Abstract
Many Helicobacter pylori genetic studies would benefit from an ability to move DNA sequences easily between strains by transformation and homologous recombination, without needing to leave a conventional drug resistance determinant at the targeted locus. Presented here is a two-gene cassette that can be selected both (i) against, due to a Campylobacter jejuni rpsL gene (dominant streptomycin susceptibility in cells also carrying an rpsL-strr allele), and (ii) for, due to an erm gene (erythromycin resistance). This rpsL,erm cassette's utility was assessed by using it to replace four gene loci (mdaB, frxA, fur, and nikR) in four streptomycin-resistant [Strr] strain backgrounds (derivatives of 26695, SS1, X47, and G27MA). The resultant 16 strains (phenotypically erythromycin resistant [Ermr] and Strs) were each transformed with wild-type genomic DNAs, and Strr derivatives were selected. The desired Erms Strr isolates were obtained at frequencies that ranged from 17 to 96% among Strr transformants, with the Erms yield apparently depending on the strain background and genome location of the targeted locus. The ease of isolating unmarked transformants described here should be valuable for many H. pylori molecular genetic and evolutionary analyses.
Genetic studies of the gastric pathogen Helicobacter pylori (21) often entail (i) the construction of cloned DNAs or PCR products containing a drug resistance determinant inserted into or near a gene of interest, (ii) DNA transformation, and (iii) selection for resistant transformants, which arise by homologous recombination and replacement of recipient DNA sequences by corresponding sequences from donor DNA. Although this strategy has been used in hundreds of H. pylori studies, successfully overcoming the rarity of natural transformation events, it becomes seriously limiting or flawed in at least four interesting situations, in each case because transformants retain resistance determinants at targeted loci: (i) if changes at numerous loci in the same strain are needed, e.g., in studies of phenotypes determined by multiple genes with additive or redundant effects (because only a few selectable resistance markers are available for H. pylori); (ii) if donor resistance determinants might affect downstream gene expression, or cellular physiology more generally (e.g., in many cases the resistance enzymes use cellular metabolites to modify antibiotics and thereby confer resistance to them [9]); (iii) if alleles with subtle (e.g., point mutation) differences are to be compared; or (iv) if alleles from many strains are to be studied in a common genetic background. Such limitations can be overcome by using a recipient strain that contains a contraselectable marker at the locus of interest. This allows the desired transformants to be selected by the loss of this recipient marker, thereby bypassing the need for a conventional resistance determinant in donor DNA.
Three genes that have been used for contraselection in other systems are thyA (thymidylate requirement; trimethoprim sensitivity) (4, 34), sacB (sucrose sensitivity) (5, 27, 33), and rpsL (streptomycin sensitivity) (25, 33). H. pylori is naturally trimethoprim resistant. This could be ascribed to (i) its apparent lack of a thyA gene (based on BLASTP homolog searches [31]); (ii) an intrinsic resistance of its enzyme for reduced folate synthesis, an apparent dihydrofolate reductase-dihydropteroate synthase chimaera (18); and/or (iii) other factors. An early report had indicated that sacB could serve as a contraselectable marker in H. pylori but did not describe the details of efficiency or complications that may have been encountered (8). In our H. pylori sacB experiments, however, selection for resistance to a range of sucrose concentrations gave far more background growth of nominally sensitive cells than was expected based on Escherichia coli experiences (33, 34), and conditions for sucrose-based differential killing of sacB-containing H. pylori cells varied among trials (unpublished data). Other groups had also found contraselection of sacB to be difficult in H. pylori although better than having no such marker at all (T. L. Cover, unpublished data; R. Haas, unpublished data). A third contraselection strategy, implemented here, uses the rpsL (ribosomal protein S12) gene and is based on the dominance of wild-type streptomycin-sensitive (Strs) alleles to resistance-conferring mutant alleles (16, 25).
Historically, streptomycin killing has been associated with diverse effects, including membrane damage and irreversible streptomycin uptake. Paradoxically, these were all blocked by the bacteriostatic translation inhibitor chloramphenicol. This implicated the capacity to synthesize proteins, even though streptomycin also inhibited protein synthesis. Sublethal streptomycin concentrations suppressed nonsense mutations, increased mutation, and caused membrane fragility. Biochemical studies showed that streptomycin allowed the continuation of translation once begun, but with errors in translation (misreading), and that it also allowed ribosomes to bind to mRNAs but blocked them from initiating translation (10). Davis' early unifying explanation for streptomycin's lethality (10) focused on errors it induced during translation and a resultant accumulation of defective proteins. He focused in particular on membrane proteins, interpreting that defects in them were responsible for the disruption of cell membrane integrity, massive leakage of ions and molecules, and cell death. This could explain the dominance of streptomycin sensitivity over resistance, as could the binding of Strs ribosomes to mRNAs, blocking the access of resistant ribosomes to these mRNAs, failure to translate them, and their ensuing degradation (10).
