Abstract
Nifedipine, a drug used for treatment of hypertension and angina, exerts its effect by calcium channel blockade and nitric oxide production. We report here a previously uncharacterized action of nifedipine on central synaptic transmission that may partially explain its side effects. Nifedipine causes a long-lasting facilitation of tetrodotoxin-insensitive spontaneous glutamate release. This effect is independent of its L-type calcium channel blocking effect, and is not mimicked by other dihydropyridines such as nimodipine, nicardipine, or Bay K 8644. The effect was dose dependent, with EC50 of 7.8 μM, with the lowest effective dose being 100 nM, a clinically relevant dose. At 10 μM, the increase is 14.7-fold. This effect is largely calcium-independent, because Cd2+, thapsigargin, or BAPTA-AM [1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid-acetoxymethyl ester] did not inhibit the nifedipine effect. Thus, nifedipine seems to act on the release process downstream of calcium entry or release. Protein kinases A or C do not mediate its effect, because it is not blocked by inhibitors of these kinases. Our finding indicates that nifedipine may be a useful tool as a secretagogue to directly target the release process, but raises caution for its use as an L-type calcium channel blocker.
Nifedipine has been a commonly prescribed compound for treatment of angina and hypertension. Its clinical effect is attributed to its blocking action on L-type calcium channels or release of nitric oxide (NO) from the vascular endothelium, which will result in relaxation or prevention of cardiac or vascular smooth muscle contraction. Compared with other dihydropyridines (DHPs), nifedipine has been reported to have relatively high incidence of neurologic adverse reactions, such as dizziness (4.1–27%) and nervousness (>7.0%) (information obtained from www.drugdigest.org/DD/SE/DisplayDrug/1,3997,488,00.html?DVHName=Nifedipine). Nifedipine can easily cross the brain–blood barrier (1); thus, it may have a direct effect in the brain. The present report introduces a previously uncharacterized action of nifedipine on synaptic transmission in the central nervous system. Nifedipine induces a profound increase in spontaneous glutamate release in a calcium-independent manner. This effect was unique to nifedipine and could not be mimicked by other DHPs; thus, its action is not through blockade of L-type calcium channels. Such synaptic activation in the central nervous system may underlie some of its adverse neurologic reactions.
Spontaneous, action potential-independent transmitter release occurs when a synaptic vesicle fuses spontaneously to the presynaptic plasma membrane and releases its content. It is not yet clear what may be the role of spontaneous release for the function of the nervous system. Recent studies indicate that spontaneously released glutamate acts to maintain the postsynaptic dendritic structure (2) and to induce clustering of AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptors on the postsynaptic membrane (3), possibly brought about by triggering spontaneous calcium transients in the postsynaptic structure (4). Spontaneous release can also have transient impact on the electrical activity of the postsynaptic cells (5). Although it has been considered to be a stochastic event, finding of possible physiological functions of spontaneous release may suggest an existence of regulatory mechanisms. A number of secretagogues promote spontaneous transmitter release from nerve terminals independently of action potential-triggered calcium influx through voltage-dependent calcium channels (VDCCs). These secretagogues include hypertonic solution, α-latrotoxin, and ruthenium red (6–8). Although their mechanisms of action remain largely unknown, they have been found useful for investigation of transmitter release processes downstream of calcium entry. The present study suggests that nifedipine may be used as another agent for the study of release process.
Methods
Slice Preparation.
All experiments were carried out in accordance with the Canadian Council on Animal Care guidelines and were approved by the University of Calgary Animal Care Committee.
Adult male Sprague–Dawley rats (150–250 g) were decapitated under halothane anesthesia, and the brain was removed to generate 200- to 300-μm-thick coronal brain slices at 0°C in a buffer solution (in mM): 87 NaCl, 2.5 KCl, 1.25 NaH2PO4, 7 MgCl2, 0.5 CaCl2, 25 NaHCO3, 25 glucose, and 20 sucrose. Slices were then incubated at 32–34°C in artificial cerebrospinal fluid (ACSF) (in mM): 126 NaCl, 2.5 KCl, 1.2 NaH2PO4, 1.2 MgCl2, 2.4 CaCl2, 18 NaHCO3, and 11 glucose. Both solutions were continuously bubbled with O2 (95%) and CO2 (5%).
Electrophysiological Recording.
