Abstract
Estrogens control many physiological and behavioral processes, some of which are connected to reproduction. These include sexual and other social behaviors. Here we implicate four gene products in a micronet required for mammalian social recognition, through which an individual learns to recognize other individuals. Female mice whose genes for the neuropeptide oxytocin (OT) or the estrogen receptor (ER)-β or ER-α had been selectively “knocked out” were deficient specifically in social recognition and social anxiety. There was a remarkable parallelism among results from three separate gene knockouts. The data strongly suggest the involvement in social recognition of the four genes coding for ER-α, ER-β, OT, and the OT receptor. We thus propose here a four-gene micronet, which links hypothalamic and limbic forebrain neurons in the estrogen control over the OT regulation of social recognition. In our model, estrogens act on the OT system at two levels: through ER-β, they regulate the production of OT in the hypothalamic paraventricular nucleus, and through ER-α, they drive the transcription of the OT receptor in the amygdala. The proper operation of a social recognition mechanism allows for the expression of appropriate social behaviors, aggressive or affiliative.
Estrogenic regulation of social behavior has been investigated in mice whose genes for estrogen receptor (ER)-α or ER-β had been disrupted [ER knockout mice (ERKO), α-ERKO and β-ERKO]. Social behaviors were studied both in male and female α- and β-ERKO mice. Interestingly, α- and β-ERKO male mice showed opposite behavioral phenotypes when tested for aggressive behavior, the α-ERKO males being less (1) and the β-ERKO males more (2) aggressive than their respective WT littermates. As well, the elimination of ER-α but not -β impaired or reduced sexual behavior in male and female mice (3).
Neural systems shown to be involved in social recognition in rodents include the two neuropeptides oxytocin (OT) and vasopressin (4). The latter is more abundant in the male than the female brain, and it was shown to be necessary for social recognition only in male rats and mice, with its effects depending on androgen hormones (5). OT, on the other hand, is equally expressed in female and male brains and modulates social recognition in both sexes, with estrogens being directly involved in its action in females (6, 7). Interestingly, male mice deficient of the gene for OT [OT knockout mice (OTKO)] showed a deficit in social recognition (8) that could be rescued by infusion of OT in the medial amygdala (9). Similarly, α-ERKO male mice were shown to have impaired social behavior, including impaired social recognition (10). Although both ER-α and -β are expressed in the amygdala (11, 12), only ER-β is present in the OT-synthesizing neurons of the hypothalamus (12, 13), which makes ER-β a good candidate for the regulation of OT gene expression and its control of social recognition. The role of ER-β in social recognition has not been investigated.
Rodent models of social behavior have been used as a tool for better understanding disorders of social behavior in humans (recently reviewed in ref. 4), including the biobehavioral responses to stress of women (14). However, our understanding of the regulation of OT gene expression and its control of social recognition in female animals is limited. The test for social recognition is based on the natural propensity for mice to investigate an intruder mouse placed in their home cage. When the same intruder mouse is presented repeatedly, the social response of the resident mouse declines to very low levels (habituation), at which point the initial level of social investigation can be reinstated (dishabituation) by presenting the resident mouse with a different, novel, intruder conspecific (15). In this series of experiments with female mice, we compared β-ERKO, α-ERKO, and OTKO mice in social recognition as well as other behavioral responses to other females (15).
Materials and Methods
Animals.
ER-β disruption was created by insertion of a neomycin resistance gene into exon 3 of the coding gene by using homologous recombination in embryonic stem cells (16). ER-α disruption and the creation of the OTKO mice are described elsewhere (refs. 17 and 18, respectively). The founders of the β-ERKO colony were originally acquired from the National Institute of Environmental Health Sciences. The founders of the α-ERKO colony were from two colonies originally maintained at National Institute on Environmental Health Sciences. The founders of the OTKO colony were obtained from the Washington University School of Medicine. All mice used in these studies were bred in the vivarium of The Rockefeller University from heterozygous (HT) × HT pairs whose offspring were genotyped by PCR amplification of tail DNA. After weaning, the mice were housed in same-sex groups of four to five in polyethylene cages (26 × 16 × 12 cm) and provided with β-chip bedding. The colony room was kept under a 12-h light/12-h dark cycle (lights off at 11:00 a.m.) at 20 ± 2°C. Food (Purina Rat Chow) and water were available ad libitum. Four to five days before testing, female mice were transferred from group to individual housing to permit establishment of a home cage territory. Eleven intact β-ERKO and 10 β-WT littermate, 12 α-ERKO and 12 α-WT littermate, 9 OTKO, 12 OTHT, and 13 OTWT littermate female mice were the experimental animals, whereas the stimulus mice were 10 ovariectomized group-housed Swiss Webster mice. At testing, all female mice were 3–5 months old. This research was approved by and conducted in accordance with the Institutional Animal Care and Use Committee of the Rockefeller University.