Recent exquisitely detailed molecular and genetic analyses provide mechanistic understanding. Streptomycin binds several specific 16S rRNA loops and thereby diminishes ribosome flexibility and the changes in conformation that charged tRNA binding normally induces. It stabilizes a “ribosomal ambiguity” (ram) state in which there is little if any of the proofreading needed for error-free protein synthesis (7, 15, 22, 24). Protein S12, which is mutated in Strr strains, binds rRNA sequences near sites of streptomycin binding. Most positions in which change confers resistance are in S12 domains that interact with the rRNA target. In addition, most Strr mutant S12 proteins increase the accuracy of translation, apparently by destabilizing the ram state that streptomycin itself induces; the most extreme of such rpsL (S12) alleles make growth streptomycin dependent. This can be overcome by mutations in ribosomal proteins S4 and S5, that in turn stabilize the ram state in this dynamic and finely tuned ribonucleoprotein machine (7, 14).
The two S12 residues that are most frequently changed in streptomycin-resistant H. pylori are Lys43 (Lys42 in E. coli numbering), which contacts the rRNA-bound streptomycin directly, and Lys88 (Lys87 in E. coli numbering), which is nearby in the structure (7, 14, 32). Although the Lys-to-Arg changes at these codons seem to be quite innocuous (14), other amino acid replacements can markedly diminish bacterial fitness. In particular, a large fitness cost and a resulting selection for compensatory mutations in other genes have been documented in E. coli and Salmonella enterica serovar Typhimurium using strains with Lys42 replaced with Asn (19, 26) (not Arg). More critically, the Lys88Arg replacement used here, and also the Lys43Arg allele, have each been incorporated into H. pylori strains used for mouse infections, without obvious negative effects on fitness (12, 13, 23).
Streptomycin contraselection for chromosomal gene replacement had been developed for H. pylori previously (13) but has not been much used, perhaps because very few of the Strr isolates recovered after transformation had sustained the desired replacement. This, we suspected, was due to the near identity of the strs and strr H. pylori rpsL alleles used: frequent gene conversion between them would result in unwanted Strr gene convertants vastly outnumbering the desired Strr transformants. If correct, it seemed that the rpsL gene might still be useful for H. pylori genetic engineering if the rpsL gene conversion could be reduced.
Generalized recombination (gene conversion included) depends on close matches between participating DNA molecules and is reduced by sequence divergence, even in species that, like H. pylori, lack the ability to cleave mismatch-containing (heteroduplex) DNAs (11, 20, 31). The rpsL genes of H. pylori and Campylobacter jejuni differ by some 18% in overall DNA sequence, but the encoded S12 proteins are 95% similar in amino acid sequence. The present study was initiated with an expectation or hope that H. pylori ribosomes containing the C. jejuni S12 protein would be functional and Strs and that DNA sequence divergence between the rpsL genes of C. jejuni and H. pylori would diminish gene conversion sufficiently for the effective recovery of transformants by streptomycin contraselection. The rpsL,erm (streptomycin susceptibility, erythromycin resistance) cassette that we constructed and tested in these studies is diagrammed in Fig. 1.
FIG. 1.
Structure of the 1.5-kb rpsL,erm cassette, which confers dominant streptomycin susceptibility and selectable erythromycin resistance. Open boxes designate open reading frames; solid line indicates noncoding sequences. The cassette contains 272 bp of C. jejuni sequence upstream of the C. jejuni rpsL gene (408 bp), a 145-bp spacer, and then the 735-nucleotide erm gene.
MATERIALS AND METHODS
H. pylori strains and general methods.
The H. pylori strains used here were cultured at 37C in a standard microaerobic (5% O2, 10% CO2) atmosphere on brain heart infusion-horse blood agar plates, with 0.4% Isovitalex and the antibiotics amphotericin B (8 μg/ml), trimethoprim (5 μg/ml), and vancomycin (6 μg/ml). Erythromycin (10 μg/ml) and streptomycin (10 μg/ml) were added to this agar as needed to select for transformants or screen colonies for resistance to either drug. Natural transformation was carried out by adding ∼1 μg of genomic DNA or PCR product to a lawn of cells growing exponentially on nonselective medium and, after overnight incubation, restreaking the population on selective (streptomycin- or erythromycin-containing) medium to obtain transformant colonies. Standard methods were used for PCR and for sequencing of the PCR products (2, 30).
Four unrelated H. pylori strains were used here, each chosen because it is much used in other molecular genetic studies: the genome-sequenced reference strain 26695 (31), which we use to identify the numerous genes that contribute to high-level metronidazole resistance (2); unrelated strains SS1 and X47 (X47 was originally called X47-2AL) (12, 17), which colonize mice, preferentially occupy different regions of the stomach (1, 28), and differ in the phenotypic consequences of inactivation of at least certain metabolic genes (30); and G27MA, a cell-culture-adapted derivative of the G27 strain that is frequently used for studies of H. pylori-host cell interaction (3) and that can efficiently colonize DBA/2 mice (W.-K. Lee, D. Dailidiene, and D. E. Berg, unpublished data). The erm gene used in the rpsL,erm cassette (Fig. 1) was originally from plasmid pRH151, kindly provided by Rainer Haas, and was matched in sequence to that of pNE131 (GenBank accession no. NC_001390). The C. jejuni rpsL gene used in this cassette was from plasmid pDRF265, kindly provided by David Hendrixson and Victor DiRita.