ACSF submerged slices were visualized by a differential interference contrast microscopy (DIC)-IR microscope, and nystatin-perforated patch whole cell recording (series/access resistance: 10–40 MΩ) was performed at 30–32°C with electrodes having tip resistance of 3–7 MΩ. The internal recording solution (pH 7.3) contained (in mM): 120 potassium-acetate, 5 MgCl2, 10 EGTA, and 40 Hepes. Nystatin was dissolved in DMSO with Pluronic F127 and added to the internal solution (final concentration: 450 μg/ml). All experiments were performed on magnocellular neurons (MCNs) in the supraoptic nucleus (SON), voltage clamped at −80 mV by using an Axopatch 1D amplifier (Axon Instruments, Foster City, CA). MCNs were identified based on the delayed onset to action potential generation in response to positive current injection (9, 10). Membrane currents were recorded without series resistance compensation, filtered at 1 kHz, and digitized at 2–10 kHz and stored for off-line analysis. Input resistance and series/access resistance were monitored regularly throughout each experiment by applying 20-mV, 75-ms hyperpolarizing pulses and recording current responses; i.e., steady-state current and decay rate (τ) of the capacitance transient. Cells that showed >15% change in these parameters were excluded from further analysis. γ-Aminobutyric acid type A receptor antagonist, picrotoxin 50 μM, was added to ACSF to pharmacologically isolate excitatory postsynaptic currents (EPSCs). A bipolar tungsten-stimulating electrode was placed in the hypothalamic region dorsal-medial to the SON to evoke synaptic responses, and a stimulus intensity giving 50–60% of the maximum evoked response was used. All data were acquired by using pclamp (Clampex 7; Axon Instruments). Hard copy chart records were also captured on a Gould (Cleveland) Recorder.
Data Analysis.
Spontaneous or miniature EPSCs (mEPSCs) were detected by using minianalysis software (Synaptosoft, Decatur, GA) and counted if amplitude was larger than four to five times the rms noise with fast rise times (1–4 ms measured from baseline to peak) and exponential decay. For amplitude measurement, only events with a clearly defined preceding baseline (>3 ms) were used. Amplitude-distribution histograms of mEPSCs were fitted with either one or the sum of several Gaussian curves, by simplex nonlinear least-squares algorithm. The quantal coefficient of variation (c.v.) of mEPSC amplitude was calculated as (variance of amplitudes in smallest peak − noise variance)1/2/smallest peak amplitude × 100 (%), assuming that noise variance stays constant independently of the quantal variance and added linearly (11). Every cell served as its own control for testing drug effects. Kolomogorov–Smirnov test and Student's paired t test were used for statistical analysis. P < 0.05 was taken as significant. All values are stated as mean ± standard error.
Drugs.
All drugs were bath perfused at final concentrations as indicated, by diluting aliquots of 1,000× stock in the ACSF, immediately before use. DHP solutions were foil covered due to their photolability (12). The final concentration of DMSO used as a vehicle (≦0.1%) had no effect on the mEPSC frequency by itself (101.8 ± 20.7% of control; P > 0.05, n = 3). The experimental setup was kept in the dark while DHPs were being applied to the brain slices during recordings. Thirty micromolar and 100 μM nifedipine was prepared by making 1,000× stock solution in DMSO immediately before diluting it into ACSF to minimize precipitation. For application of Cd2+, NaH2PO4 in ACSF was substituted by equimolar NaCl to prevent precipitation.
Nifedipine was obtained from Sigma and Tocris Cookson (Ellisville, MO); phorbol 12-myristate 13-acetate from Tocris Cookson; thapsigargin from Alomone Labs (Jerusalem); CdSO4 from Fisher Scientific; and Pluronic F127 from BASF Bioresearch (Cambridge, MA). All other drugs were from Sigma.
Results
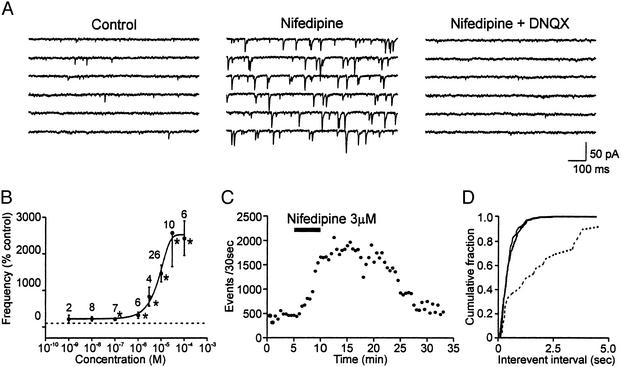
Application of nifedipine induced a profound increase in frequency of spontaneous EPSCs in 79.9% (107/134) of the cells tested (100 nM to 100 μM; Fig. 1), without a significant change in the input resistance of the recorded postsynaptic cell (737.8 ± 198.0 MΩ vs. 735.0 ± 190.4 MΩ; P > 0.05, n = 5). The nifedipine effect was dose dependent, with EC50 = 7.8 μM (Fig. 1B). A significant increase in spontaneous EPSC frequency could be detected with concentration as low as 100 nM (213.7 ± 34.5% of control; P < 0.05, n = 7). At 10 nM, although the effect was statistically insignificant as a group (223.0 ± 62.8%; P > 0.05, n = 8), 3 of 8 cells showed a clear response (P < 0.05 by Kolomogorov–Smirnov test), up to a 6-fold increase. Ten to 20 μM was used for all of the following experiments. The latency of the effect varied from 2 to 15 min, with the mean of 6.4 ± 0.6 min (n = 33; Fig. 1C). On washout of the agent, it took 5–23 min to return to baseline level in 14 cells in which washout kinetics were followed, and, in 1 cell, it lasted for the duration of the recording (up to 60 min; Fig. 1C). Cells that showed recovery responded repeatedly to nifedipine. Most of these EPSCs were action potential-independent mEPSCs as shown by their insensitivity to tetrodotoxin (1 μM; P > 0.05, n = 7; Fig. 1D). These mEPSCs were mediated by non-N-methyl-d-aspartate receptors because they were abolished by 6,7-dinitroquinoxaline-2,3-dione (DNQX; 10 μM, P < 0.05, n = 6; Fig. 1A).