All tests were run in the home cage of the mouse to be assayed, having a clear Plexiglas (23 × 33 cm) top. Stimulus mice were placed in a clear Plexiglas cylinder (7 cm in diameter, 12 cm high) and 36 holes (4 mm diameter) drilled in the bottom allowed the passage of olfactory cues while preventing direct interaction between the experimental and the stimulus mouse. This means of presentation of the stimulus mouse, while reducing variability due to behavioral differences across different intruder stimuli mice, was shown to elicit high social interest in the subject mice (19).
Social Recognition Paradigm.
Individual mice were tested five times (tests 1–5) in their home cage, where a cylinder containing a stimulus mouse was introduced. Each test lasted 5 min, and tests were repeated at 15-min intervals. In tests 1–4, the same stimulus mouse was used, whereas in test 5, a novel stimulus mouse was used. During testing, the mice were left undisturbed in the room, and their behavior was videotaped [8-mm Sony (Tokyo) Handycam]) for subsequent analysis. At the onset of the dark phase of the day of testing, experimental and stimulus mice were moved to a darkened holding space next to the testing room and left undisturbed for 90–120 min. Before testing, all mice were moved to the darkened testing room (with a small shielded red light in a corner), and experimental mice were habituated to the presence of an empty cylinder in their home cage for 10 min. Similarly, the stimulus mice were placed into a clean cylinder 5 min before being placed, while in the cylinder, into the home cage of the experimental animal. A new cylinder was used for each test, so the factor of novelty of the cylinder was maintained constantly throughout the experiment. During the inter-test 15-min intervals, the same empty cylinder was placed back in the cage. The position of all the cylinders introduced into the mouse home cage was kept constant throughout the experiment. The clean cylinders had been thoroughly washed with unscented soap, then paper towel and air dried.
Behavioral Analysis.
A trained investigator unaware of the test animal's genotype scored the videotapes by using specific software (Observer Video Analysis, Noldus, The Netherlands). The behavioral elements collected are described in Table 1 and allowed for the evaluation, within the context of the social test, of behaviors that are indicative of various motivational states of the animals. The behaviors collected include social investigation (active sniffing of the holes), nonsocial investigation (sniffing of the upper part of the cylinder, without holes), horizontal and vertical activity (not in relation to the cylinder), anxiety-related behaviors [stretched approaches (20–22)], and other active (self-grooming, digging in bedding) as well as nonactive (sitting) behaviors.
Table 1.
Behaviors of resident mouse
| Behavior | Description |
|---|---|
| Social investigation | The resident mouse sniffs at the holes at the bottom of the cylinder containing the intruder mouse. |
| Nonsocial investigation | The resident mouse sniffs the part of the intruder-containing cylinder without holes. |
| Stretched approach | Risk assessment behavior involving the mouse stretching its body toward the cylinder containing the intruder mouse (20–22). The ventral surface of the body is kept flat against the floor, and the mouse sniffs the air or the floor. The back feet do not move, whereas the head and forefeet are stretched forward. The behavior is typically completed with a “step back” of the front feet, so that the body is no longer stretched. The step back can be incomplete when either a series of stretch attends is performed or a stretch attend is followed by locomotor activity. In each of these cases, one “stretched approach” for each complete body stretch was counted. |
| Vertical activity | The mouse lifts both forefeet off the floor. When rearing along the walls, the mouse typically leans against them. |
| Horizontal activity | Locomotor activity with investigative sniffing of the air and floor of the cage. |
| Dig | Rapid forefeet movements of digging in the bedding. |
| Self-groom | Rapid cleaning movements of the forefeet toward the face and/or body. A typical complete grooming bout starts with the mouse scratching its face, progressively moving along the body, and terminating with the tip of the tail. Both complete and noncomplete grooming bouts (interrupted at some point along the body) were counted as “groom.” |
| Inactivity | The mouse sits with no locomotor activity, mostly sniffing the air with the eyes open. The minimum nonlocomotion time to be defined as an “inactivity” bout was 3 s. |
Statistical Analysis.
Duration and frequency of each behavior were analyzed with ANOVA and multivariate ANOVA. Mean comparisons were planned a priori in the analysis models (SuperANOVA, Abacus Concepts, Berkeley, CA). When necessary, data were normalized by means of natural log transformation before analysis. The results of the analyses run on the frequency of each behavior were mostly in line with those of its respective duration and are not presented here.