To prepare for the development and testing of the rpsL,erm cassette, an strr allele of the normal chromosomal H. pylori rpsL gene was constructed and introduced into strain 26695 to generate 26695-str. This was achieved by PCR of rpsL DNA from 26695 with the primers rpsL1 and rpsL2 (Table 1) to generate a 203-bp PCR product that contained the A-to-G changes at each of the two sites most frequently responsible for streptomycin resistance (codons 43 and 88; Lys to Arg in each case) (32). This product was used to transform strain 26695 to streptomycin resistance to generate “26695-str.” PCR-based DNA sequencing showed that each of several such 26695-str transformants contained an A-to-G change at nucleotide position 263 but was wild type at nucleotide position 129 (i.e., a change of Lys to Arg at codon 88 only). Genomic DNA from one such transformant was used to transform SS1 and G27MA to streptomycin resistance (generating SS1-str and G27MA-str). Strain X47 is already streptomycin resistant (10) and was found to contain the same rpsL A263G (Lys88Arg) allele.
TABLE 1.
PCR primers used in this study and features of their amplification products
| Process | Primera | Sequence (5′-3′)b | Product size and/or comments |
|---|---|---|---|
| Creation of streptomycin resistance allele (strr) of H. pylori rpsL gene | rpsL1 | AGGGGTTTGTACTAGGGTTTATACGACTACCCCTAAGAAGCCTAACTCG-3′ | 203 bp; the rpsL fragment containing the Lys43Arg mutation (AAA129AGA) strr allele in primer rpsL1 and the Lys88Arg mutation (AAG263AGG) strr allele in rpsL2 are shown (mutations are in boldface) |
| rpsL2 | GAACGATGTGGTATTTCACACCGGGTAAATCCCTAACCCTACCCCCACG-3′ | ||
| H. pylori rpsL gene sequencing | rpsL-F | GGGAACAGGCATGTATAAGA-3′ | 675 bp |
| rpsL-R | CGTCGAACATCATCTTATTGAT-3′ | ||
| erm gene amplification from plasmid pRH151 | erm-F | CAATAATCGCATCAGATTGCAGTA-3′ | 853 bp; the 5′ ends of the erm-F and erm-R primers are 118 bp upstream of the 5′ end and exactly at the 3′ end of the erm open reading frame |
| erm-R | TTACTTATTAAATAATTTATAGCTATTGAA-3′ | ||
| ΔmdaB-erm allele construction | 1. m1 | CCTTCTACCATTAAAATGTAATTG-3′ | 454 bp |
| 2. mermF1 | TACTGCAATCTGATGCGATTATTGCTAATTAAGGAGTGGTCATGTTC-3′ | ||
| 3. erm-F | CAATAATCGCATCAGATTGCAGTA-3′ | 853 bp | |
| 4. erm-R | TTACTTATTAAATAATTTATAGCTATTGAA-3′ | ||
| 5. mermR1 | TTCAATAGCTATAAATTATTTAATAAGTAAGGCTTGTTTATTCCACAATAAAGTC-3′ | 526 bp | |
| 6. m4 | GAGCTTATGGAAGAATACAGCTCCTTG-3′ | ||
| All | The 5′ ends of primers are as follows: primer 1, 596 bp upstream of the 5′ end of mdaB; primer 2, 165 bp upstream of the 5′ end of mdaB; primer 5, 62 bp downstream of the 3′ end of mdaB; primer 6, 563 bp downstream of the 3′ end of mdaB; the PCR products with primers 1 and 6 are 1,744 and 1,833 bp long from the wild-type and ΔmdaB-erm DNAs, respectively | ||
| ΔmdaB-rpsL,erm allele construction (C. jejuni rpsL insertion in the ΔmdaB-erm allele) | 1. m1 | CCTTCTACCATTAAAATGTAATTG-3′ | 454 bp |
| 2. mrpsL | CTAATTAAGGAGTGGTCATGTTC-3′ | ||
| 3. rpsLCj-F | GAACATGACCACTCCTTAATTAGGATGCTTTATAACTATGGATTAAACAC-3′ | 686 bp | |
| 4. rpsLCj-R | TACTGCAATCTGATGCGATTATTGATCTAACGGATTTGTCTGTATG-3′ | ||
| 5. erm-F | CAATAATCGCATCAGATTGCAGTA-3′ | 1,379 bp | |
| 6. m4 | GAGCTTATGGAAGAATACAGCTCCTTG-3′ | ||
| All | The PCR products with primers 1 and 6 are 1,744 and 2,518 bp long from the wild-type and ΔmdaB-rpsL,erm DNAs, respectively; primers 3 and 4 were used for amplification of the C. jejuni rpsL gene from plasmid DRH265; the 5′ ends of primers 3 and 4 are 273 bp upstream of the 5′ end and 27 bp downstream of the 3′ end of the rpsL open reading frame | ||
| ΔnikR-rpsL,erm allele construction (replacement of nikR with the rpsL,erm cassette) | 1. 1338A1-F | TAATAAGCCCACATAAGGCGCG-3′ | 547 bp |
| 2. 1338A2-R | TGTTTAATCCATAGTTATAAAGCATCATCCTTTTTTGGCATGAGTTCG-3′ | ||
