Abstract
Streptococcus gordonii is a bacterial vaccine vector which has previously been shown to activate dendritic cells in vitro and to induce local and systemic immune responses in vivo. In the present study, human monocytes (THP-1 cell line and peripheral blood monocytes) were characterized following interaction with S. gordonii. Treatment of human monocytes with S. gordonii but not latex beads induced a clear up-regulation of CD83, CD40, CD80, and CD54 and the down-regulation of CD14. Furthermore, bacterial treatment stimulated an increased expression of Toll-like receptor 5 (TLR5), TLR6, and TLR7, production of the proinflammatory cytokines tumor necrosis factor alpha and interleukin 1 beta, and reduction of the phagocytic activity. This work shows that the immunostimulatory activity of S. gordonii is not restricted to induction of dendritic-cell maturation but also affects the differentiation process of human monocytes.
Streptococcus gordonii is a nonpathogenic gram-positive commensal bacterium and component of the normal microbial flora of the human oral cavity (13) which was developed as a vaccine vector (20, 24, 26). A variety of antigens of different origins and sizes have been expressed on the surface of S. gordonii and were shown to be immunogenic by the systemic and mucosal routes (oral, nasal, vaginal, and intragastric) both in mice and in monkeys (6, 17-21, 23-25, 31). Using the model of adoptive transfer of transgenic T lymphocytes, we recently demonstrated that an antigen-specific primary activation of CD4+ T cells is induced in the nasal mucosa-associated lymphoid tissue, draining lymph nodes, and spleen following intranasal immunization with recombinant S. gordonii (16). It has also been shown in a phase I clinical trial that S. gordonii is safe in humans when administered by the nasal/oral route and that it can be rapidly eradicated either spontaneously or with antibiotic treatment (14).
The mechanisms involved in the immunostimulating activity of this vaccine vector need to be further investigated. We have previously demonstrated that S. gordonii is efficiently internalized by both murine and human dendritic cells (DCs), and it induces their maturation and activation as shown by phenotypic and functional changes (3, 4, 30). The model antigens expressed on the surfaces of recombinant bacteria are processed and presented by DCs not only in association with major histocompatibility complex (MHC) class II but also MHC class I molecules much more efficiently than the soluble antigen (30). Furthermore, human DCs were more efficient than B cells at presenting the heterologous antigen expressed on the surface of S. gordonii (4).
Migrating DCs can originate from monocytes that continuously exit the bloodstream and enter body tissues, where they encounter differentiation (8, 28, 29, 36). Monocytes are immature precursors with a double differentiation potential (27). It has been demonstrated that macrophages and monocyte-derived DCs can readily interconvert into one another until the late stages of their differentiation/maturation process (27). Maturation signals, including those of bacterial origin and cytokines, are the factors determining whether monocytes differentiate into DCs or into macrophages (27). Recent studies indicate that whole bacteria can influence the differentiation of monocytes and maturation of DCs (1, 3, 9-12, 30, 32-34).
In the present study, the human monocytic THP-1 cell line and human peripheral blood monocytes were characterized following interaction with S. gordonii. Phenotypic modifications, expression of Toll-like receptors (TLRs), cytokine production, and phagocytic activity were assessed for monocytes stimulated with S. gordonii.
MATERIALS AND METHODS
THP-1 cell line and culture conditions.
The human acute monocytic leukemia THP-1 cell line (ATCC number TIB-202) (35) was obtained from the Istituto Zooprofilattico Sperimentale della Lombardia e dell'Emilia (Brescia) and cultured in suspension flasks (Pbi International) at 37°C with 5% CO2. The culture medium RPMI 1640 (Gibco BRL, Life Technologies, Scotland) was supplemented with 10% heat-inactivated fetal bovine serum (Gibco), 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamine (all from Sigma-Aldrich). Cells were kept no more than 2 months in culture from the original stocks.
Human peripheral blood monocytes.
Peripheral blood monocytes were isolated from buffy coats of healthy donors (Immunoematologia e Servizio Trasfusionale, Policlinico Le Scotte, Siena, Italy) by using Ficoll-Hypaque density gradient centrifugation (Pharmacia Biotech, Sweden) and by magnetic microbead isolation using CD14-conjugated microbeads (Miltenyi Biotec GmbH, Germany) according to the manufacturer's instructions. Cells were separated on LS+ columns positioned on a MidiMACS magnet (Miltenyi Biotec GmbH, Germany). The purity of the monocyte population was >95% as determined by flow cytometry with anti-CD14 antibody. Cell viability of monocytes at isolation was >90% as determined by trypan blue exclusion in a cell counting chamber and by propidium iodide staining and flow cytometry analysis. Monocytes were cultured in RPMI 1640 supplemented with 5% fetal bovine serum and 2 mM l-glutamine.
Bacterial strains and growth conditions.
S. gordonii was grown at 37°C in tryptic soy broth without dextrose (Difco) and harvested by centrifugation at the end of the exponential-growth phase. Bacterial cells were then washed and resuspended at 1:500 of the original culture volume in fresh medium containing 10% glycerol. Aliquots were stored frozen at −70°C until used. Killed bacteria were obtained by incubating S. gordonii for an hour at 60°C. The killing efficiency was evaluated by plating bacteria on blood agar plates.
Stimulation of THP-1 cells and peripheral blood monocytes with S. gordonii.
THP-1 cells were seeded in 24-well tissue culture plates (Pbi International) at a density of 2.5 × 105/ml in a volume of 2 ml per well. Cells were incubated with different doses (from 1:1 to 100:1) of S. gordonii or latex beads (1.1 μm; Sigma-Aldrich) per cell. When peripheral blood monocytes were used, the doses of bacteria per cell were 1 and 10. Plates were incubated at 37°C with 5% CO2 for 18 h. In the time course experiment, phenotypic modifications of THP-1 cells were analyzed following 18, 48, and 96 h of treatment with the single dose of 100 bacteria per cell. Antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin) were added 2 h after bacterial addition.
Flow cytometric analysis and intracellular cytokine detection.