Results
The results reveal striking parallels between the social behavior of β-ERKO, α-ERKO, and OTKO females (Figs. 1–3). Mice of all three KO strains had impaired social recognition and lower social anxiety when compared with their respective WT littermates.
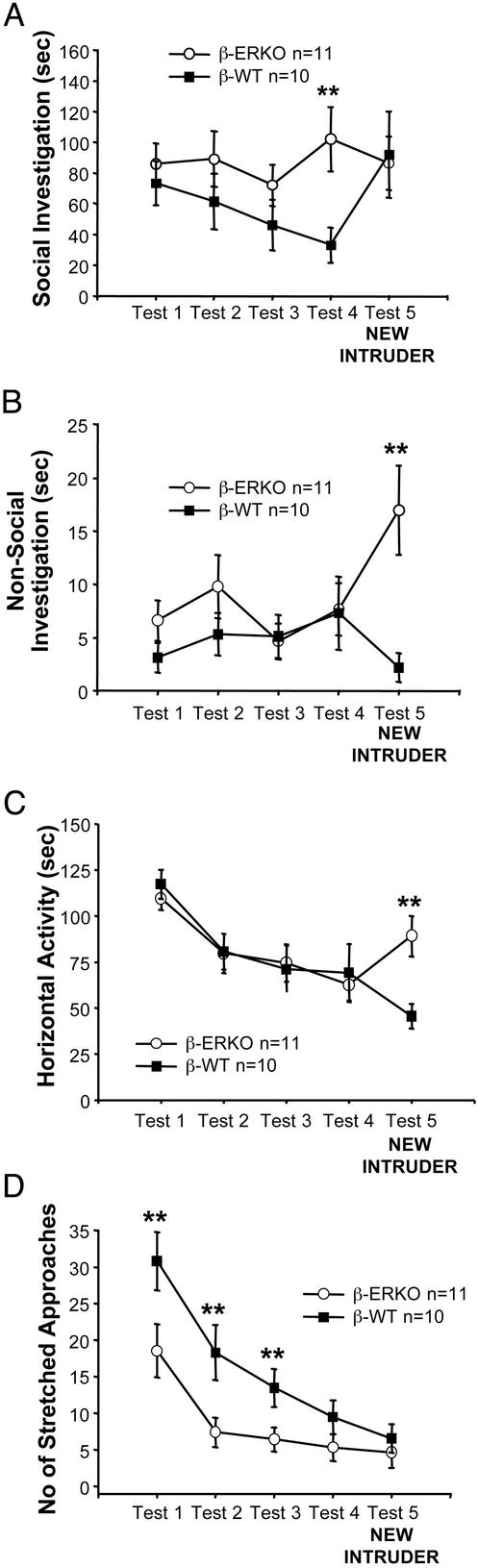
Figure 1.
Behavior of β-ERKO (○) and β-WT (■) mice in the social recognition test. Unlike their WT littermates, β-ERKO mice showed neither decreasing investigation of a repeatedly presented intruder mouse (tests 1–4) nor increased investigation of a new intruder at test 5. (A) β-ERKO mice were selectively impaired in social recognition but not in overall activity, because at test 5, they showed augmented nonsocial investigation (B) as well as a generalized increased activity (C). Consistently, their social anxiety-related behavior of stretched approaches toward the intruder was lower than the β-WT mice (D). **, P < 0.01 in comparison β-ERKO vs. β-WT.