| 3. rpsL-F-1 | GATGCTTTATAACTATGGATTAAACAC-3′ | 1,539 bp | |
| 4. erm-R | TTACTTATTAAATAATTTATAGCTATTGAA-3′ | ||
| 5. 1338A3-F | AATAGCTATAAATTATTTAATAAGTAAGGGGTTAAATTCGCTAAATTGAC-3′ | 481 bp | |
| 6. 1338AA4-R | TTGGATCTCTTCATAGCCAATCC-3′ | ||
| All | The 5′ ends of the primers are as follows: primer 1, 556 bp upstream of the 5′ end of nikR; primer 2, 10 bp upstream of the 5′ end of nikR; primer 5, inside nikR, 54 bp from the 3′ end; primer 6, 427 bp downstream of the 3′ end of nikR; the PCR products with primers 1 and 6 are 1,430 and 2,567 bp long from the wild-type and ΔnikR-rpsL,erm DNAs, respectively | ||
| ΔfrxA-rpsL,erm allele construction (replacement of frxA with the rpsL,erm cassette) | 1. frxA1-F | GTGCGCTTCAAAGCTTGGGTTA-3′ | 444 bp |
| 2. frxA2-R | GTGTTTAATCCATAGTTATAAAGCATCGCAACCACTTGTTCTCTGTCCA-3′ | ||
| 3. rpsL-F-1 | GATGCTTTATAACTATGGATTAAACAC-3′ | 1,539 bp | |
| 4. erm-R | TTACTTATTAAATAATTTATAGCTATTGAA-3′ | ||
| 5. frxA3-F | TCAATAGCTATAAATTATTTAATAAGTAAGCTTGGCGTTAGCCAAGTGCT-3′ | 275 bp | |
| 6. frxA4-R2 | GCCTTCAATGTTGCGCTCTTTGT-3′ | ||
| All | The 5′ ends of the primers are as follows: primer 1, 421 bp upstream of the 5′ end of frxA; primer 2, within frxA, 23 bp from its 5′ end; primer 5, 36 bp downstream of the 3′ end of frxA; primer 6, 311 bp downstream of the 3′ end of frxA; the PCR products with primers 1 and 6 are 1,386 and 2,258 bp long from the wild-type and ΔfrxA-rpsL,erm DNAs, respectively | ||
| Δfur-rpsL,erm allele construction (replacement of fur with rpsL,erm cassette) | 1. x5k-F | CCTTAATTTAGCCGCTTCTTGTTTG-3′ | 553 bp |
| 2. fur-R-A2 | AAGTGTTTAATCCATAGTTATAAAGCATCCTGATATCTTCCTTATCCGTA-3′ | ||
| 3. rpsL-F-1 | GATGCTTTATAACTATGGATTAAACAC-3′ | 1,539 bp | |
| 4. erm-R | TTACTTATTAAATAATTTATAGCTATTGAA-3′ | ||
| 5. fur F-A3 | TTCAATAGCTATAAATTATTTAATAAGTAAGCTTAGATAGGGCTATCTTT-3′ | 454 bp | |
| 6. x4-R | CTGTAGAGTTGCCTGGAATTTATCA-3′ | ||
| All | The 5′ ends of the primers are as follows: primer 1, 554 bp upstream of the fur 5′ end; primer 2, 2 bp upstream of the fur 5′ end; primer 5, 18 bp downstream of the fur 3′ end; primer 6, 471 bp downstream of the fur 3′ end; the PCR products with primers 1 and 6 are 1,478 and 2,546 bp long from the wild-type and ΔfrxA-rpsL,erm DNAs, respectively |
Generic primer designations 1, 2, 3, 4, 5, and 6 for the construction of insertion and deletion alleles are as diagrammed in Fig 2.
The portions of the primer sequences in italics constitute complement with another primer (primer 2 with primer 3 or vice versa and primer 4 with primer 5 or vice versa); the portions of these hybrid primers in boldface indicate regions matching the H. pylori genomic DNA used for amplification. All, primers 1 to 6.
PCR-based construction of strains with insertion and deletion alleles.
The alleles used here were constructed by assembling individual PCR products without the need for recombinant DNA plasmid cloning, as first described by Chalker et al. (6). In brief, assembly depends on overlaps of ≥20 bp at the ends of DNAs to be joined together, which, in turn, result from the design of PCR primers used in amplification (see Table 1, sequences in italics) (6, 29).
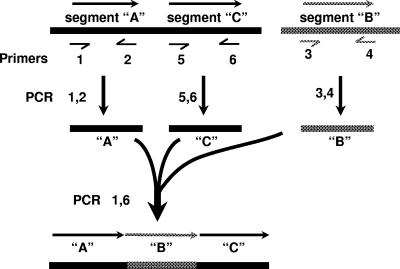
To construct the rpsL,erm cassette, we first replaced the mdaB quinone reductase gene in the H. pylori chromosome with the erm gene and then used genomic DNA carrying this ΔmdaB-erm allele for PCR to insert the C. jejuni rpsL gene just upstream of erm. Each of these manipulations involved a three-fragment assembly as in Fig. 2, transformation and selection for erythromycin resistance (Ermr), and PCR verification of structure. Two sequential three fragment assemblies were used because three-fragment assemblies have been more reliable for us than four-fragment assemblies.
FIG. 2.