The surface marker expression of THP-1 cells and peripheral blood monocytes following treatment with S. gordonii was assayed by flow cytometry. Phosphate-buffered saline (PBS) with 0.5% bovine serum albumin (Sigma-Aldrich) was used as diluent/wash buffer. Cells were incubated for 30 min at 4°C with the following monoclonal antibodies (20 μl/106 cells): fluorescein isothiocyanate (FITC)-conjugated mouse anti-human CD14 (clone M5E2, immunoglobulin G2a [IgG2a]), CD80 (L307.4, IgG1), HLA-DR (G46-6, IgG2a), CD40 (5C3, IgG1), R-phycoerythrin (R-PE)-conjugated mouse anti-human CD83 (HB15e, IgG1), CD54 (HA58, IgG1), HLA-A, -B, and -C (G46-2.6, IgG1), and CD86 (IT2.2, IgG2b). Appropriate FITC or PE-conjugated isotype-matched antibodies were used as controls. All antibodies were purchased from BD PharMingen (San Diego, CA). Cells were fixed in freshly prepared 1% paraformaldehyde (Sigma-Aldrich) and analyzed by flow cytometry (FACScan; Becton Dickinson). Data analysis was performed with CellQuest software (Becton Dickinson).
Intracellular cytokine production was detected in THP-1 cells treated with live or killed S. gordonii (100 bacteria per cell), latex beads (100 beads per cell), or lipoteichoic acid (LTA) (10 μg/ml) (Sigma-Aldrich) for 6 and 24 h. Brefeldin A (10 μg/ml, Sigma-Aldrich) was added 4 h before the completion of incubation with the stimuli. THP-1 cells were permeabilized in the dark by using FACS permeabilizing solution (diluted 1:10 in distillate sterile water; Becton Dickinson) for 10 min at room temperature. After being washed with wash FACS buffer (PBS with 0.5% bovine serum albumin and 0.1% NaN3), cells were incubated for 30 min at room temperature with the following monoclonal antibodies: FITC-conjugated anti-human interleukin 1 beta (IL-1β) (AS10, IgG1), IL-6 (AS12, IgG1), tumor necrosis factor alpha (TNF-α) (6401.1111, IgG1), purchased from Becton Dickinson (San Jose, CA), PE-conjugated anti-human IL-10 (JES3-19F1, IgG2a), IL-12 (C11.5, IgG1), and IgG subclass-matched control antibody obtained from BD Pharmingen (San Diego, CA). Cells were washed, resuspended in 1% paraformaldehyde, and analyzed by flow cytometry.
Quantitative real-time RT-PCR for detection of TLR expression.
Quantitative real-time RT-PCR, performed as previously described (22), was used for detecting the expression of TLRs. THP-1 cells were incubated with S. gordonii at the ratio of 10, 30, and 100 bacteria per cell for 6 h at 37°C with 5% CO2. Positive controls were obtained by treating cells with phorbol myristate acetate (10 ng/ml; Sigma-Aldrich) for 12 h and then with lipopolysaccharide (100 ng/ml; Sigma-Aldrich) for 6 h. Cells were lysed, and mRNA was extracted by using the poliA-column μMACS mRNA isolation kit (Miltenyi Biotec) according to the manufacture's instructions and eluted in diethyl pyrocarbonate water. The presence of contaminating DNA was verified by PCR using hemoglobin gene primers (forward, 5′-GAAGAGCCAAGGACAGGTAC-3′; reverse, 5′-GGAAAATAGACCAATAGGCAG-3′), and positive samples were treated with DNase (Ambion) for 15 min at 37°C. mRNA (300 ng) was retrotranscribed using a reaction mixture consisting of 40 pmol oligo(dT) primers, 40 pmol random hexamers (both from MWG-BIOTECH, Germany), 50 μM deoxynucleoside triphosphates (EuroClone, Italy), 20 U RNase inhibitor (Promega), and 8 mM dithiothreitol (Sigma-Aldrich). Samples were incubated for 2 min at 37°C and immediately chilled on ice. ImPromII reverse transcriptase (Promega) was added to the mix, followed by incubation for 50 min at 37°C. Reverse transcriptase was inactivated by incubation for 15 min at 70°C. Amplification and detection of specific products were performed using the DNA Engine Opticon System apparatus (MJ Research). Primers for quantification were designed to amplify fragments of 250 to 300 bp in length (Table 1). PCR amplification was performed in a 20-μl mixture containing LightCycler DNA Master SYBR green I 1X (Roche), 2.5 mM MgCl2, 5 pmol of each primer, and 2 μl of reverse transcriptase reaction mixture as a template. PCRs were carried out according to the following cycle profile: 95°C for 30 s (one cycle); 95°C for 1 s, 55°C for 10 s, and 72°C for 20 s (35 cycles); and 72°C for 5 min (one cycle). The DNA dissociation curve was determined for each amplicon, and no primer-dimer artifact was detected. Relative quantification was performed by determining the threshold value as suggested by the supplier. The gene expression was analyzed by the 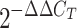 method (15) using the cyclophilin B gene as an internal control gene. Results are reported as the degree of increase in expression compared to that for untreated THP-1 cells.
method (15) using the cyclophilin B gene as an internal control gene. Results are reported as the degree of increase in expression compared to that for untreated THP-1 cells.
TABLE 1.
Oligonucleotide primers for human TLRs
| Namea | Sequence | GenBank accession no. | Amplicon size (bp) |
|---|---|---|---|
| TLR1 | NM_003263 | 302 | |
| for | GTTAGAAGAAATCAGGATAACA | ||
| rev | GTCCAAAGCTCAGATATATAAT | ||
| TLR2 | NM_003264 | 304 | |
| for | GCAACAACAATCTCAATTTA | ||
| rev | GAGTCACACAGGTAATTTG | ||
| TLR3 | |||
| for | CTTTCCTACAACAAGTACCTGC | ||
| rev | GTTTCCAGAGCCGTGCTAAG | NM_003265 | 256 |
| TLR4 | |||
| for | CGACAACCTCCCCTTCTC | ||
| rev | GATTGTGAGCCACATTAAGTTC | NM_003266 | 335 |
| TLR5 | |||
| for | GTCTTGGATATAACTCATAACA | ||
| rev | GAACTTTGTGACTGTGAGGA | NM_003268 | 273 |
| TLR6 | |||
| for | CTGCCCATCTGTAAGGA | ||
| rev | TGATATGTTCACTTGGATAGC | NM_006068 | 243 |
| TLR7 | |||
| for | ATGTCACAGCCGTCCCTA | ||
| rev | CACATGCTGAAGAGAGTTAC | AF245702 | 272 |
| TLR8 | |||
| for | ACAATCAACAAATCCGCACT | ||
| rev | AGGGCAGCCAACATAACCA | AF245703 | 293 |
| TLR9 | |||
| for | CTGGGACCTCTGGTACTG | ||
| rev | TCTTGCGGCTGCCATAGA | AF245704 | 278 |
| TLR10 | |||
| for | CTGTGGTCAATATGAATCTGT | ||
| rev | GAATGGATTTCTTCCCGCA | AF296673 | 308 |
| Cyclophilin B | |||
| for | GCCCAAAGTCACCGTC | ||
| rev | TTCAGTTTGAAGTTCTCATC | M60857 | 276 |
for, forward; rev, reverse.