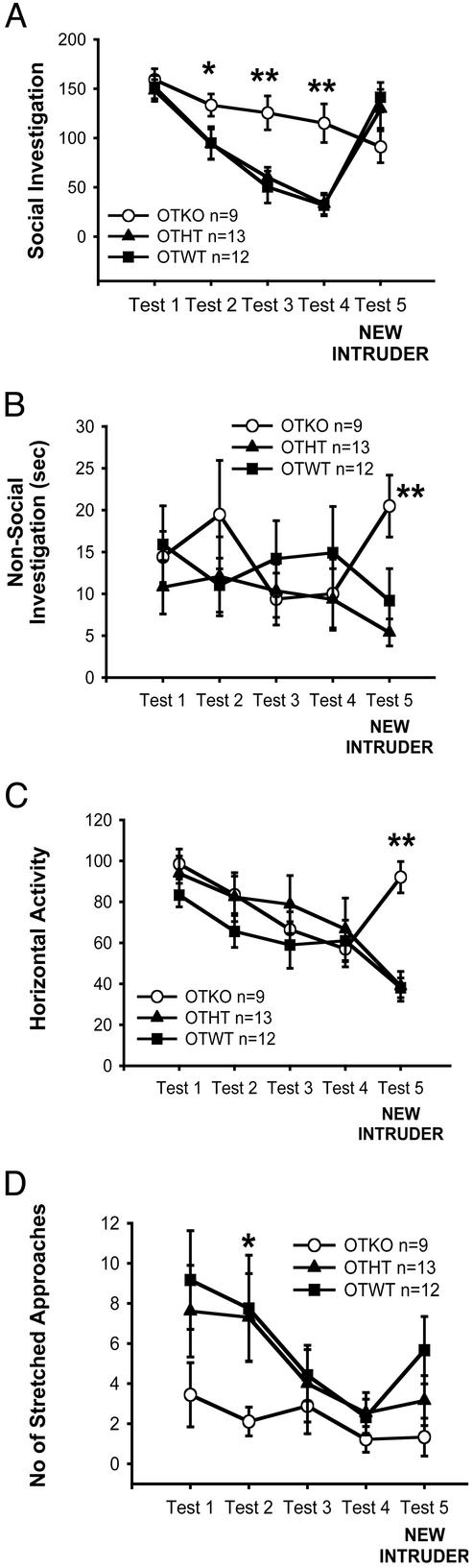
Figure 3.
Behavior of OTKO (○), OTWT (■), and heterozygous OTHT (▴) mice in the social recognition test. Unlike WT and HT, OTKO mice showed neither decreasing investigation of a repeatedly presented intruder mouse (tests 1–4) nor increased investigation of a new intruder at test 5. (A) OTKO mice were selectively impaired in social recognition but not in overall activity, because at test 5, they showed augmented nonsocial investigation (B) as well as a generalized increased activity (C). Consistently, their social anxiety-related behavior of stretched approaches toward the intruder was lower than WT and HT mice (D). *, 0.01 < P < 0.05; **, P < 0.01 in comparison OTKO vs. OTWT; †, 0.01 < P < 0.05; ‡‡, P < 0.01 compared with test 1.
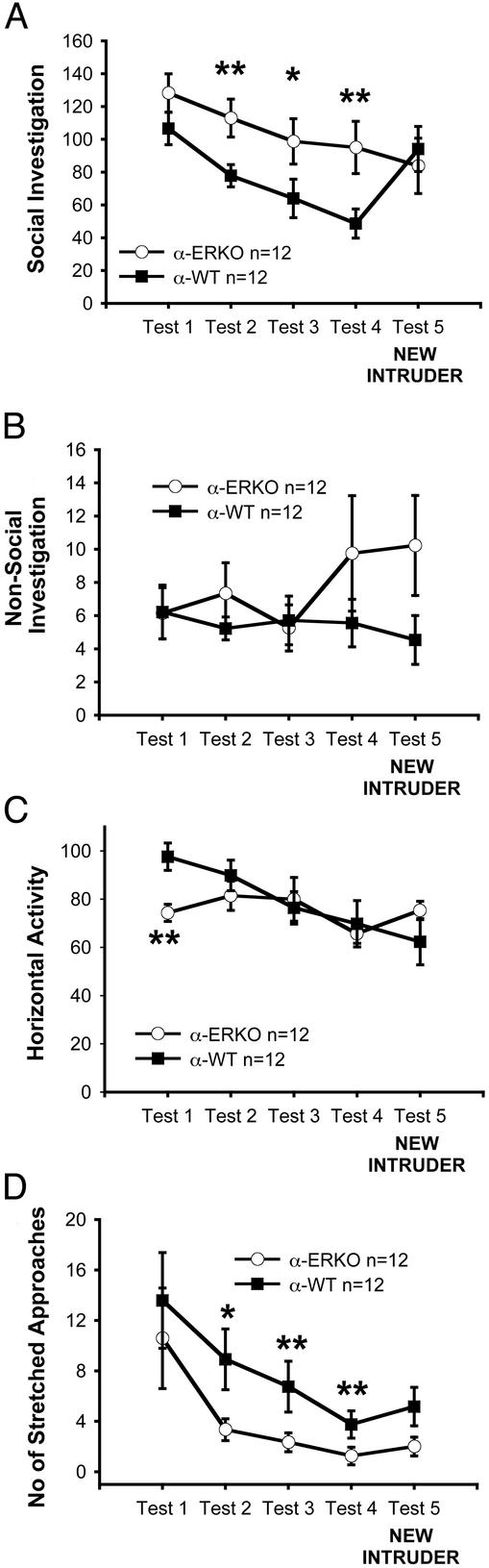
For the duration of the social investigation, the ANOVA showed significant genotype × test interactions in the ER-β (P < 0.003), ER-α (P < 0.02), and OT mice (P < 0.0001). In all three experiments, WT and KO mice behaved differently during the series of tests. The WT mice showed normally decreasing social response throughout tests 1–4 [comparison tests 1 vs. 4 in β-WT, P < 0.003; α-WT, P < 0.0001; OTWT, P < 0.0001]. Then, differently from their KO littermates, the WT mice showed the normal augmented social interest at TEST 5 when presented with a novel animal (comparison test 4 vs. test 5 in β-WT, P < 0.0001; α-WT, P < 0.0007; OTWT, P < 0.0001).