Figure 1.
Nifedipine induces increases in the frequency of miniature postsynaptic currents. (A) Sample EPSC traces recorded from a typical supraoptic neuron. Holding potential, −80 mV. Nifedipine increased mEPSCs (Middle), which were abolished by DNQX (Right). (B) Dose–response curve of nifedipine effect on the frequency of mEPSCs. Numbers above each data point indicate number of cells tested. *, P < 0.05 compared with control (pretreatment) by Student's paired t test. (C) Time–effect plot of the frequency of mEPSCs in a representative cell. (D) Cumulative plot of interevent interval of EPSCs. Dashed line, control; solid lines, in the presence of nifedipine or nifedipine and tetrodotoxin.
This effect of nifedipine was not selective to SON excitatory synapses because similar facilitation of mEPSC frequency was also observed in other brain areas such as paraventricular nucleus, suprachiasmatic nucleus, dorsomotor nucleus of the vagus, and nucleus accumbens (data not shown). In addition, miniature inhibitory postsynaptic currents recorded in the SON were similarly facilitated.
This effect was replicated with two different lots of nifedipine from Sigma, and another purchased from Tocris Cookson, suggesting that it is indeed an effect unique to nifedipine. Contamination of nifedipine by its photodegraded product, 2-nitroso-pyridine, is also improbable, because light-illuminated nifedipine did not have any effect. Nifedipine stock solution was left under a desk light for 24 h, a procedure shown to degrade nifedipine (13). This procedure abolished the facilitatory effect of nifedipine (119.0 ± 33.6% of control; P > 0.05, n = 4).
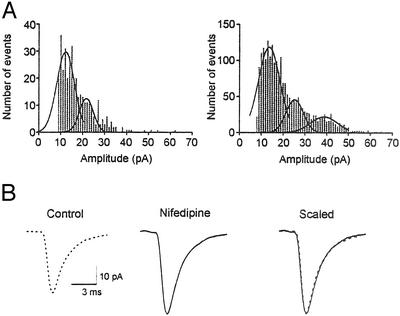
Nifedipine application increased not only the frequency of mEPSCs but also, to a lesser effect, their mean amplitude (19.4 ± 4.6 pA vs. 26.1 ± 5.8 pA; P < 0.05, n = 10, Figs. 1A and 2A). This finding may indicate both a pre- and postsynaptic effect. However, large miniature events may also occur if the spontaneous release is not uniquantal (5, 14). If the amplitude increase was due to postsynaptic change, the peak of mEPSC amplitude distribution should shift to the right, leaving the relative distribution unchanged. The largest peak, however, remained the same with the distribution more skewed to the right, with increased number of roughly equidistant peaks in the presence of nifedipine (Fig. 2A). In control condition, mEPSC amplitude distribution was best fitted by one to three Gaussian curves, with mean smallest peak amplitude of 15.0 ± 1.2 pA, coefficient of variation (c.v.) = 14.5 ± 1.0%. In the presence of nifedipine, two to four Gaussian curves could be best fitted to mEPSC amplitude distribution, with mean smallest peak amplitude of 15.2 ± 1.9 pA, c.v. = 14.5 ± 0.5% (P > 0.05 vs. control, n = 5). Thus, the apparent increase in mean amplitude may reflect multiquantal release. Another possibility is an increase in the size of individual quanta, also a presynaptic change.
Figure 2.
Nifedipine effects on the amplitude of mEPSCs. (A) Amplitude distribution histograms of a representative cell in control (Left) and in the presence of nifedipine (Right). (B) Averaged (46–50 events) mEPSC waveforms in control and in nifedipine. Scaled and superimposed traces (Right) show that the time course of the events has not changed.
Whereas the above results seem to indicate the presynaptic origin of increased amplitude, changes in AMPA receptor kinetics or numbers cannot be excluded. However, no detectable change was observed in mEPSC kinetics, i.e., rise and decay times (Fig. 2B). In addition, in contrast to the amplitude increase in mEPSCs, current induced by brief application of AMPA was decreased by nifedipine (89.5 ± 3.2% control; P < 0.05, n = 4). Such change was considered to be due to an effect on postsynaptic L-type calcium channels, because nicardipine had a similar effect on postsynaptic AMPA currents (76.3 ± 8.8% control; P < 0.05, n = 5). Therefore, it is unlikely that changes in kinetics or numbers of AMPA receptors underlie the increase in mEPSC amplitude or could be responsible for increased frequency due to altered ability to detect more events.