Construction of insertion and deletion alleles by assembly of three fragments with overlapping ends. Half arrows indicate the positions of the primers, whose sequences are given in Table 1. Segments A and C represent DNA segments flanking the locus to be deleted or the site of insertion (depending on needs of experiment). Segment B represents (i) the erm (resistance) gene used initially to replace the mdaB locus; (ii) the rpsL streptomycin susceptibility gene from C. jejuni that was inserted just upstream of erm in a strain carrying erm in place of mdaB; or (iii) the rpsL,erm cassette, which can be moved to many loci.
In detail, for the first stage (and using the nomenclature presented in Fig. 2) PCR products were generated with primers 1 and 2 (fragment A, upstream of mdaB), primers 5 and 6 (fragment C, downstream of mdaB), and primers 3 and 4 (fragment B, erm) (Fig. 2; the primers are listed in Table 1). Primers 2 and 3 overlap, as do primers 4 and 5. A second round of PCR amplification using a mixture of fragments A, B, and C and primers 1 and 6 only yielded a 1.8-kp product. Transformation of strain 26695 with this product and selection for erythromycin resistance resulted in replacement of mdaB with the ΔmdaB-erm allele.
The C. jejuni rpsL gene was inserted upstream of erm in the ΔmdaB-erm allele to generate the 1.5-kb rpsL,erm cassette (Fig. 1). This was achieved by amplification of three PCR products and their assembly into a composite PCR product (again as in Fig. 2 and Table 1), use of this product to transform strain 26695-str, selection of Ermr transformant colonies, and identification of those products that had become phenotypically Strs. In terms of the depiction in Fig. 2, fragment A contained sequences just upstream of erm in the ΔmdaB-erm allele; fragment C contained erm and also H. pylori sequences downstream of mdaB; and fragment B contained the Cj.rpsL (strs allele) gene. PCR of the mixture of segments A, B, and C with primers 1 and 6 yielded the desired 2.5-kb assembly.
Replacements of genes frxA, fur, and nikR with the rpsL,erm cassette were made similarly by (i) using DNAs from upstream and downstream of these target genes (amplified with appropriate primers 1 and 2 and primers 5 and 6) and also from the rpsL,erm cassette (amplified with primers 3 and 4); (ii) assembly from the three-fragment mixture using the appropriate locus-specific primers 1 and 6 (Table 1); (iii) transformation of 26695-str, selection of Ermr colonies, and identification of those that were phenotypically Strs; and (iv) verification by PCR of the replacement of wild-type alleles by the rpsL,erm-marked deletion alleles.
RESULTS
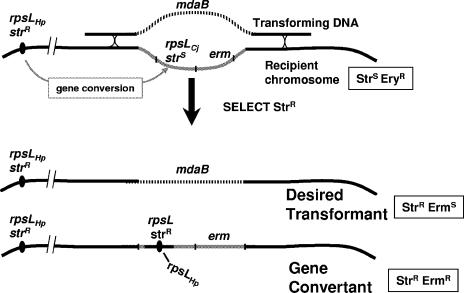
A two-gene (1.5-kb) cassette containing the C. jejuni rpsL gene (strs allele) upstream of an erythromycin resistance gene (erm) (Fig. 1), flanked by H. pylori chromosomal sequences that normally flank the mdaB quinone reductase gene (allele designated ΔmdaB-rpsL,erm) was constructed by PCR (Fig. 2) and recovered in the H. pylori chromosome after the transformation of 26695-str (see Materials and Methods). PCR tests showed that each of four Ermr Strs transformants tested contained the expected ΔmdaB-rpsL,erm allele in place of the full-length mdaB gene. Streaks of such colonies to streptomycin-containing agar typically yielded a few Strr derivative colonies after prolonged incubation. This slight instability is not seen in haploid strains containing only an rpsL-strs allele. These rare Strr derivatives remained Ermr and, based on PCR tests, retained the ΔmdaB-rpsL,erm allele in place of mdaB. These derivatives likely result from occasional gene conversion, despite H. pylori-C. jejuni rpsL sequence divergence.
In initial characterizations, nine recipient populations, each representing a different Strs ΔmdaB-rpsL,erm transformant of 26695-str, were used for transformation with genomic DNAs from wild-type 26695. About 30 Strr colonies from each transformation were streaked with toothpicks to erythromycin-containing agar: between 63 and 96% of Strr colonies obtained were Erms (average, 84% ± 13%) (Table 2). PCR tests confirmed ΔmdaB-rpsL,erm replacement by full-length mdaB in each of eight Strr Erms colonies tested, and retention of ΔmdaB-rpsL,erm in four Strr Ermr colonies, as expected (Fig. 3). In related tests, PCR products containing full-length mdaB plus several hundred base pairs of flanking sequences (made with mdaB primers 1 and 6; Table 1) were used in equivalent transformations of ΔmdaB-rpsL,erm derivatives of 26695-str or isogenic metronidazole-resistant strains (mutation in mdaB is implicated in the development of high-level metronidazole resistance [2]). Approximately 30 to 86% of the Strr colonies obtained (average of 68%; ten transformations) were of the desired Erms type.
TABLE 2.