α-ERKO (Fig. 2A) and OTKO (Fig. 3A) females also showed a decline in the duration of social investigations toward the repeatedly introduced stimulus mouse [comparison tests 1 vs. 4 in β-ERKO, not significant (NS); α-ERKO, P < 0.02; OTKO, P < 0.009], but they failed to show an increase of social investigation at test 5 [comparison tests 4 vs. 5, all Ps NS]. The habituation shown by the α-ERKO and OTKO mice was lower that that shown by the WT mice. As a result, the KO mice of the three strains investigated the intruder more than their respective WT littermates at tests 2–4 (Figs. 1A, 2A, and 3A) (test 2, ER-β strain, NS; ER-α strain, P < 0.02; OT strain, P < 0.09), (test 3: ER-β strain, NS; ER-α strain, P < 0.07; OT strain, P < 0.002) (test 4: ER-β strain, P < 0.02; ER-α strain, P < 0.02; OT strain, P < 0.0002). At test 5, when the social investigation of the WT mice increased, the KO and the WT mice of the three strains were not different.
Figure 2.
Behavior of α-ERKO (○) and α-WT (■) mice in the social recognition test. Unlike their WT littermates, α-ERKO mice showed neither decreasing investigation of a repeatedly presented intruder mouse (tests 1–4) nor increased investigation of a new intruder at test 5. (A) α-ERKO mice were impaired in social recognition and, at test 1 only (C), also in horizontal activity. (D) Their social anxiety-related behavior of stretched approaches toward the intruder were lower than the α-WT mice. **, P < 0.01 in comparison β-ERKO vs. β-WT.
In parallel, the levels of anxiety-related behavior (Figs. 1D, 2D, and 3D) in the KO mice of the three strains were lower than those of their WT littermates, when initially presented with the cylinder containing the intruder conspecific at tests 1–3 (test 1, ER-β strain, P < 0.03; ER-α strain, NS; OT strain, P < 0.09) (test 2, ER-β strain, P < 0.02; ER-α strain, P < 0.04; OT strain, P < 0.09) and (test 3, ER-β strain, P < 0.03; ER-α strain, P < 0.05; OT strain, NS).
The impairment of social recognition in mice lacking OT and ER-β genes was very specific to the social component of the test, because at test 5, all the mice showed a behavioral arousal, which only in the WT animals was directed to an augmented social investigation. That is, compared with test 4, at test 5, both the β-ERKO and OTKO mice showed increased horizontal activity (β-ERKO, P < 0.03; OTKO, P < 0.0006) (Figs. 1C and 3C) and nonsocial investigation of the cylinder (β-ERKO, P < 0.009; OTKO, P < 0.05) (Figs. 1B and 3B), suggesting that they detected the factor of “novelty” but could not identify this novelty with the social aspect of the test. Mice lacking the ER-α gene did not show a similar behavioral arousal at test 5 but showed at test 1 (Fig. 2C) a level of horizontal activity lower than that of their WT littermates (P < 0.003).
There were no effects of genotype on other behaviors quantified, described in Table 1 (data not shown), thus demonstrating the specificity of the effects of the gene KOs on social recognition and anxiety. As well, statistical information about the OTHT mice is not shown, because their behavior never differed from that of the OTWT mice.
Discussion
This series of experiments shows that female mice whose genes for the neuropeptide OT, or the ER-β or -α, had been selectively “knocked out” were deficient, specifically, in social recognition. In all three strains, the gene KO mice, α-ERKO, β-ERKO, and OTKO females showed a reduced habituation to a repeatedly presented conspecific female and also failed to show a normal dishabituation response when, at test 5, the intruder mouse was replaced with a novel mouse. In parallel, anxiety-related behaviors were lower in the KO mice of the three strains than in their respective WT littermates (Figs. 1–3).
