci USA. 2001;98:7200–7205. doi: 10.1073/pnas.121173298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gratton J P, Morales-Ruiz M, Kureishi Y, Fulton D, Walsh K, Sessa W C. J Biol Chem. 2001;276:30359–30365. doi: 10.1074/jbc.M009698200. [DOI] [PubMed] [Google Scholar]
- 24.Jamieson C A, Yamamoto K R. Proc Natl Acad Sci USA. 2000;97:7319–7324. doi: 10.1073/pnas.97.13.7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zimmermann S, Moelling K. Science. 1999;286:1741–1744. doi: 10.1126/science.286.5445.1741. [DOI] [PubMed] [Google Scholar]
- 26.Panos R J, Rubin J S, Csaky K G, Aaronson S A, Mason R J. J Clin Invest. 1993;92:969–977. doi: 10.1172/JCI116673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ulich T R, Yi E S, Longmuir K, Yin S, Biltz R, Morris C F, Housley R M, Pierce G F. J Clin Invest. 1994;93:1298–1306. doi: 10.1172/JCI117086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tichelaar J W, Lu W, Whitsett J A. J Biol Chem. 2000;275:11858–11864. doi: 10.1074/jbc.275.16.11858. [DOI] [PubMed] [Google Scholar]
- 29.Panos R J, Bak P M, Simonet W S, Rubin J S, Smith L J. J Clin Invest. 1995;96:2026–2033. doi: 10.1172/JCI118250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yano T, Deterding R R, Simonet W S, Shannon J M, Mason R J. Am J Respir Cell Mol Biol. 1996;15:433–442. doi: 10.1165/ajrcmb.15.4.8879176. [DOI] [PubMed] [Google Scholar]
- 31.Yi E S, Williams S T, Lee H, Malicki D M, Chin E M, Yin S, Tarpley J, Ulich T R. Am J Pathol. 1996;149:1963–1970. [PMC free article] [PubMed] [Google Scholar]
- 32.Werner S. Cytokine Growth Factor Rev. 1998;9:153–165. doi: 10.1016/s1359-6101(98)00010-0. [DOI] [PubMed] [Google Scholar]
- 33.Barazzone C, Donati Y R, Rochat A F, Vesin C, Kan C D, Pache J C, Piguet P F. Am J Pathol. 1999;154:1479–1487. doi: 10.1016/S0002-9440(10)65402-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brauchle M, Angermeyer K, Hubner G, Werner S. Oncogene. 1994;9:3199–3204. [PubMed] [Google Scholar]
- 35.Waters C M, Savla U, Panos R J. Am J Physiol. 1997;272:L681–L689. doi: 10.1152/ajplung.1997.272.4.L681. [DOI] [PubMed] [Google Scholar]
- 36.Borok Z, Danto S I, Dimen L L, Zhang X L, Lubman R L. Am J Physiol. 1998;274:L149–L158. doi: 10.1152/ajplung.1998.274.1.L149. [DOI] [PubMed] [Google Scholar]
- 37.Wu K I, Pollack N, Panos R J, Sporn P H, Kamp D W. Am J Physiol. 1998;275:L780–L787. doi: 10.1152/ajplung.1998.275.4.L780. [DOI] [PubMed] [Google Scholar]
- 38.Ray P, Tang W L, Wang P, Homer R, Kuhn C, III, Flavell R A, Elias J A. J Clin Invest. 1997;100:2501–2511. doi: 10.1172/JCI119792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forster K, Helbl V, Lederer T, Urlinger S, Wittenburg N, Hillen W. Nucleic Acids Res. 1999;27:708–710. doi: 10.1093/nar/27.2.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gossen M, Freundlieb S, Bender G, Muller G, Hillen W, Bujard H. Science. 1995;268:1766–1769. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- 41.Ray P, Higgins K M, Tan J C, Chu T Y, Yee N S, Nguyen H, Lacy E, Besmer P. Genes Dev. 1991;5:2265–2273. doi: 10.1101/gad.5.12a.2265. [DOI] [PubMed] [Google Scholar]
- 42.Nocka K, Majumder S, Chabot B, Ray P, Cervone M, Bernstein A, Besmer P. Genes Dev. 1989;3:816–826. doi: 10.1101/gad.3.6.816. [DOI] [PubMed] [Google Scholar]