Taken together, these data suggest that the nifedipine effect is mainly on excitatory presynaptic terminals to induce increase in glutamate release. Because the mEPSC frequency is a sensitive measure of presynaptic modulation, the remainder of the study deals with the frequency of mEPSCs.
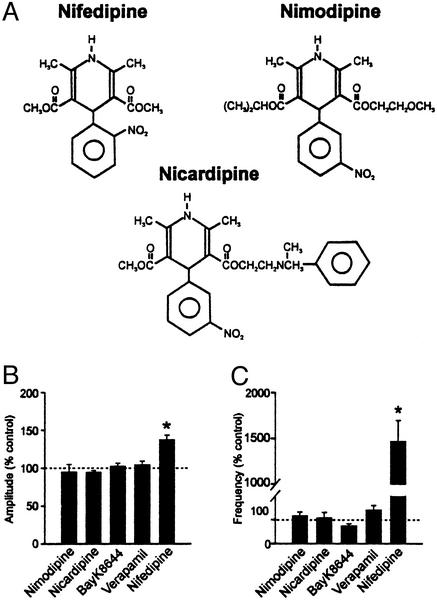
If nifedipine is acting on L-type calcium channels to induce this massive increase in mEPSCs, other compounds that affect these channels could be expected to mimic its effect. The following compounds that are known to bind to L-type channels were tested for their potency in affecting the mEPSCs: nimodipine (20–50 μM, n = 7) and nicardipine (10–20 μM, n = 13), DHPs structurally and functionally similar to nifedipine (Fig. 3A); Bay K 8644, a DHP that activates L-type channels (10 μM, n = 6); and verapamil, a phenylalkylamine that blocks L-type channels (50 μM, n = 5). As shown in Fig. 3B, none of these L-type calcium channel modulators had a significant effect on the amplitude (Fig. 3B) or frequency (Fig. 3C) of mEPSCs (P > 0.05) when applied for more than 10 min. Interestingly, when the slice was pretreated with nicardipine (10–20 μM) for 5–7 min before nifedipine challenge, the stimulatory effect of nifedipine was still observed (1,209.3 ± 421.5% of control, n = 7; P < 0.05). This result suggests that nifedipine acts on a site distinct to a conventional DHP binding site to induce this unique effect.
Figure 3.
Effect of different L-type calcium channel modulators on mEPSCs. (A) Chemical structures of DHP class L-type channel blockers. (B) Effect of L-type channel modulators on mEPSC amplitude. (C) Effect of L-type channel modulators on mEPSC frequency. *, P < 0.05 vs. control.
It has been found in the preoptic area that DHPs, including nifedipine, induce miniature inhibitory postsynaptic current (mIPSCs) by causing inhibition of calcium-activated potassium conductance. This effect was mimicked by BK or SK channel blockers (15). Although, in the present study, other L-type channel modulators failed to induce an effect similar to nifedipine, the possibility remains that a class of channels highly sensitive to nifedipine exist in the presynaptic terminals in the SON. Subclasses of L-type channels showing different sensitivities to different DHPs have been reported (16). However, such a mechanism cannot explain the effect observed in the SON because direct blockade of BK or SK by their specific blockers, iberiotoxin (100 nM, 125.2 ± 35.6% of control; P > 0.05, n = 4) or apamin (1 μM, 115.7 ± 6.5% of control; P > 0.05, n = 6), respectively, did not alter the frequency of mEPSCs.
Effects of nifedipine unrelated to its calcium channel blocking property have been previously observed (17). For example, nifedipine is known to interact with the adenosine system by acting as an antagonist to its receptor and/or interfering with its uptake system at a micromolar potency (18). It is possible that the massive increase in mEPSC frequency induced by nifedipine is due to disinhibition of inhibitory modulation by adenosine (19). In that case, blocking endogenous adenosine by an antagonist should mimic the nifedipine effect. However, an adenosine receptor antagonist 8-(p-sulfophenyl)theophylline (8-SPT; 10 μM) had no effect on the frequency of mEPSCs (115.9 ± 7.6% of control; P > 0.05, n = 3).
In some preparations, nifedipine has been shown to induce production of NO (20). To examine whether NO mediates the effect of nifedipine, an NO synthase inhibitor, NG-nitro-l-arginine methyl ester (l-NAME) was tested. Application of l-NAME (100 μM) did not block the facilitatory effect of nifedipine on mEPSCs (1,481.5 ± 529.2% of control; P > 0.05, n = 5). In addition, a NO donor, sodium nitroprusside (10 μM), did not increase mEPSC frequency (70.3 ± 9.4% of control; P > 0.05, n = 7), which is consistent with a previous report (21), thus excluding NO as the mediator. All these results show that none of the above previously known effects of nifedipine, which might alter transmitter release, are involved in this effect.