Phagocytosis assay.
THP-1 cells were cultured (5 × 104 per well) in 96-well flat-bottomed microtiter plates (Greiner, Frickenhausen, Germany) with S. gordonii (100 bacteria per cell) or medium alone for 18 h at 37°C. Subsequently, fluorescent beads (0.87 μm, SPHERO fluorescent particles, Yellow; PharMingen) were added at a dose of 300 per cell and incubated for 24 h at 37°C or at 4°C. Cells were collected, washed with cold PBS, and analyzed by flow cytometry.
Statistical analysis.
Results of surface marker expression, cytokine production, and TLR expression were reported as means ± standard deviations (SDs) from three, six, or five independent experiments, respectively. The statistical significance of surface marker expression and cytokine production between bacterium-stimulated and control cells was determined with Student's t test. The statistical significance of the reduction of phagocytosis between bacterium-treated THP-1 and the control was calculated using the chi-square test. The significance level was set at a P value of ≤0.05.
RESULTS
Expression of surface markers in human monocytes stimulated with different doses of S. gordonii.
THP-1 cells were treated for 18 h with S. gordonii at a ratio of 100 bacteria per cell, and the surface expression of CD14 and CD83, being the monocytic and mature DC markers, respectively, was evaluated by flow cytometric analysis. As shown in Fig. 1A, bacterial treatment induced a considerable increase of CD83+ cells (72%) compared to control (untreated) cells (6.38%). CD14 was still expressed on 95% of stimulated cells, even if the mean fluorescence intensity (MFI) value decreased from 57 (untreated cells) to 32 (bacterially treated cells). To evaluate whether the modifications induced by bacterial treatment were dose dependent, THP-1 cells were stimulated with different doses of S. gordonii, starting from a ratio of 1:1 to 100:1 bacteria per cell (Fig. 1B). The phenotypic modifications induced by bacterial treatment were compared to the ones induced by latex beads. A significant decrease of CD14 and increase of the expression of CD83 and CD54 molecules was observed. These effects were strictly dose dependent and could not be detected following treatment with the same doses of latex beads (Fig. 1B). In contrast, the expression of costimulatory molecules, such as CD80 and CD86, and of MHC class I was slightly affected by the bacterial treatment in comparison to latex beads. Interestingly, the HLA-DR expression showed a significant down-regulation with the increase of the bacterial dose (Fig. 1B). A ratio of 100 bacteria per cell was chosen for subsequent experiments of interaction of bacteria with THP-1 cells, since it showed optimal results with little effect on cell viability (data not shown).
FIG. 1.
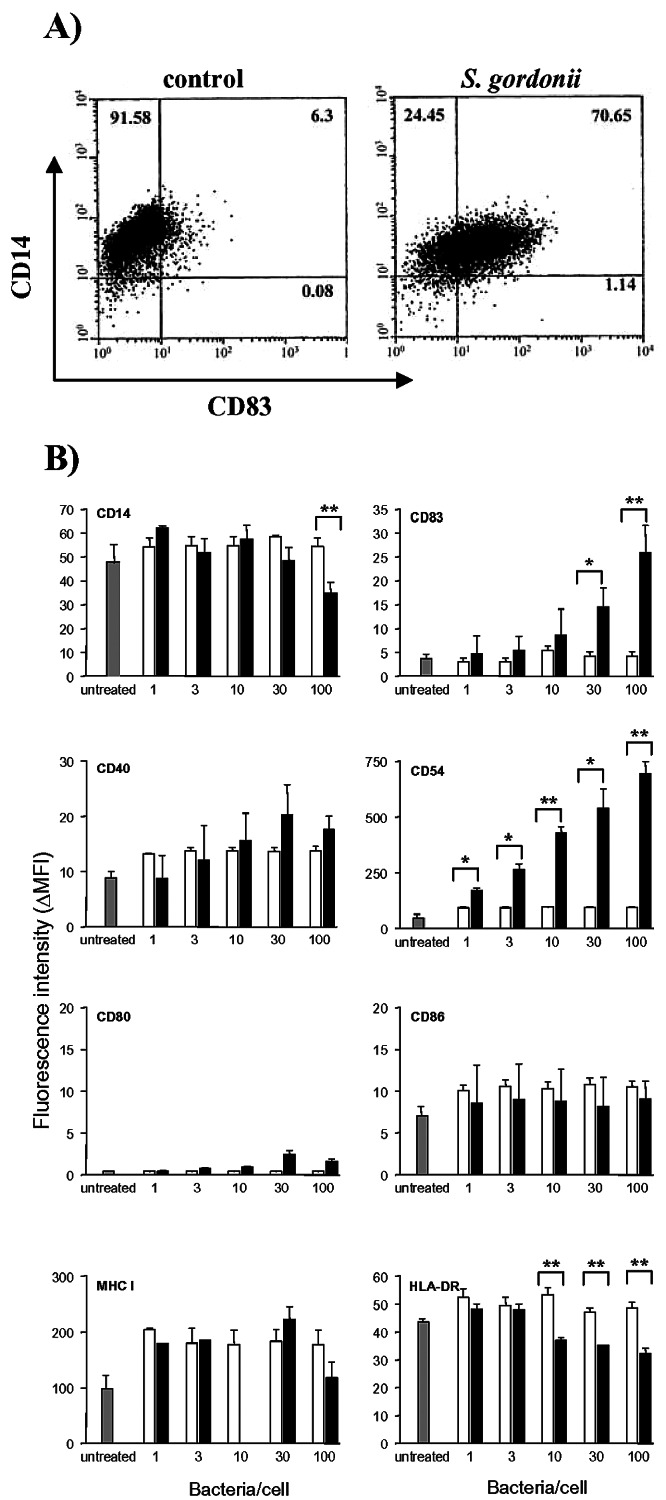
Phenotypic characterization of THP-1 cells following S. gordonii stimulation. A. Dot plot analysis of THP-1 cells cultured for 18 h with S. gordonii (100 bacteria per cell) or in medium alone (control) and stained with FITC-conjugated anti-human CD14 and PE-conjugated anti-human CD83. Percentage values for each quadrant are reported. B. Expression of surface markers on THP-1 cells following 18 h of stimulation with S. gordonii (▪) or latex beads (□) at different doses (1, 3, 10, 30, or 100 per cell) or no treatment ( ). The mean fluorescence intensity minus the fluorescence of matched-isotype control antibody (ΔMFI) was calculated from each sample. Values are reported as means ± standard deviations from three independent experiments. *, P ≤ 0.05; **, P ≤ 0.01, compared to results for cells treated with the same dose of latex beads.