- 43.Lu Y B, Parkyn L, Otterbein L, Kureishi Y, Walsh K, Ray A, Ray P. J Exp Med. 2001;193:545–549. doi: 10.1084/jem.193.4.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ray P, Yang L, Zhang D H, Ghosh S, Ray A. J Biol Chem. 1997;272:20191–20197. doi: 10.1074/jbc.272.32.20191. [DOI] [PubMed] [Google Scholar]
- 45.Yang L, Cohn L, Zhang D H, Homer R, Ray A, Ray P. J Exp Med. 1998;188:1739–1750. doi: 10.1084/jem.188.9.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang D H, Yang L, Cohn L, Parkyn L, Homer R, Ray P, Ray A. Immunity. 1999;11:473–482. doi: 10.1016/s1074-7613(00)80122-3. [DOI] [PubMed] [Google Scholar]
- 47.Datta S R, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg M E. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 48.She Q B, Ma W Y, Zhong S, Dong Z. J Biol Chem. 2002;277:24039–24048. doi: 10.1074/jbc.M109907200. [DOI] [PubMed] [Google Scholar]
- 49.Gnesutta N, Qu J, Minden A. J Biol Chem. 2001;276:14414–14419. doi: 10.1074/jbc.M011046200. [DOI] [PubMed] [Google Scholar]
- 50.Scheid M P, Schubert K M, Duronio V. J Biol Chem. 1999;274:31108–31113. doi: 10.1074/jbc.274.43.31108. [DOI] [PubMed] [Google Scholar]
- 51.Tan Y, Demeter M R, Ruan H, Comb M J. J Biol Chem. 2000;275:25865–25869. doi: 10.1074/jbc.M004199200. [DOI] [PubMed] [Google Scholar]
- 52.Harada H, Becknell B, Wilm M, Mann M, Huang L J, Taylor S S, Scott J D, Korsmeyer S J. Mol Cell. 1999;3:413–422. doi: 10.1016/s1097-2765(00)80469-4. [DOI] [PubMed] [Google Scholar]
- 53.Bonni A, Brunet A, West A E, Datta S R, Takasu M A, Greenberg M E. Science. 1999;286:1358–1362. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- 54.Jyonouchi H, Sun S, Abiru T, Chareancholvanich S, Ingbar D H. Am J Respir Cell Mol Biol. 1998;19:426–436. doi: 10.1165/ajrcmb.19.3.2862m. [DOI] [PubMed] [Google Scholar]
- 55.Pan Z Z, Parkyn L, Ray A, Ray P. Am J Lung Cell Mol Biol Physiol. 2000;279:L658–L666. doi: 10.1152/ajplung.2000.279.4.L658. [DOI] [PubMed] [Google Scholar]
- 56.Guo J, Yi E S, Havill A M, Sarosi I, Whitcomb L, Yin S, Middleton S C, Piguet P, Ulich T R. Am J Physiol. 1998;275:L800–L805. doi: 10.1152/ajplung.1998.275.4.L800. [DOI] [PubMed] [Google Scholar]
- 57.Yi E S, Salgado M, Williams S, Kim S J, Masliah E, Yin S, Ulich T R. Inflammation. 1998;22:315–325. doi: 10.1023/a:1022304317111. [DOI] [PubMed] [Google Scholar]
- 58.Matthay M A, Wiener-Kronish J P. Am Rev Respir Dis. 1990;142:1250–1257. doi: 10.1164/ajrccm/142.6_Pt_1.1250. [DOI] [PubMed] [Google Scholar]
- 59.Revest J-M, Spencer-Dene B, Kerr K, De Moerlooze L, Rosewell I, Dickson C. Dev Biol. 2001;231:47–62. doi: 10.1006/dbio.2000.0144. [DOI] [PubMed] [Google Scholar]
- 60.Werner S, Smola H, Liao X, Longaker M T, Krieg T, Hofschneider P H, Williams L T. Science. 1994;266:819–822. doi: 10.1126/science.7973639. [DOI] [PubMed] [Google Scholar]
- 61.Rossi S, Blazar B R, Farrell C L, Danilenko D M, Lacey D L, Weinberg K I, Krenger W, Hollander G A. Blood. 2002;100:682–691. doi: 10.1182/blood.v100.2.682. [DOI] [PubMed] [Google Scholar]
- 62.Fujio Y, Walsh K. J Biol Chem. 1999;274:16349–16354. doi: 10.1074/jbc.274.23.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rudich A, Tirosh A, Potashnik R, Khamaishi M, Bashan N. Diabetologia. 1999;42:949–957. doi: 10.1007/s001250051253. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.