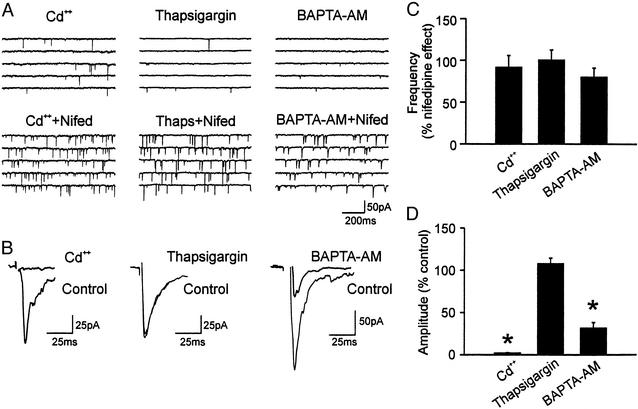
Elevated intracellular calcium level has been observed to increase spontaneous exocytosis in a number of preparations (6, 22, 23). Thus, one possible explanation of the nifedipine effect is that intraterminal calcium concentration is elevated. Major sources of intraterminal calcium elevation are extracellular calcium through VDCCs and release from intracellular stores. When cells were pretreated with Cd2+ (200 μM), a VDCC blocker, nifedipine still increased mEPSCs whereas evoked EPSCs were blocked (n = 4; Fig. 4 A, B, and D). To perform a paired test, Cd2+ was applied to other cells after the nifedipine effect was induced, and it did not reverse the effect on mEPSCs (91.6 ± 14.0% of nifedipine effect; P > 0.05, n = 6, Fig. 4C) whereas it completely blocked evoked EPSCs. This result indicates that these events are independent of calcium influx through VDCCs, and also strengthens our contention that the nifedipine action is not via L-type channels.
Figure 4.
Nifedipine action is independent of calcium. (A) Sample current traces of three different neurons, treated with Cd2+, thapsigargin, or BAPTA-AM (Upper) and the effect of nifedipine on these treated cells (Lower). (B) Sample evoked EPSCs of three different neurons, showing the effect of Cd2+, thapsigargin, or BAPTA-AM. (C) Summary of the effect of Cd2+, thapsigargin, or BAPTA-AM on nifedipine-induced mEPSC frequency. In these cells, nifedipine was applied first. (D) Summary of the effect of Cd2+, thapsigargin, or BAPTA-AM on evoked EPSC amplitude. *, P < 0.05 vs. control. Thaps, thapsigargin; Nifed, nifedipine.
To investigate the contribution of internal calcium stores, thapsigargin (1 μM) was used to deplete these stores. Nifedipine was capable of inducing mEPSCs in the presence of thapsigargin (n = 3, Fig. 4A). In addition, thapsigargin had no effect in reversing nifedipine-induced mEPSCs (100.3 ± 12.2% of nifedipine effect; n = 5, P > 0.05, Fig. 4B), excluding these stores as a major source of calcium for the nifedipine effect. This result contrasts with a report describing an action of DHPs to induce calcium release from internal stores in skeletal muscles (24). To further elucidate the involvement of intracellular calcium, BAPTA-AM (25 μM), a membrane-permeable analogue of the calcium chelator BAPTA, was bath applied until inhibition of evoked EPSCs had reached a plateau. It took 7–26 min to reach its maximum effect of reducing the evoked EPSCs by 68.3 ± 8.2% (P < 0.05, n = 6, Fig. 4 B and D). This result of partial reduction of evoked transmitter release by BAPTA-AM is similar to other reports (25, 26). In contrast to its effect on evoked EPSCs, BAPTA-AM induced only a slight, statistically insignificant reduction of the frequency of mEPSCs induced by nifedipine (80.0 ± 5.2% of nifedipine effect; P > 0.05, n = 6, Fig. 4B). Nifedipine was also able to induce increase in mEPSCs in the presence of BAPTA-AM (n = 2, Fig. 4A). Taken together, our result indicated that the nifedipine effect is largely calcium independent.