Yields of Erms isolates among Strr colonies after transformationa
| rpsL,erm marked gene deletion | Yield of Strs Ermr (rpsL,erm) derivative of recipient strain ± SD:
|
|||
|---|---|---|---|---|
| 26695-str | SS1-str | X47 | G27MA-str | |
| mdaB | 0.84 ± 0.13 (9) | 0.40 ± 0.10 (2) | 0.88 ± 0.14 (2) | 0.21 ± 0.14 (4) |
| frxA | 0.53 ± 0.22 (10) | 0.90 ± 0.04 (2) | 0.87 ± 0.03 (2) | 0.17 ± 0.15 (4) |
| fur | 0.28 ± 0.3 (8) | 0.22 ± 0.11 (2) | 0.71 ± 0.03 (2) | 0.56 ± 0.26 (4) |
| nikR | 0.54 ± 0.42 (2) | 0.86 ± 0.18 (2) | 0.96 ± 0.02 (2) | 0.38 ± 0.35 (4) |
Average fraction of Strr colonies ± standard deviation that were Erms after transformation of the indicated rspL,erm cassette containing recipient strain with isogenic wild-type genomic DNA. These Erms colonies were products of transformation and replacement of the contraselectable cassette, not gene conversion. These fractions are based on erythromycin resistance tests of approximately 30 colonies per transformation experiment. Listed in parentheses are the numbers of independent transformations, each with a different single colony isolate of the indicated recipient strain.
FIG. 3.
Strategy for the use of rpsL from C. jejuni as a contraselectable determinant of streptomycin susceptibility in H. pylori. Diagrammed here is a recipient strain carrying an strr allele of its normal (H. pylori) rpsL gene and also the rpsL,erm cassette that had (in an earlier transformation) replaced the mdaB gene, now being transformed with genomic DNA from a strain with full-length mdaB, and acquisition of mdaB plus flanking sequences by crossing over in regions of sequence homology. This results in replacement of the rpsL,erm cassette and a change from Ermr to Erms in phenotype. Unwanted gene conversion events also result in an Strr phenotype, but the Ermr phenotype is retained.
To test the generality of this allele replacement strategy, the ΔmdaB-rpsL,erm allele was moved by transformation into the streptomycin-resistant (rpsL) strains SS1-str, G27MA-str, and X47 by using genomic DNA from 26695-str ΔmdaB-rpsL,erm and selection for erythromycin resistance. Each of the ∼30 Ermr transformants in each of these strains were Strs, as expected. Two ΔmdaB-rpsL,erm derivatives of each strain, found by PCR to have the expected structure, were used as recipients in transformations with DNA from isogenic wild-type parents. Table 2 shows that the yields of Strr Erms transformants among Strr colonies ranged from ∼21% in the case of G27MA-str to 88% in the case of X47.
To further test the general utility of this contraselection strategy, we constructed deletion alleles marked with the rpsL,erm cassette of three other genes—frxA (nitroreductase), fur (iron- and pH-responsive regulation), and nikR (nickel-responsive regulation)—by PCR (Fig. 2) and transformation of 26695-str (Materials and Methods). DNAs from transformants with the desired Strs phenotypes and PCR-verified structures were then used to move each rpsL,erm-tagged deletion allele to the three other strains (G27MA-str, SS1-str, and X47). The yields of Ermr Strs transformants among Ermr colonies made with these genomic DNAs varied from ∼50% to ∼100% (data not shown). Since just one genomic DNA preparation for each locus was used to move a given allele among H. pylori strains (in each case from the 26695-str derivative), this variation in Strs colony yield might reflect an interplay of the strain background (e.g., nuclease or DNA repair activities) and the sequence of the transforming DNA and/or the targeted region.
These new rpsL,erm-marked strains were then transformed with isogenic wild-type genomic DNAs, and Strr colonies were selected and tested as described above. Table 2 shows that the yields of Erms Strr transformants among Strr colonies varied with the locus under study (from ∼17% to ∼90%) and with the strain background. PCR tests of a few representative Strr Erms transformants of each of these various rpsL,erm-marked deletion strains verified the expected replacement of the rpsL,erm allele in each case.
DISCUSSION
We constructed a two-gene cassette containing an erm (resistance) gene for selection and a C. jejuni rpsL gene (dominant streptomycin susceptibility; ribosomal protein S12) that is 18% divergent from H. pylori rpsL for contraselection. Each rpsL,erm-marked allele was first made by transformation of a PCR product into one strain and then moved to other strains by transformation with genomic DNA from this first PCR-verified transformant. In the future such strains might equally be generated using PCR products to strictly limit the amount of DNA adjacent to the rpsL,erm cassette that is acquired during transformation. It would also eliminate any risk of the acquisition of unlinked genes (although this was not a major concern here because simultaneous transformation for two unlinked markers is rare).
Our results with four rpsL,erm-marked loci in four unrelated strains suggest that this cassette can be placed at any nonessential site in H. pylori strains of interest. Once inserted, other DNAs can be moved to that marked locus by transformation and homologous recombination. The desired transformants are identified simply by screening a relatively few Strr colonies to identify those with the desired Erms phenotype. The yields of these replacements among selected Strr colonies varied from ∼17% to ∼90%, depending on the gene targeted and the strain background. That this variation was seen with genomic DNAs from isogenic wild-type donor strains indicates that it is not due to DNA restriction. Rather, it may reflect local and genomewide effects on relative efficiencies of transformation versus gene conversion between related but divergent sequences. If further improvement in efficiency were needed, this could likely be achieved with a synthetic rpsL gene that is even more divergent from the H. pylori rpsL in DNA sequence (to further inhibit gene conversion), while exploiting the degeneracy of the code to ensure that it still encodes an H. pylori-like S12 protein.