). The mean fluorescence intensity minus the fluorescence of matched-isotype control antibody (ΔMFI) was calculated from each sample. Values are reported as means ± standard deviations from three independent experiments. *, P ≤ 0.05; **, P ≤ 0.01, compared to results for cells treated with the same dose of latex beads.
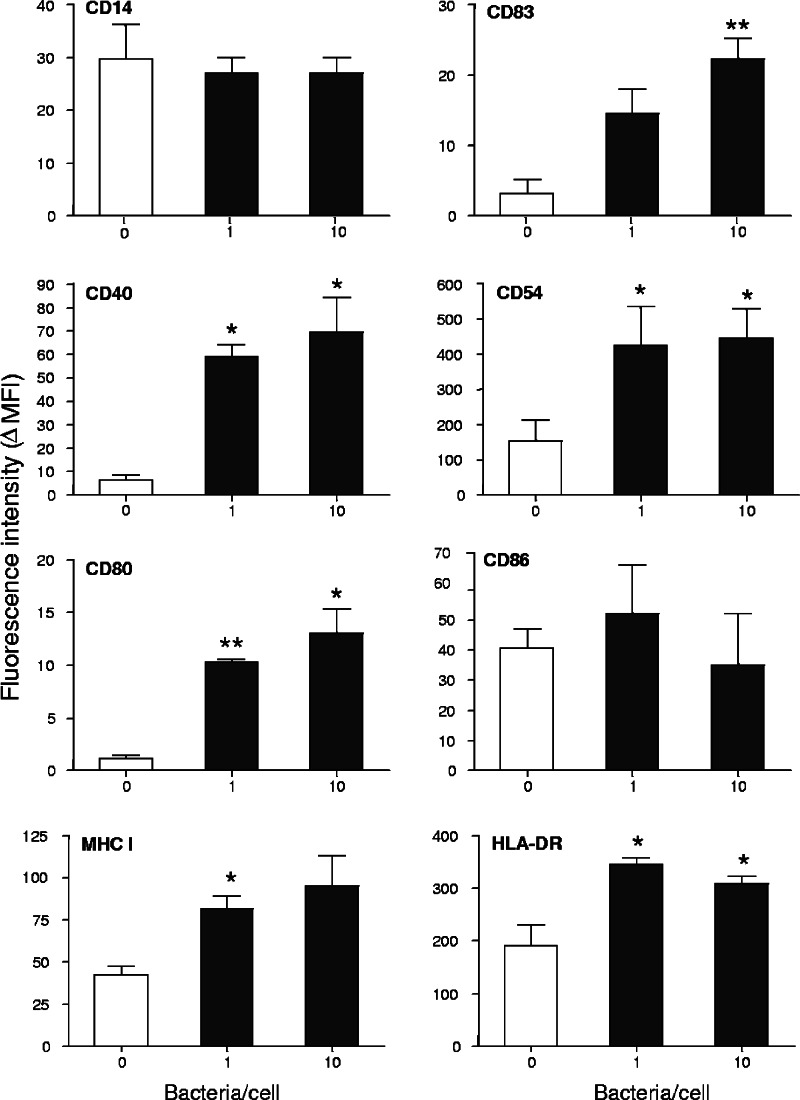
The effects of S. gordonii were also investigated on human peripheral blood monocytes collected from three different donors. Cells were stimulated for 18 h with S. gordonii at the doses of 1 and 10 bacteria per cell, showing a significant up-regulation of CD83 and CD54 compared to results with untreated cells, as previously observed with THP-1 cells. Furthermore, peripheral monocytes showed increased expression of CD40, CD80, and MHC class I and II molecules (Fig. 2).
FIG. 2.
Surface antigen expression on human monocytes treated with different doses of S. gordonii. Monocytes, isolated from buffy coats of healthy donors using magnetic CD14-conjugated beads, were cultured with S. gordonii at a concentration of 1 or 10 bacteria per cell (▪) or in medium alone (□). Cells were harvested after 18 h and analyzed by flow cytometry. The mean fluorescence intensity minus the fluorescence of the matched-isotype control antibody (ΔMFI) was calculated for each sample. Values are reported as means ± standard deviations from three independent experiments. *, P ≤ 0.05; **, P ≤ 0.01, compared to results for untreated cells.
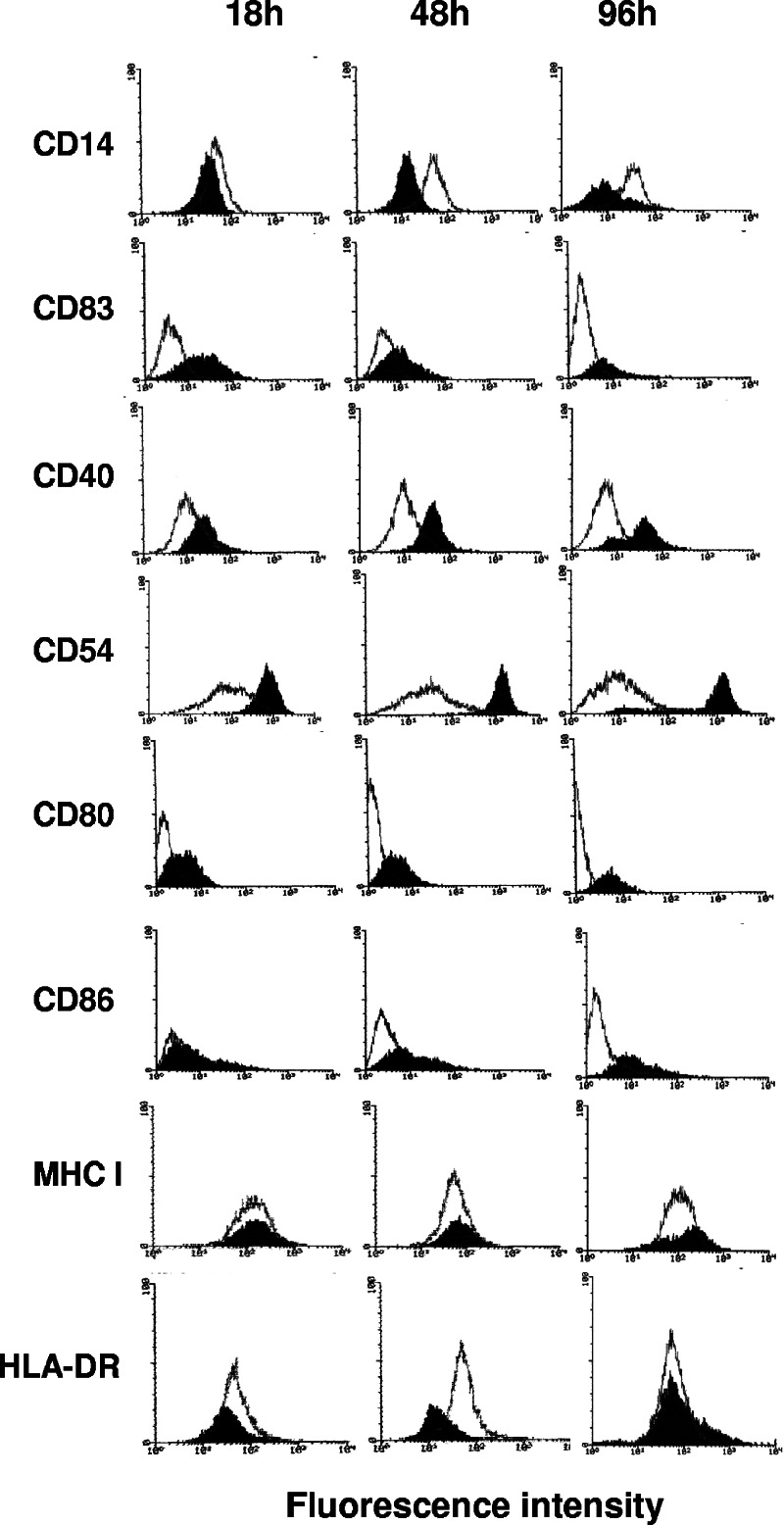
Time course of surface marker expression.