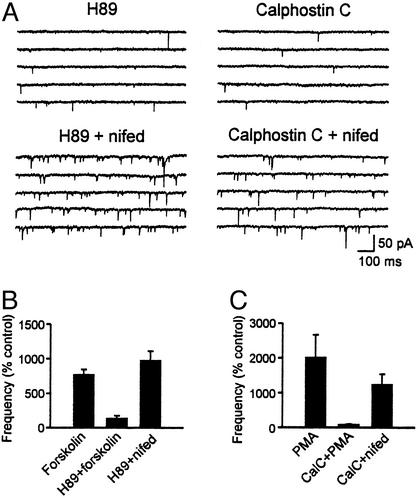
Activation of PKA or PKC pathways can lead to increase in mEPSCs (27–30); thus, we tested the hypothesis that these kinases mediate the effect of nifedipine. The slices were pretreated with a PKA inhibitor (H89, 20 μM) for 1–2 h, but nifedipine still induced a significant increase in mEPSC frequency (969.8 ± 142.2% of control; P < 0.05 vs. control, n = 4, Fig. 5 A and B). In contrast, H89 was able to inhibit the effect of a PKA activator, forskolin (10–20 μM), which also increased the frequency of mEPSCs (Fig. 5B). Similarly, application of PKC inhibitors calphostin C (0.1–1 μM) or chelerythrine chloride (10 μM) did not inhibit the nifedipine effect, suggesting that nifedipine does not act through activation of PKC (1,234.8 ± 301.1% of control; P < 0.05, n = 8, Fig. 5 A and C). However, calphostin C was effective in blocking the facilitation of mEPSCs by a phorbor ester, phorbol 12-myristate 13-acetate (100 nM; Fig. 5C).
Figure 5.
PKA or PKC does not mediate nifedipine effect. (A) Sample current traces from neurons pretreated with H89 or calphostin C. Nifedipine was effective in inducing mEPSCs in the presence of these inhibitors. (B) H89 inhibited forskolin-induced but not nifedipine-induced EPSCs. (C) Calphostin C blocked phorbor ester-induced mEPSCs but not nifedipine effect. PMA, phorbol 12-myristate 13-acetate; CalC, calphostin C; nifed, nifedipine.
Discussion
In this study, we demonstrate a previously uncharacterized effect of nifedipine, acting as a secretagogue to increase spontaneous transmitter release in central synapses. The facilitation seems to be due to a direct action on the release process, independent of its well-known action on L-type calcium channels.
The precise mechanism of the nifedipine effect is yet unknown. It cannot be attributed to the already known action of nifedipine to interfere with the adenosine system (18), increase production of NO (20), or block calcium-dependent potassium currents (15). Also, we have shown that its action is not due to activation of a PKA or PKC pathway. The finding that nifedipine effect is independent of PKC activation may indicate that its action is not due to an increase in the size of a readily releasable pool of synaptic vesicles, because PKC has been shown to increase the refilling rate and the size of a readily releasable pool (6, 31).
Activation of silent synapses can also give rise to increased frequency of mEPSCs; however, this is an unlikely possibility. Available evidence shows that AMPA receptor insertion to the synapse depends on N-methyl-d-aspartate (NMDA) receptor activation (32–35). We cannot expect NMDA receptor or VDCC activation in our recording condition of normal magnesium concentration and holding potential of −80 mV. In addition, blocking calcium influx through VDCCs by Cd2+ or chelating intracellular calcium by BAPTA-AM did not prevent the nifedipine effect. Thus, the most likely site of action is at the presynaptic release process, downstream of calcium entry/calcium release from internal stores.
Among the three DHP class L-type channel blockers used in this study, namely nifedipine, nimodipine and nicardipine, there are two major differences in the structural characteristics between nifedipine and others that were ineffective (Fig. 3A). First, nifedipine has an ortho-nitro substituent on its aromatic ring whereas the other two have a meta-nitro substituent. The substitution of the aromatic ring is thought to be important in locking the compound in its active conformation and hence activity (36). Second, nifedipine has two identical ester side chains on the 1,4-DHP ring at positions 3 and 5. In contrast, nimodipine and nicardipine have nonidentical esters on these positions. Variation of the esters alters the pharmacokinetic properties, such as the potency, duration of action, and latency (37, 38). These differences may account for the selectivity for nifedipine on a yet undetermined target. It may be relevant that a report by Aiello et al. (39) showed that nifedipine produced a local rigidity of a phospholipid bilayer, a feature not shared by lacidipine, another 1,4-DHP. Such a change in the local rigidity may create distortion in the membrane that would promote fusion. Alternatively, nifedipine action may involve an intracellular site, which might account for the long latency and washout of the effect.
It is important to note that nifedipine showed its effect at submicromolar dose (100 nM, and in some cases 10 nM). In normal human subjects, it has been shown that, after a single oral administration of clinical dose of nifedipine (10 mg), its plasma concentration reaches up to 56 ng/ml (= 162 nM) (40). Therefore, it is conceivable that nifedipine exerts its facilitatory effect on central synapses in vivo. Its effect was not specific to excitatory synapses in the SON: other excitatory synapses in other brain areas, as well as inhibitory synapses in the SON, responded similarly. This result may be an indication that the nifedipine target is something generally found in nerve terminals. Thus, in addition to a central effect, nifedipine may have a similar effect on the peripheral nervous system that needs investigation. Previously, it has been shown that nifedipine induced an increase in circulating norepinephrine level without increase in muscle sympathetic nerve activity in human subjects (41). This finding could be due to nifedipine acting on the nerve terminals to facilitate spontaneous transmitter release. Vasopressin released from posterior pituitary, possibly resulting from increased spontaneous excitatory synaptic activity in the SON (5), may also oppose nifedipine's antihypertensive action, not only by direct action on blood vessels through V1 receptor stimulation but also by facilitation of sympathetic neurotransmission and potentiation of constrictor effects of norepinephrine as shown in human saphenous veins (42). It will require further investigation to clarify whether the facilitatory effect of nifedipine on the synaptic transmission underlies some of its neurologic adverse effects. Nonetheless, our results suggest that use of nifedipine in neuropharmacological or neurophysiological studies of L-type calcium channels should be interpreted with caution, given the facilitatory action of nifedipine on synaptic transmission.