In other experiments to date, we used this rpsL,erm cassette to replace point mutant alleles with wild-type alleles and vice versa, to replace full-length genes with unmarked deletion alleles, and to create a novel triple mutant allele of the fur regulatory gene (itself generated by PCR without cloning) for functional analyses of iron and pH-responsive transcriptional regulation. We anticipate additional applications for this cassette, including (i) the functional characterization of genes from divergent clinical isolates; (ii) altering key regulatory sites; (iii) engineering new domains or epitopes in surface-exposed or -secreted proteins; (iv) adding new genes to genomes of interest; and (v) deletion or other changes in some or all members of multigene families, unimpeded by the numbers of changes that might be needed.
Acknowledgments
We thank Manuel Amieva, Peter Chivers, Tim Cover, Vic DiRita, Steven Gregory, Rainer Haas, Dave Hendrixson, and Phil Youderian for stimulating discussions and/or gifts of strains and reagents.
This study was supported by grants RO1 DK063041 and P30 DK52574 from the U.S. National Institutes of Health.
REFERENCES
- 1.Akada, J. K., K. Ogura, D. Dailidiene, G. Dailide, J. M. Cheverud, and D. E. Berg. 2003. Helicobacter pylori tissue tropism: mouse-colonizing strains can target different gastric niches. Microbiology 149:1901-1909. [DOI] [PubMed] [Google Scholar]
- 2.Albert, T. J., D. Dailidiene, G. Dailide, J. E. Norton, A. Kalia, T. A. Richmond, M. Molla, J. Singh, R. D. Green, and D. E. Berg. 2005. Mutation discovery in bacterial genomes: metronidazole resistance in Helicobacter pylori. Nat. Methods 2:951-953. [DOI] [PubMed] [Google Scholar]
- 3.Amieva, M. R., R. Vogelmann, A. Covacci, L. S. Tompkins, W. J. Nelson, and S. Falkow. 2003. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science 300:1430-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amyes, S. G., and J. T. Smith. 1975. Thymineless mutants and their resistance to trimethoprim. J. Antimicrob. Chemother. 1:85-89. [DOI] [PubMed] [Google Scholar]
- 5.Blomfield, I. C., V. Vaughn, R. F. Rest, and B. I. Eisenstein. 1991. Allelic exchange in Escherichia coli using the Bacillus subtilis sacB gene and a temperature-sensitive pSC101 replicon. Mol. Microbiol. 5:1447-1457. [DOI] [PubMed] [Google Scholar]
- 6.Chalker, A. F., H. W. Minehart, N. J. Hughes, K. K. Koretke, M. A. Lonetto, K. K. Brinkman, P. V. Warren, A. Lupas, M. J. Stanhope, J. R. Brown, and P. S. Hoffman. 2001. Systematic identification of selective essential genes in Helicobacter pylori by genome prioritization and allelic replacement mutagenesis. J. Bacteriol. 183:1259-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carter, A. P., W. M. Clemons, D. E. Brodersen, R. J. Morgan-Warren, B. T. Wimberly, and V. Ramakrishnan. 2000. Functional insights from the structure of the 30S ribosomal subunit and its interactions with antibiotics. Nature 407:340-348. [DOI] [PubMed] [Google Scholar]
- 8.Copass, M., G. Grandi, and R. Rappuoli. 1997. Introduction of unmarked mutations in the Helicobacter pylori vacA gene with a sucrose sensitivity marker. Infect. Immun. 65:1949-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies, J. E. 1997. Origins, acquisition and dissemination of antibiotic resistance determinants. Ciba Found. Symp. 207:15-35. [PubMed] [Google Scholar]
- 10.Davis, B. D. 1987. Mechanism of bactericidal action of aminoglycosides. Microbiol. Rev. 51:341-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisen, J. A. 1998. A phylogenomic study of the MutS family of proteins. Nucleic Acids Res. 26:4291-4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ermak, T. H., P. J. Giannasca, R. Nichols, G. A. Myers, J. Nedrud, R. Weltzin, C. K. Lee, H. Kleanthous, and T. P. Monath. 1998. Immunization of mice with urease vaccine affords protection against Helicobacter pylori infection in the absence of antibodies and is mediated by MHC class II-restricted responses. J. Exp. Med. 188:2277-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer. W., D. Schwan, E. Gerland, G. E. Erlenfeld, S. Odenbreit, and R. Haas. 1999. A plasmid-based vector system for the cloning and expression of Helicobacter pylori genes encoding outer membrane proteins. Mol. Gen. Genet. 262:501-507. [DOI] [PubMed] [Google Scholar]
- 14.Gregory, S. T., J. H. Cate, and A. E. Dahlberg. 2001. Streptomycin-resistant and streptomycin-dependent mutants of the extreme thermophile Thermus thermophilus. J. Mol. Biol. 309:333-338. [DOI] [PubMed] [Google Scholar]