THP-1 cells were treated with S. gordonii, and the phenotypic modifications were analyzed by flow cytometry after 18, 48, and 96 h. CD14 expression was down-regulated during the time course analysis, while a clear up-regulation was observed for CD40 and CD54 molecules. CD83 reached the highest level of expression at 18 h and then slightly declined at 48 and 96 h (Fig. 3). A sixfold increase in the MFI of CD80 and CD86 costimulatory molecules was observed 96 h after the bacterial addition. The expression of MHC class I was not influenced by bacterial treatment at any time points.
FIG. 3.
Time course analysis of phenotypic modification of THP-1 cells treated with S. gordonii. Flow cytometric analysis of THP-1 cells cultured with S. gordonii at a ratio of 100 bacteria per cell and harvested 18, 48, and 96 h after addition of bacteria. The expression of different surface markers on treated (solid histogram) or untreated (open histogram) cells is reported. In all experiments, the isotype-matched control peak was between a linear value of 0 and 10 on the x axis (data not shown). Data are representative of four independent experiments.
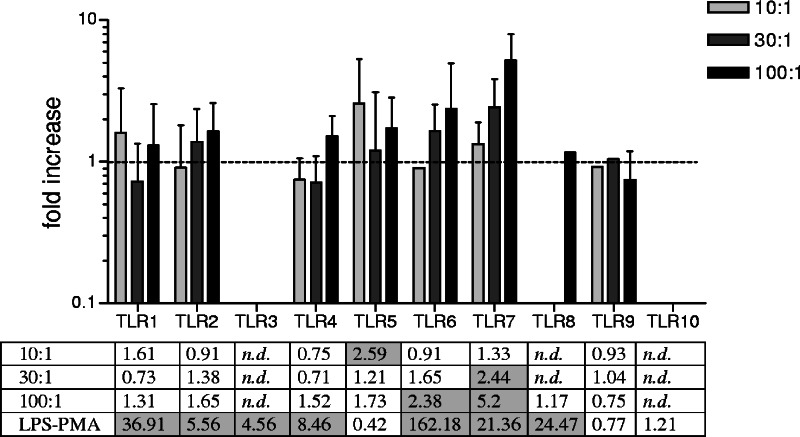
TLR expression.
The expression of TLRs was assessed by real-time RT-PCR in THP-1 cells treated for 6 h with different doses of S. gordonii (10, 30, and 100 bacteria per cell). Treatment of THP-1 cells with bacteria increased the expression of several TLR mRNAs, as observed for TLR5 and TLR7 and at a lower level for TLR6 (Fig. 4). The strongest effect was detected on the expression of TLR7, with the higher bacterial dose showing a 5.2-fold increase over levels for untreated cells, while a 2.59-fold increase of TLR5 was induced at the dose of 10 bacteria per cell.
FIG. 4.
TLR expression in THP-1 cells following stimulation with S. gordonii. THP-1 cells were stimulated for 6 h with different doses of bacteria (10, 30, or 100 bacteria per cell) or with phorbol myristate acetate (10 ng/ml) for 12 h and then with lipopolysaccharide (100 ng/ml) for 6 h as a positive control. Data are normalized to cyclophilin-B expression. Values, expressed as increases (n-fold) over values for untreated cells, are reported as means ± standard deviations from five independent experiments. Shaded squares indicate an increase (n-fold) of ≥2. n.d., not detectable.
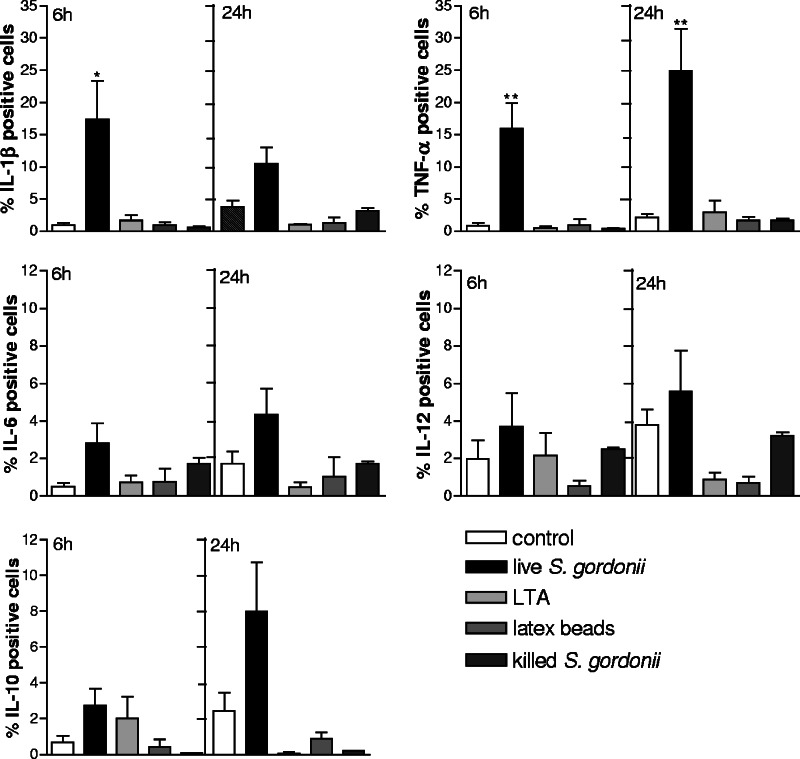
Production of cytokines.