Acknowledgments
We thank Drs. G. W. Zamponi, J. S. Bains, J. E. A. Braun, and J. R. Coorssen for critical suggestions, and L. Bauce for technical assistance. This work is supported by the Canadian Institutes of Health Research, the Heart and Stroke Foundation of Canada, and the Alberta Heritage Foundation for Medical Research.
Abbreviations
- DHP
dihydropyridine
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- VDCC
voltage-dependent calcium channel
- ACSF
artificial cerebrospinal fluid
- SON
supraoptic nucleus
- EPSC
excitatory postsynaptic current
- mEPSC
miniature EPSC
- BAPTA-AM
1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid-acetoxymethyl ester
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
See commentary on page 5589.
References
- 1.Janicki P K, Siembab D, Paulo E A, Krzascik P. Pharmacology. 1988;36:183–187. doi: 10.1159/000138382. [DOI] [PubMed] [Google Scholar]
- 2.McKinney R A, Capogna M, Durr R, Gahwiler B H, Thompson S M. Nat Neurosci. 1999;2:44–49. doi: 10.1038/4548. [DOI] [PubMed] [Google Scholar]
- 3.Saitoe M, Schwarz T L, Umbach J A, Gundersen C B, Kidokoro Y. Science. 2001;293:514–517. doi: 10.1126/science.1061270. [DOI] [PubMed] [Google Scholar]
- 4.Murthy V N, Sejnowski T J, Stevens C F. Proc Natl Acad Sci USA. 2000;97:901–906. doi: 10.1073/pnas.97.2.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kombian S B, Hirasawa M, Mouginot D, Chen X, Pittman Q J. J Neurophysiol. 2000;83:2542–2553. doi: 10.1152/jn.2000.83.5.2542. [DOI] [PubMed] [Google Scholar]
- 6.Khvotchev M, Lonart G, Sudhof T C. Neuroscience. 2000;101:793–802. doi: 10.1016/s0306-4522(00)00378-x. [DOI] [PubMed] [Google Scholar]
- 7.Capogna M, Gahwiler B H, Thompson S M. J Neurophysiol. 1996;76:3149–3158. doi: 10.1152/jn.1996.76.5.3149. [DOI] [PubMed] [Google Scholar]
- 8.Trudeau L E, Doyle R T, Emery D G, Haydon P G. J Neurosci. 1996;16:46–54. doi: 10.1523/JNEUROSCI.16-01-00046.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armstrong W E. Prog Neurobiol. 1995;47:291–339. [PubMed] [Google Scholar]
- 10.Tasker J G, Hoffman N W, Dudek F E. J Neurosci Methods. 1991;38:129–143. doi: 10.1016/0165-0270(91)90163-t. [DOI] [PubMed] [Google Scholar]
- 11.Edwards F A, Konnerth A, Sakmann B. J Physiol (London) 1990;430:213–249. doi: 10.1113/jphysiol.1990.sp018289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanguinetti M C, Kass R S. Biophys J. 1984;45:873–880. doi: 10.1016/S0006-3495(84)84233-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diaz-Araya G, Godoy L, Naranjo L, Squella J A, Letelier M E, Nunez-Vergara L J. Gen Pharmacol. 1998;31:385–391. doi: 10.1016/s0306-3623(98)00034-2. [DOI] [PubMed] [Google Scholar]
- 14.Llano I, Gonzalez J, Caputo C, Lai F A, Blayney L M, Tan Y P, Marty A. Nat Neurosci. 2000;3:1256–1265. doi: 10.1038/81781. [DOI] [PubMed] [Google Scholar]
- 15.Druzin M, Haage D, Malinina E, Johansson S. J Physiol (London) 2002;542:131–146. doi: 10.1113/jphysiol.2001.015610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charles A C, Hales T G. J Neurophysiol. 1995;73:56–64. doi: 10.1152/jn.1995.73.1.56. [DOI] [PubMed] [Google Scholar]
- 17.Zernig G. Trends Pharmacol Sci. 1990;11:38–44. doi: 10.1016/0165-6147(90)90040-f. [DOI] [PubMed] [Google Scholar]
- 18.Morgan P F, Tamborska E, Patel J, Marangos P J. Neuropharmacology. 1987;26:1693–1699. doi: 10.1016/0028-3908(87)90119-5. [DOI] [PubMed] [Google Scholar]