These results cannot be explained by a generalized difference in total activity, because there were no overall differences in this measure between the KO and the WT mice at test 5. Consistent with the literature on these mice and on the effects of estrogens and the estrous cycle on female activity (3, 23–25), horizontal activity was reduced in α-ERKO females. However, this was true only at test 1, so this effect cannot account for the reduced social investigation at test 5. Actually, at test 1, the social investigation of the α-ERKO mice was not lower than that of the α-WT mice. There is a possibility that KO mice habituated to a small extent to the testing situation as a whole. This minor phenomenon was not habituation to the plastic cylinder, because that was changed on every test. We note that slight differences among the three WT strains are not relevant to similarities of the three KO effects.
Overall, these results show remarkable parallels between the β-ERKO, α-ERKO, and OTKO mice and prove that genes for both ER-α and -β play a crucial role in OT-dependent social recognition. The striking phenotypic similarity of the β-ERKO, α-ERKO, and OTKO mice in our current study points to linked signaling of the ER-β, ER-α, and OT gene products on the central nervous system's control of social recognition (Fig. 4). Whether the crucial alteration of KO mouse behavior was due to disruption of the OT pathway itself or to its interactions with other systems remains to be determined. OT gene expression relevant to social behavior is in the paraventricular nucleus (PVN) of the hypothalamus and its mediation of social recognition in mice (8) is in the amygdala (9). When OT-receptor KO mice are proven viable, their behavior will justify new studies.
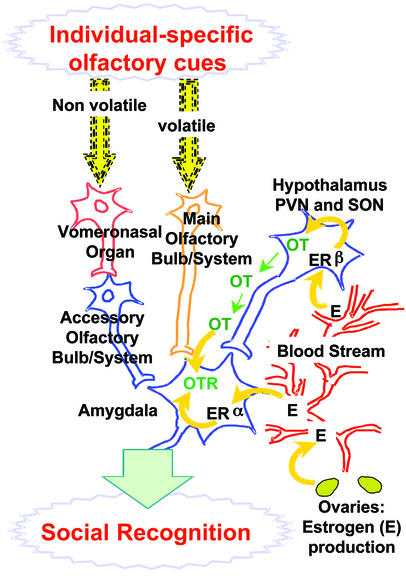
Figure 4.
Cartoon of four-gene micronet regulating social recognition. Ovarian estrogens regulate OT secretion in the PVN of the hypothalamus through binding to ER-β. OT, through axonal projections of the PVN neurons, reaches the amygdala, where estrogens regulate the expression of OT receptors through binding to ER-α. Estrogen-mediated OT-OTR activation in the amygdala ultimately facilitates social recognition.
Therefore, we propose a model of the mechanism underlying social recognition in animals involving the regulation of a four-gene “micronet” (Fig. 4) acting in the forebrain. The process of social recognition begins with individual specific volatile and nonvolatile odorous compounds that are detected respectively by the main (26) and accessory (vomeronasal organ) olfactory systems (27–29). These, through synapses in the main or accessory olfactory bulb, reach the medial and cortical nuclei of the amygdala where OT receptors mediate OT effects on social recognition (9).
OT mediation of social recognition itself is regulated by ovarian circulating estrogens that reach various areas of the brain where they bind to ER-α or -β (likely gene duplication products) and regulate gene expression. Specifically, estrogens regulate the production of OT by specialized neurosecretory neurons in the PVN of the hypothalamus by means of the highly expressed ER-β, whereas ER-α is absent in these neurons (12, 13, 30–33). Accordingly, estrogen-induced OT production in the PVN is absent in β-ERKO mice from the same colony as the present study (34). OT neurons then project to various extrahypothalamic areas, including the amygdala (extensively reviewed in ref. 35), where estrogens regulate OT receptor density (6) especially through the action of ER-α (12, 13, 36).
The reduced social anxiety found in the β-ERKO, α-ERKO, and OTKO mice of the present study resonates with the ability of estrogens to regulate anxiety (25) and the anxiolytic action of OT (37). OT involvement in human psychiatric disorders that involve social deficits such as social phobias and autism has been suggested (38). As well, acute OT in the amygdala modulates behavior in animal models of anxiety (39). The reduced anxiety shown by the OTKO mice, where OT is absent throughout development, suggests differential roles of OT in the developmental vs. the adult regulation of anxiety. OT may also be differently involved in anxiety-related behaviors in social vs. nonsocial contexts. Psychosocial, compared with nonsocial, anxiety was affected differently by anxiolytic drugs, suggesting differences in their neuromodulatory mechanisms (4, 40). Similarly, reduced anxiety behavior in β-ERKO mice emerged in social conditions, whereas in nonsocial tests of anxiety, β- but not α-ERKO female mice showed heightened levels of anxiety (24). Our results with KO mice reinforce the apparent need for assays of social as well as nonsocial animal models when evaluating anxiety profiles in mice.