THP-1 cells treated with S. gordonii were analyzed for cytokine production. The flow cytometric analysis was performed by immunofluorescent staining of intracellular cytokines on permeabilized cells. Production of IL-1β, TNF-α, IL-6, IL-12, and IL-10 induced following 6 or 24 h of treatment with live S. gordonii was compared to that induced by other stimuli, such as LTA, latex beads, and heat-killed S. gordonii (Fig. 5). A significant increase in the percentages of THP-1 cells expressing IL-1β and TNF-α was observed following 6 h of bacterial stimulation compared to those of untreated cells (P = 0.039 and P = 0.009, respectively). TNF-α production further increased after 24 h of bacterial treatment, with 25% of cells being positive (P = 0.01). On the contrary, the production of IL-6, IL-10, and IL-12 was only slightly affected by bacterial treatment (Fig. 5). The treatment with LTA (10 μg/ml), latex beads (100 per cell), or heat-killed bacteria (100 per cell) induced a lower level of cytokine production, in some cases comparable to that for untreated cells (Fig. 5). The cytokine production also has been assessed in human monocytes from two different donors, and the increase of the proinflammatory cytokines TNF-α and IL-β has been confirmed (data not shown).
FIG. 5.
Cytokine production of THP-1 cells treated with live S. gordonii (100 bacteria per cell), LTA (10 μg/ml), latex beads (100 beads per cell), heat-killed S. gordonii (100 bacteria per cell), or medium alone (control). The cytokine production was evaluated by flow cytometric analysis on permeabilized THP-1 cells following 6 or 24 h of incubation with the different stimuli. The percentage of positive cells minus the isotype control value is reported. Data are expressed as means ± standard deviations from six independent experiments. *, P ≤ 0.05; **, P ≤ 0.01, compared to results for untreated cells.
Effect on phagocytic activity.
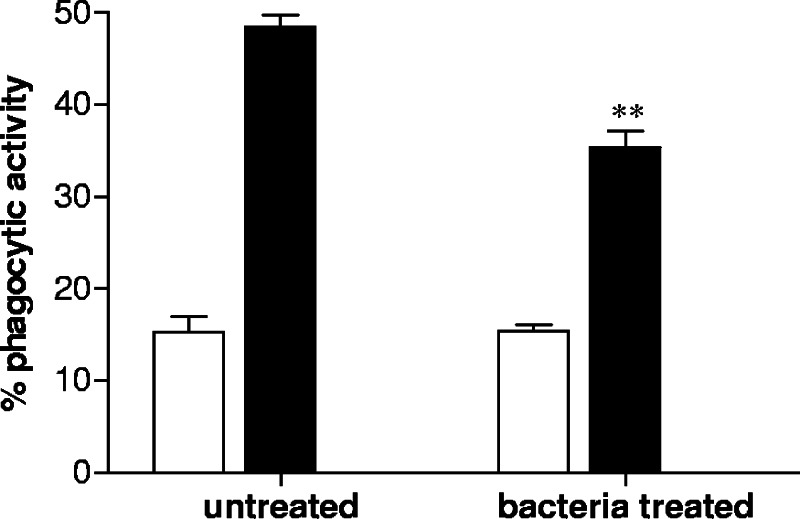
THP-1 cells were stimulated with S. gordonii for 18 h and then incubated with fluorescent beads in order to assess whether the phagocytic ability of cells was influenced by bacterial treatment. As shown in Fig. 6, the capacity of THP-1 cells to internalize particles was significantly reduced by bacterial treatment (P ≤ 0.01). While 48% of untreated cells were able to internalize fluorescent beads, only the 35% of THP-1 cells previously treated with S. gordonii maintained the phagocytic ability. The same experiment was performed at 4°C in order to control the aspecific association of beads with cells (background value) (Fig. 6). A 40% reduction of the phagocytic activity of THP-1 cells stimulated with bacteria was calculated after subtraction of the background value.
FIG. 6.
Phagocytic activity of THP-1 cells treated with S. gordonii. THP-1 cells were cultured with S. gordonii (100 bacteria per cell) or medium alone for 18 h and then incubated with fluorescent beads at 37°C (▪) or at 4°C (□) as a control for phagocytosis. Cells were analyzed after 24 h by flow cytometry. The percentage of cells which have internalized fluorescent beads is reported. Data are expressed as means ± standard deviations from three experiments. **, P ≤ 0.01 compared to results for untreated cells.
DISCUSSION
In this work, human monocytes (THP-1 cell line and peripheral blood monocytes) were characterized following interaction with the bacterial vaccine vector S. gordonii. We show that upon bacterial treatment, human monocytes display the following: (i) up-regulation of surface markers CD83, CD40, CD80, and CD54; (ii) down-regulation of surface marker CD14; (iii) increased expression of mRNA for TLR5, TLR6, and TLR7; (iv) production of proinflammatory cytokines IL-1-β and TNF-α; and (v) reduction of phagocytic activity.
The phenotypic modifications induced by bacterial treatment were dose dependent and were not observed following treatment with latex beads, demonstrating that a phagocytic stimulus itself was not responsible for the changes in surface molecule expression. The observed up-regulation of CD83, a typical marker of mature DCs, also was recently reported for monocyte-derived DCs treated with Lactobacillus plantarum and Salmonella enterica serovar Typhimurium (32). CD54, an adhesion molecule involved in leukocyte trafficking toward inflammatory stimuli, also increased in a dose-dependent way, and an up-regulation was observed during the time course analysis. The expression of costimulatory molecules, such as CD80 and CD86, also increased following 96 h of bacterial stimulation. Interestingly, the expression of HLA-DR was down-regulated by bacterial treatment, as previously observed with heat-killed Mycobacterium bovis and Escherichia coli (5). Using peripheral blood monocytes we have demonstrated that the phenotypic modifications observed following bacterial stimulation were not restricted to the monocytic THP-1 cell line. In fact, the interaction of peripheral blood monocytes with S. gordonii induced the up-regulation of CD83, CD40, CD54, CD80, and also HLA-DR.