- 15.Gromadski, K. B., and M. V. Rodnina. 2004. Streptomycin interferes with conformational coupling between codon recognition and GTPase activation on the ribosome. Nat. Struct. Mol. Biol. 11:316-322. [DOI] [PubMed] [Google Scholar]
- 16.Lederberg. J. 1951. Streptomycin resistance: a genetically recessive mutation. J. Bacteriol. 61:549-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee, A., J. O'Rourke, M. C. De Ungria, B. Robertson, G. Daskalopoulos, and M. F. Dixon. 1997. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology 112:1386-1397. [DOI] [PubMed] [Google Scholar]
- 18.Levin, I., M. Giladi, N. Altman-Price, R. Ortenberg, and M. Mevarech. 2004. An alternative pathway for reduced folate biosynthesis in bacteria and halophilic archaea. Mol. Microbiol. 54:1307-1318. [DOI] [PubMed] [Google Scholar]
- 19.Maisnier-Patin, S., O. G. Berg, L. Liljas, and D. I. Andersson. 2002. Compensatory adaptation to the deleterious effect of antibiotic resistance in Salmonella typhimurium. Mol. Microbiol. 46:355-366. [DOI] [PubMed] [Google Scholar]
- 20.Matic, I., F. Taddei, and M. Radman. 1996. Genetic barriers among bacteria. Trends Microbiol. 4:69-72. [DOI] [PubMed] [Google Scholar]
- 21.Mobley, H. L. T., G. L. Mendz, and S. L. Hazell (ed.). 2001. Helicobacter pylori. ASM Press, Washington, D.C. [PubMed]
- 22.Ogle, J. M., A. P. Carter, and V. Ramakrishnan. 2003. Insights into the decoding mechanism from recent ribosome structures. Trends Biochem. Sci. 28:259-266. [DOI] [PubMed] [Google Scholar]
- 23.Panthel, K., P. Dietz, R. Haas, and D. Beier. 2003. Two-component systems of Helicobacter pylori contribute to virulence in a mouse infection model. Infect. Immun. 71:5381-5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peske, F., A. Savelsbergh, V. I. Katunin, M. V. Rodnina, and W. Wintermeyer. 2004. Conformational changes of the small ribosomal subunit during elongation factor G-dependent tRNA-mRNA translocation. J. Mol. Biol. 343:1183-1194. [DOI] [PubMed] [Google Scholar]
- 25.Russell, C. B., and F. W. Dahlquist. 1989. Exchange of chromosomal and plasmid alleles in Escherichia coli by selection for loss of a dominant antibiotic sensitivity marker. J. Bacteriol. 171:2614-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schrag, S. J., Perrot, V., and B. R. Levin. 1997. Adaptation to the fitness costs of antibiotic resistance in Escherichia coli. Proc. Biol. Sci. 264:1287-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steinmetz, M., D. Le Coq, H. H. Djemia, and P. Gay. 1983. Genetic analysis of sacB, the structural gene of a secreted enzyme, levansucrase of Bacillus subtilis Marburg. Mol. Gen. Genet. 191:138-144. [DOI] [PubMed] [Google Scholar]
- 28.Suto, H., M. Zhang, and D. E. Berg. 2005. Age-dependent changes in susceptibility of suckling mice to individual strains of Helicobacter pylori. Infect. Immun. 73:1232-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan, S., and D. E. Berg. 2004. Motility of urease-deficient derivatives of Helicobacter pylori. J. Bacteriol. 186:885-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan, S., C. D. Fraley, M. Zhang, D. Dailidiene, A. Kornberg, and D. E. Berg. 2005. Diverse phenotypes resulting from polyphosphate kinase gene (ppk1) inactivation in different strains of Helicobacter pylori. J. Bacteriol. 187:7687-7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomb, J. F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. A. Ketchum, H. P. Klenk, S. Gill, B. A. Dougherty, K. Nelson, J. Quackenbush, L. Zhou, E. F. Kirkness, S. Peterson, B. Loftus, D. Richardson, R. Dodson, H. G. Khalak, A. Glodek, K. McKenney, L. M. Fitzegerald, N. Lee, M. D. Adams, E. K. Hickey, D. E. Berg, J. D. Gocayne, T. R. Utterback, J. D. Peterson, J. M. Kelley, M. D. Cotton, J. M. Weidman, C. Fujii, C. Bowman, L. Watthey, E. Wallin, W. S. Hayes, M. Borodovsky, P. D. Karp, H. O. Smith, C. M. Fraser, and J. C. Venter. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539-547. [DOI] [PubMed] [Google Scholar]
- 32.Torii. N., T. Nozaki, M. Masutani, H. Nakagama, T. Sugiyama, D. Saito, M. Asaka, T. Sugimura, and K. Miki. 2003. Spontaneous mutations in the Helicobacter pylori rpsL gene. Mutat. Res. 535:141-145. [DOI] [PubMed] [Google Scholar]
- 33.Wang, G., R. W. Blakesley, D. E. Berg, and C. M. Berg. 1993. pDUAL: a transposon-based cosmid cloning vector for generating nested deletions and DNA sequencing templates in vivo. Proc. Natl. Acad. Sci. USA 90:7874-7878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang, G., X. Xu, J. M. Chen, D. E. Berg, and C. M. Berg. 1994. Inversions and deletions generated by a mini-gamma delta (Tn1000) transposon. J. Bacteriol. 176:1332-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]