Our results further suggest that estrogen modulation of female psychosocial anxiety depends on both ER-α and -β (Figs. 1 and 2) (1, 3, 24). It has been suggested that in females, including women, biobehavioral responses to stress involve the formation of female–female social bonds in a tend-and-be-friend response as opposed to the fight-or-flight response more typical of males. The tend-and-be-friend response of females was linked to actions of “the peptide of affiliation” OT (41) and to those of estrogens (14). Our results with female mice, suggesting an ER-α- and -β-mediated OT–estrogen interplay in the regulation of social recognition as well as psychosocial anxiety, have potential important implications for understanding the social behavior of females, particularly in relation to their social befriending management of stress.
Abbreviations
- ER
estrogen receptor
- KO
knockout
- OT
oxytocin
- PVN
paraventricular nucleus
- HT
heterozygous
- NS
not significant
References
- 1.Ogawa S, Lubahn D B, Korach K S, Pfaff D W. Proc Natl Acad Sci USA. 1997;94:1476–1481. doi: 10.1073/pnas.94.4.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nomura M, Durbak L, Chan J, Smithies O, Gustafsson J-Å, Korach K S, Pfaff D W, Ogawa S. Horm Behav. 2002;41:288–296. doi: 10.1006/hbeh.2002.1773. [DOI] [PubMed] [Google Scholar]
- 3.Ogawa S, Eng V, Taylor J, Lubahn D B, Korach K S, Pfaff D W. Endocrinology. 1998;139:5070–5081. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- 4.Young L J. Biol Psychiatr. 2002;51:18–26. doi: 10.1016/s0006-3223(01)01268-9. [DOI] [PubMed] [Google Scholar]
- 5.Blunthé R-M, Dantzer R. Brain Res. 1993;604:205–210. doi: 10.1016/0006-8993(93)90370-3. [DOI] [PubMed] [Google Scholar]
- 6.de Kloet E R, Voorhuis A M, Boxhma Y, Elands J. Neuroendocrinology. 1986;44:415–421. doi: 10.1159/000124680. [DOI] [PubMed] [Google Scholar]
- 7.Hliòák Z. Horm Behav. 1993;27:159–166. doi: 10.1006/hbeh.1993.1012. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson J N, Young L J, Hearn E F, Matzuk M M, Insel T R, Winslow J T. Nat Genet. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- 9.Ferguson J N, Aldag J M, Insel T R, Young L J. J Neurosci. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imwalle D B, Scordakales E M, Rissman E F. Horm Behav. 2002;42:484–491. doi: 10.1006/hbeh.2002.1837. [DOI] [PubMed] [Google Scholar]
- 11.Kuiper G G J M, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson J-Å. Proc Natl Acad Sci USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shughrue P J, Lane M V, Merchenthaler I. Endocrinology. 1999;140:2613–2620. doi: 10.1210/endo.140.6.6876. [DOI] [PubMed] [Google Scholar]
- 13.Shughrue P J, Askew R G, Dellovade T J, Merchenthaler I. Endocrinology. 2002;143:1643–1650. doi: 10.1210/endo.143.5.8772. [DOI] [PubMed] [Google Scholar]
- 14.Taylor S E, Cousino Klein L, Lewis B P, Gruenewald T L, Gurung R A R, Updegraff J A. Psychol Rev. 2000;107:411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- 15.Gheusi G, Blunthé R-M, Goodall G, Dantzer R. Behav Process. 1994;33:59–88. doi: 10.1016/0376-6357(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 16.Krege J H, Hodgin J B, Couse J F, Enmark E, Warner M, Mahler J F, Sar M, Korach K S, Gustafsson J A, Smithies O. Proc Natl Acad Sci USA. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lubahn D B, Moyer J S, Golding T S, Couse, Korach K S, Smithies O. Proc Natl Acad Sci USA. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gross G, Imamura T, Luedke C, Vogt S K, Olson L M, Nelson D M, Sadovksy Y, Muglia L J. Proc Natl Acad Sci USA. 1998;95:11871–11875. doi: 10.1073/pnas.95.20.11875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kudryavtseva N N, Bondar N P, Avgustinovich D F. Behav Brain Res. 2002;133:83–93. doi: 10.1016/s0166-4328(01)00443-0. [DOI] [PubMed] [Google Scholar]