TLRs are recognition receptors for different microbial ligands, including bacteria. The expression of TLR mRNAs was influenced in a dose-dependent manner by the interaction with S. gordonii. An increased expression of TLR5, TLR6, and TLR7 was observed, with the highest level detected for TLR7. The increased expression of TLR7 has been previously found in THP-1 cells and human monocytes treated with the gram-positive bacterium Staphylococcus aureus (38). In contrast, no increase in the expression of TLR2 and TLR4 was observed; this is in agreement with what was reported previously for human monocytes treated with L. plantarum and E. coli (12).
We have also investigated the pattern of cytokines produced by THP-1 cells stimulated with S. gordonii. A significant increase in the synthesis of proinflammatory cytokines, such as IL-1β and TNF-α, was observed. In particular, 25% of THP-1 cells were positive for the production of TNF-α 24 h from stimulation. In contrast, the production of IL-6, IL-12, and IL-10 was only slightly affected by the bacterial treatment. The low production of IL-12 could depend on the absence of MHC class II and CD40 triggering, since it occurs during T-cell antigen presentation (2, 17, 37). A similar pattern of cytokine production was recently found in human monocytes and monocyte-derived DCs treated with gram-positive commensal bacteria (9, 10, 12, 32). We have also demonstrated that THP-1 cells previously stimulated with S gordonii show a reduction of their ability to engulf fluorescent beads compared with untreated THP-1 cells.
In conclusion, this work shows that the immunostimulatory activity of the vaccine vector S. gordonii is not restricted to the induction of DC maturation (3, 4, 30) but also affects the differentiation process of human monocytes.
Acknowledgments
We gratefully acknowledge the valuable collaboration of the late Giuseppe Fanetti and Giuseppe Campoccia (Immunoematologia e Servizio Trasfusionale, Policlinico Santa Maria alle Scotte, Siena, Italy).
This study has been carried out with financial support from the Commission of the European Communities, Sixth Framework Programme, contract LSHP-CT-2003-503240, “Mucosal Vaccines for Poverty-Related Diseases” (MUVAPRED), Fifth Framework Programme, contract QLK2-CT-2002-00882, “Mucosal Vaccines Against Human and Simian Immunodeficiency Viruses Based on Dendritic Cells” (MUVADEN), from “Azione Concertata Italiana per lo Sviluppo di un Vaccino HIV/AIDS” (ICAV) of the Istituto Superiore di Sanità, contract no. 45F.29, MIUR (FIRB RBNE01RB9B_009 and FIRB RBNE01N9EE) and PAR Progetti di Ricerca 2005.
REFERENCES
- 1.Braat, H., E. C. de Jong, J. M. H. van den Brande, M. L. Kapsenberg, M. P. Peppelenbosch, E. A. F. van Tol, and S. J. H. van Deventer. 2004. Dichotomy between Lactobacillus rhamnosus and Klebsiella pneumoniae on dendritic cell phenotype and function. J. Mol. Med. 82:197-205. [DOI] [PubMed] [Google Scholar]
- 2.Cella, M., D. Scheidegger, K. Palmer-Lehmann, P. Lane, A. Lanzavecchia, and G. Alber. 1996. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J. Exp. Med. 184:747-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corinti, S., D. Medaglini, A. Cavani, M. Rescigno, G. Pozzi, P. Ricciardi-Castagnoli, and G. Girolomoni. 1999. Human dendritic cells very efficiently present a heterologous antigen expressed on the surface of recombinant Gram-positive bacteria to CD4+ T lymphocytes. J. Immunol. 163:3029-3036. [PubMed] [Google Scholar]
- 4.Corinti, S., D. Medaglini, C. Prezzi, G. Pozzi, and G. Girolomoni. 2000. Human dendritic cells are superior to B cells at presenting a major histocompatibility complex class-II restricted heterologous antigen expressed on recombinant Streptococcus gordonii. Infect. Immun. 68:1879-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Lerma Barbaro, A., G. Tosi, M. T. Valle, A. M. Megiovanni, S. Sartoris, A. D'Agostino, O. Soro, M. C. Mingari, G. W. Canonica, F. Manca, and R. S. Accolla. 1999. Distinct regulation of HLA class II and class I cell surface expression in the THP-1 macrophage cell line after bacterial phagocytosis. Eur. J. Immunol. 29:499-511. [DOI] [PubMed] [Google Scholar]
- 6.Di Fabio, S., D. Medaglini, C. M. Rush, G. Corrias, G. L. Panzini, M. Pace, P. Verani, G. Pozzi, and F. Titti. 1998. Vaginal immunization of Cynomolgus monkeys with Streptococcus gordonii expressing HIV-1 and HPV 16 antigens. Vaccine 16:485-492. [DOI] [PubMed] [Google Scholar]
- 7.Fujii, S., K. Liu, C. Smith, A. J. Bonito, and R. M. Steinman. 2004. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J. Exp. Med. 199:1607-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geissmann, F., S. Jung, and D. R. Littman. 2003. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19:71-82. [DOI] [PubMed] [Google Scholar]
- 9.Grangette, C., S. Nutten, E. Palumbo, S. Morath, C. Hermann, J. Dewulf, B. Pot, T. Hartung, H. Pascal, and A. Mercenier. 2005. Enhanced anti-inflammatory capacity of a Lactobacillus plantarum mutant synthesizing modified teichoic acids. Proc. Natl. Acad. Sci. USA 102:10321-10326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hessle, C. C., B. Andersson, and A. E. Wold. 2005. Gram-positive and Gram-negative bacteria elicit different patterns of pro-inflammatory cytokines in human monocytes. Cytokine 30:311-318. [DOI] [PubMed] [Google Scholar]
- 11.Jones, M. A., S. Totemeyer, D. J. Maskell, C. E. Bryant, and P. A. Barrow. 2003. Induction of pro-inflammatory responses in the human monocytic cell line THP-1 by Campylobacter jejuni. Infect. Immun. 71:2626-2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karlsson, H., P. Larsson, A. E. Wold, and A. Rudin. 2004. Pattern of cytokine responses to gram-positive and gram-negative commensal bacteria is profoundly changed when monocytes differentiate into dendritic cells. Infect. Immun. 72:2671-2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kilian, M., L. Mikkelsen, and J. Henrichsen. 1989. Taxonomic study of viridans streptococci: description of Streptococcus gordonii sp. nov. and emended descriptions of Streptococcus sanguis (White and Niven 1946), Streptococcus oralis (Bridge and Sneath 1982), and Streptococcus mitis (Andrews and Horder 1906). Int. J. Syst. Bacteriol. 39:471-484. [Google Scholar]