- 19.Oliet S H, Poulain D A. J Physiol (London) 1999;520:815–825. doi: 10.1111/j.1469-7793.1999.00815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dhein S, Salameh A, Berkels R, Klaus W. Drugs. 1999;58:397–404. doi: 10.2165/00003495-199958030-00002. [DOI] [PubMed] [Google Scholar]
- 21.Ozaki M, Shibuya I, Kabashima N, Isse T, Noguchi J, Ueta Y, Inoue Y, Shigematsu A, Yamashita H. J Neuroendocrinol. 2000;12:273–281. doi: 10.1046/j.1365-2826.2000.00448.x. [DOI] [PubMed] [Google Scholar]
- 22.Cummings D D, Wilcox K S, Dichter M A. J Neurosci. 1996;16:5312–5323. doi: 10.1523/JNEUROSCI.16-17-05312.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grassi F, Mileo A M, Monaco L, Punturieri A, Santoni A, Eusebi F. Brain Res. 1994;659:226–230. doi: 10.1016/0006-8993(94)90883-4. [DOI] [PubMed] [Google Scholar]
- 24.Weigl L G, Hohenegger M, Kress H G. J Physiol (London) 2000;525:461–469. doi: 10.1111/j.1469-7793.2000.t01-1-00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adler E M, Augustine G J, Duffy S N, Charlton M P. J Neurosci. 1991;11:1496–1507. doi: 10.1523/JNEUROSCI.11-06-01496.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robitaille R, Garcia M L, Kaczorowski G J, Charlton M P. Neuron. 1993;11:645–655. doi: 10.1016/0896-6273(93)90076-4. [DOI] [PubMed] [Google Scholar]
- 27.Chen C, Regehr W G. J Neurosci. 1997;17:8687–8694. doi: 10.1523/JNEUROSCI.17-22-08687.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chavez-Noriega L E, Stevens C F. J Neurosci. 1994;14:310–317. doi: 10.1523/JNEUROSCI.14-01-00310.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evans D I, Jones R S, Woodhall G. J Neurophysiol. 2001;85:571–579. doi: 10.1152/jn.2001.85.2.571. [DOI] [PubMed] [Google Scholar]
- 30.Soliakov L, Wonnacott S. Br J Pharmacol. 2001;132:785–791. doi: 10.1038/sj.bjp.0703873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevens C F, Sullivan J M. Neuron. 1998;21:885–893. doi: 10.1016/s0896-6273(00)80603-0. [DOI] [PubMed] [Google Scholar]
- 32.Shi S H, Hayashi Y, Petralia R S, Zaman S H, Wenthold R J, Svoboda K, Malinow R. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- 33.Lu W, Man H, Ju W, Trimble W S, MacDonald J F, Wang Y T. Neuron. 2001;29:243–254. doi: 10.1016/s0896-6273(01)00194-5. [DOI] [PubMed] [Google Scholar]
- 34.Passafaro M, Piech V, Sheng M. Nat Neurosci. 2001;4:917–926. doi: 10.1038/nn0901-917. [DOI] [PubMed] [Google Scholar]
- 35.Broutman G, Baudry M. J Neurosci. 2001;21:27–34. doi: 10.1523/JNEUROSCI.21-01-00027.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rovnyak G C, Kimball S D, Beyer B, Cucinotta G, DiMarco J D, Gougoutas J, Hedberg A, Malley M, McCarthy J P, Zhang R, et al. J Med Chem. 1995;38:119–129. doi: 10.1021/jm00001a017. [DOI] [PubMed] [Google Scholar]
- 37.Bangalore R, Baindur N, Rutledge A, Triggle D J, Kass R S. Mol Pharmacol. 1994;46:660–666. [PubMed] [Google Scholar]
- 38.van Zwieten P A. Blood Press Suppl. 1998;2:5–9. [PubMed] [Google Scholar]
- 39.Aiello M, Moran O, Pisciotta M, Gambale F. Eur Biophys J. 1998;27:211–218. doi: 10.1007/s002490050127. [DOI] [PubMed] [Google Scholar]
- 40.Foster T S, Hamann S R, Richards V R, Bryant P J, Graves D A, McAllister R G. J Clin Pharmacol. 1983;23:161–170. doi: 10.1002/j.1552-4604.1983.tb02720.x. [DOI] [PubMed] [Google Scholar]
- 41.Binggeli C, Corti R, Sudano I, Luscher T F, Noll G. Hypertension. 2002;39:892–896. doi: 10.1161/01.hyp.0000013264.41234.24. [DOI] [PubMed] [Google Scholar]
- 42.Medina P, Acuna A, Martinez-Leon J B, Otero E, Vila J M, Aldasoro M, Lluch S. Circulation. 1998;97:865–870. doi: 10.1161/01.cir.97.9.865. [DOI] [PubMed] [Google Scholar]