- 20.Choleris E, Thomas A W, Kavaliers M, Prato F S. Neurosci Biobehav Rev. 2001;25:235–260. doi: 10.1016/s0149-7634(01)00011-2. [DOI] [PubMed] [Google Scholar]
- 21.Blanchard R J, Yudko E B, Rodgers R J, Blanchard D C. Behav Brain Res. 1993;58:155–165. doi: 10.1016/0166-4328(93)90100-5. [DOI] [PubMed] [Google Scholar]
- 22.Lister R G. Pharmacol Ther. 1990;46:321–340. doi: 10.1016/0163-7258(90)90021-s. [DOI] [PubMed] [Google Scholar]
- 23.Morgan M A, Pfaff D W. Horm Behav. 2001;40:472–482. doi: 10.1006/hbeh.2001.1716. [DOI] [PubMed] [Google Scholar]
- 24.Krȩżel W, Dupont S, Krust A, Chambon P, Chapman P F. Proc Natl Acad Sci USA. 2001;98:12278–12282. doi: 10.1073/pnas.221451898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zimmerberg B, Farley J. Physiol Behav. 1993;54:1119–1124. doi: 10.1016/0031-9384(93)90335-d. [DOI] [PubMed] [Google Scholar]
- 26.Dluzen D E, Muraoka S, Engelmann M, Landgraf R. Peptides. 1998;19:999–1005. doi: 10.1016/s0196-9781(98)00047-3. [DOI] [PubMed] [Google Scholar]
- 27.Blunthé R-M, Gheusi G, Dantzer R. Psychoneuroendocrinology. 1993;18:323–335. doi: 10.1016/0306-4530(93)90028-j. [DOI] [PubMed] [Google Scholar]
- 28.Keverne E B. Cell. 2002;108:735–738. doi: 10.1016/s0092-8674(02)00687-6. [DOI] [PubMed] [Google Scholar]
- 29.Del Punta K, Leinders-Zufall T, Rodriguez I, Jukam D, Wysocki C J, Ogawa S, Zufall F, Mombaerts P. Nature. 2002;419:70–74. doi: 10.1038/nature00955. [DOI] [PubMed] [Google Scholar]
- 30.Burbach J P, Luckman S M, Murphy D, Gainer H. Physiol Rev. 2001;81:1197–1267. doi: 10.1152/physrev.2001.81.3.1197. [DOI] [PubMed] [Google Scholar]
- 31.Hrabovszky E, Kalló I, Hajszán T, Shughrue P J, Merchenthaler I, Liposits Z. Endocrinology. 1998;139:2600–2604. doi: 10.1210/endo.139.5.6024. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J-Q, Cai W-Q, Zhou D S, Su B-Y. Brain Res. 2002;935:73–80. doi: 10.1016/s0006-8993(02)02460-5. [DOI] [PubMed] [Google Scholar]
- 33.Somponpun S, Sladek C. Endocrinology. 2002;143:2899–2904. doi: 10.1210/endo.143.8.8946. [DOI] [PubMed] [Google Scholar]
- 34.Nomura M, McKenna E, Korach K S, Pfaff D W, Ogawa S. Mol Brain Res. 2002;109:84–94. doi: 10.1016/s0169-328x(02)00525-9. [DOI] [PubMed] [Google Scholar]
- 35.Gimpl G, Fahrenholz F. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 36.Young L J, Wang Z, Donaldson R, Rissman E F. NeuroReport. 1998;9:933–936. doi: 10.1097/00001756-199803300-00031. [DOI] [PubMed] [Google Scholar]
- 37.McCarthy M M, McDonald C H, Brooks P J, Goldman D. Physiol Behav. 1996;60:1209–1215. doi: 10.1016/s0031-9384(96)00212-0. [DOI] [PubMed] [Google Scholar]
- 38.Young L J. Am J Med Gen. 2001;105:53–54. [PubMed] [Google Scholar]
- 39.Bale T L, Davis A M, Auger A P, Dorsa D M, McCarthy M. J Neurosci. 2001;21:2546–2552. doi: 10.1523/JNEUROSCI.21-07-02546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gonzalez L E, Andrews N, File S. Brain Res. 1996;732:145–153. doi: 10.1016/0006-8993(96)00517-3. [DOI] [PubMed] [Google Scholar]
- 41.Carter S C. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]