- 14.Kotloff, K. L., S. S. Wassermann, K. F. Jones, S. Livio, D. E. Hruby, C. A. Franke, and V. A. Fischetti. 2005. Clinical and microbiological responses of volunteers to combined intranasal and oral inoculation with a Streptococcus gordonii carrier strain intended for future use as a group A Streptococcus vaccine. Infect. Immun. 73:2360-2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 16.Medaglini, D., A. Ciabattini, A. M. Cuppone, C. Costa, S. Ricci, M. Costalonga, and G. Pozzi. 2006. In vivo activation of naive CD4+ T cells in nasal mucosa-associated lymphoid tissue following intranasal immunization with recombinant Streptococcus gordonii. Infect. Immun. 74:2760-2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medaglini, D., A. Ciabattini, M. R. Spinosa, T. Maggi, H. Marcotte, M. R. Oggioni, and G. Pozzi. 2001. Immunization with recombinant Streptococcus gordonii expressing tetanus toxin fragment C confers protection from lethal challenge in mice. Vaccine 19:1931-1939. [DOI] [PubMed] [Google Scholar]
- 18.Medaglini, D., M. R. Oggioni, and G. Pozzi. 1998. Vaginal immunization with recombinant Gram positive bacteria. Am. J. Reprod. Immunol. 39:199-208. [DOI] [PubMed] [Google Scholar]
- 19.Medaglini, D., G. Pozzi, T. P. King, and V. A. Fischetti. 1995. Mucosal and systemic immune responses to a recombinant protein expressed on the surface of the oral commensal bacterium Streptococcus gordonii after oral colonization. Proc. Natl. Acad. Sci. USA 92:6868-6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Medaglini, D., S. Ricci, T. Maggi, C. M. Rush, R. Manganelli, M. R. Oggioni, and G. Pozzi. 1997. Recombinant Gram-positive bacteria as vehicles of vaccine antigens. Biotechnol. Annu. Rev. 3:297-312. [Google Scholar]
- 21.Medaglini, D., C. M. Rush, P. Sestini, and G. Pozzi. 1997. Commensal bacteria as vectors for mucosal vaccines against sexually transmitted diseases: vaginal colonization with recombinant streptococci induces local and systemic antibodies in mice. Vaccine 15:1330-1337. [DOI] [PubMed] [Google Scholar]
- 22.Oggioni, M. R., F. Iannelli, S. Ricci, D. Chiavolini, R. Parigi, C. Trappetti, J. P. Claverys, and G. Pozzi. 2004. Antibacterial activity of a competence-stimulating peptide in experimental sepsis caused by Streptococcus pneumoniae. Antimicrob. Agents Chemother. 48:4725-4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oggioni, M. R., R. Manganelli, M. Contorni, M. Tommasino, and G. Pozzi. 1995. Immunization of mice by oral colonization with live recombinant commensal streptococci. Vaccine 13:775-779. [DOI] [PubMed] [Google Scholar]
- 24.Oggioni, M. R., D. Medaglini, T. Maggi, and G. Pozzi. 1999. Engineering the Gram-positive cell surface for construction of bacterial vaccine vectors. Methods 19:163-173. [DOI] [PubMed] [Google Scholar]
- 25.Oggioni, M. R., D. Medaglini, L. Romano, F. Peruzzi, T. Maggi, L. Lozzi, L. Bracci, M. Zazzi, F. Manca, P. E. Valensin, and G. Pozzi. 1999. Antigenicity and immunogenicity of the V3 domain of HIV-1 gp120 expressed on the surface of Streptococcus gordonii. AIDS Res. Hum. Retrovir. 15:451-459. [DOI] [PubMed] [Google Scholar]
- 26.Oggioni, M. R., and G. Pozzi. 1996. A host-vector system for heterologous gene expression in Streptococcus gordonii. Gene 169:85-90. [DOI] [PubMed] [Google Scholar]
- 27.Palucka, K. A., N. Taquet, F. Sanchez-Chapuis, and J. C. Gluckman. 1998. Dendritic cells as the terminal stage of monocyte differentiation. J. Immunol. 160:4587-4595. [PubMed] [Google Scholar]
- 28.Randolph, G. J., S. Beaulieu, S. Lebecque, R. M. Steinman, and W. A. Muller. 1998. Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science 282:480-483. [DOI] [PubMed] [Google Scholar]
- 29.Randolph, G. J., K. Inaba, D. F. Robbiani, R. M. Steinman, and W. A. Muller. 1999. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity 11:753-761. [DOI] [PubMed] [Google Scholar]
- 30.Rescigno, M., S. Citterio, C. Thery, M. Rittig, D. Medaglini, G. Pozzi, S. Amigorena, and P. Ricciardi-Castagnoli. 1998. Bacterial-induced neo-biosynthesis, stabilization and surface expression of functional class I molecules in mouse dendritic cells. Proc. Natl. Acad. Sci. USA 95:5229-5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ricci, S., D. Medaglini, C. M. Rush, A. Marcello, R. Manganelli, G. Palù, and G. Pozzi. 2000. Immunogenicity of the B monomer of the Escherichia coli heat-labile toxin expressed on the surface of Streptococcus gordonii. Infect. Immun. 68:760-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rimoldi, M., M. Chieppa, P. Larghi, M. Vulcano, P. Allavena, and M. Rescigno. 2005. Monocyte-derived dendritic cells activated by bacteria or by bacteria-stimulated epithelial cells are functionally different. Blood 106:2818-2826. [DOI] [PubMed] [Google Scholar]
- 33.Rotta, G., E. W. Edwards, S. Sangaletti, C. Bennett, S. Ronzoni, M. P. Colombo, R. M. Steinman, G. J. Randolph, and M. Rescigno. 2003. Lipopolysaccharide or whole bacteria block the conversion of inflammatory monocytes into dendritic cells in vivo. J. Exp. Med. 198:1253-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thornton, J., and L. S. Mc Daniel. 2005. THP-1 monocytes up-regulate intercellular adhesion molecule 1 in response to pneumolysin from Streptococcus pneumoniae. Infect. Immun. 73:6493-6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsuchiya, S., M. Yamabe, Y. Yamaguchi, Y. Kobayashi, T. Konno, and K. Tada. 1980. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Cancer 26:171-176. [DOI] [PubMed] [Google Scholar]
- 36.van Furth, R., and Z. A. Cohn. 1968. The origin and kinetics of mononuclear phagocytes. J. Exp. Med. 128:415-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winzler, C., P. Rovere, M. Rescigno, F. Granucci, G. Penna, L. Adorini, V. S. Zimmerman, J. Davoust, and P. Ricciardi-Castagnoli. 1997. Maturation stages of mouse dendritic cells in growth factor-dependent long-term cultures. J. Exp. Med. 185:317-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zarember, K. A., and P. J. Godowski. 2002. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J. Immunol. 168:554-561. [DOI] [PubMed] [Google Scholar]