Abstract
Nicotianamine (NA), a chelator of metals, is ubiquitously present in higher plants. Nicotianamine aminotransferase (NAAT) catalyzes the amino group transfer of NA in the biosynthetic pathway of phytosiderophores and is essential for iron acquisition in graminaceous plants. The gene that encodes NAAT from barley was introduced into the nongraminaceous plant tobacco, which produces NA but not phytosiderophores. Transgenic tobacco plants (naat tobacco) that constitutively expressed the NAAT gene had young leaves with interveinal chlorosis and flowers that were abnormally shaped and sterile. Endogenous NA was consumed as a result of NAAT overproduction in naat tobacco. The resulting NA shortage caused disorders in internal metal transport, leading to these abnormal phenotypes. In addition to its role in long-distance metal transport, NA may be involved in the regulation of metal transfer within the cells. These results suggest that a shortage of NA impaired the functions of metal-requiring proteins, including transcription factors.
INTRODUCTION
Metal ions, such as Fe, Mn, Zn, and Cu, are essential for normal plant growth. However, under aerobic conditions in the physiological pH range, these ions (particularly Fe) are sparingly soluble in the soil solution and are not readily available to plants. Therefore, plants have developed sophisticated and tightly regulated mechanisms to acquire Fe from the soil.
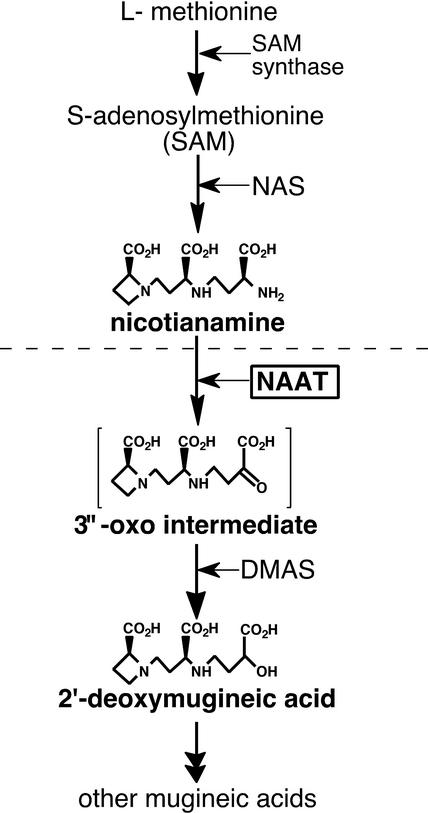
Mugineic acid family phytosiderophores (MAs) are natural Fe chelators that graminaceous plants secrete from their roots to solubilize Fe in the soil (Takagi, 1976). Nicotianamine aminotransferase (NAAT) is a critical enzyme in the biosynthetic pathway of MAs (Figure 1) that catalyzes the aminotransfer of nicotianamine (NA), an essential intermediate in the production of MAs (Mori and Nishizawa, 1987; Shojima et al., 1990). Although MAs are produced only in graminaceous plants, NA was found originally in tobacco (Noma et al., 1971) and has been found in all plants investigated to date (Noma and Noguchi, 1976; Rudolph et al., 1985). NA chelates metal cations, including Fe(II) and Fe(III) (Benes et al., 1983; von Wirén et al., 1999). Unlike MAs, NA is not secreted and is thought to play a role in the internal transport of Fe and other metals. This role is supported by the fact that the NA synthesis–defective tomato mutant chloronerva (Rudolph et al., 1985; Higuchi et al., 1996) has a phenotype indicative of Fe deficiency (Pich and Scholz, 1996; Stephan et al., 1996). NA also might function as an Fe(II) scavenger to protect cells from oxidative stress (von Wirén et al., 1999). However, the precise roles of NA in higher plants remain unclear.
Figure 1.
Biosynthetic Pathway of MAs.
The pathway downstream of the horizontal dashed line is specific to graminaceous plants. DMAS, deoxymugineic acid synthase.
In tobacco and graminaceous plants, NA is formed by the trimerization of three molecules of S-adenosyl Met, a reaction catalyzed by nicotianamine synthase (NAS) (Shojima et al., 1989a, 1990; Higuchi et al., 1995). By contrast, NAAT activity has not been detected in tobacco. Therefore, of the enzymes in the MAs biosynthetic pathway, NAS and S-adenosyl Met synthetase are ubiquitous in plants but NAAT is specific to graminaceous plants.
In a previous study, NAAT was purified from Fe-deficient barley roots (Takahashi et al., 1999). Two cDNAs (naat-A and naat-B) and one genomic DNA fragment containing naat-A and naat-B were isolated. Expression of both naat-A and naat-B was induced markedly in Fe-deficient barley roots. The barley genomic DNA fragment containing naat-A and naat-B was introduced into rice in an attempt to genetically engineer plants that could tolerate low Fe availability in soil (Takahashi et al., 2001). Under Fe-deficient conditions, transgenic rice plants had increased NAAT activity and secreted more MAs from their roots than did wild-type rice plants. Consequently, transgenic rice plants had enhanced tolerance of low Fe availability in alkaline soil.
To investigate the consequences of ectopic NAAT production in nongraminaceous plants, tobacco was transformed with a construct containing the 35S promoter of Cauliflower mosaic virus combined with the coding region of the hvnaat-A gene. Here, we describe the phenotype of transgenic tobacco plants that overexpressed barley NAAT and consumed NA in situ. The transgenic tobacco had metal transport disorders and consequently developed a striking interveinal chlorosis in young leaves. In addition, flowers were abnormally shaped and sterile. These findings demonstrate the essential role of NA in growth, flower development, and fertility in plants.
RESULTS
Integration and Expression of a Barley naat-A in Tobacco
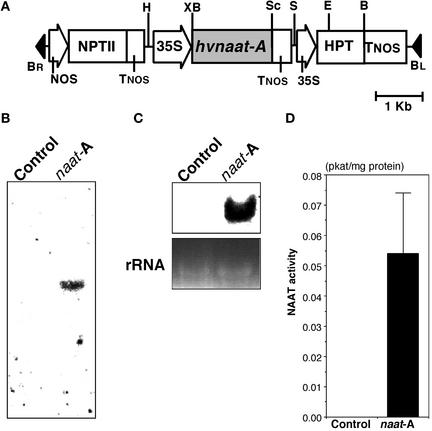
hvnaat-A cDNA was inserted behind the 35S promoter of Cauliflower mosaic virus for constitutive expression in place of the β-glucuronidase (GUS) gene in the binary vector pIG121Hm. The plasmid was used to transform tobacco plants (Figure 2A). DNA gel blot analysis confirmed that naat-A was integrated into the tobacco genome (Figure 2B). RNA gel blot analysis showed that naat-A mRNA accumulated in transgenic tobacco (Figure 2C). NAAT activity was measured in transgenic tobacco leaves to ensure that NAAT had been translated correctly. NAAT activity was detected in transgenic tobacco but not in control tobacco (Figure 2D).
Figure 2.
Construct of Binary Vector pBI121Hm-hvnaat-A and hvnaat-A Expression in Tobacco.
(A) Construct of binary vector pBI121Hm-hvnaat-A. BL, left border; BR, right border; HPT, hygromycin phosphotransferase; NOS, nopaline synthase promoter; NPT II, neomycin phosphotransferase; 35S, 35S promoter; B, BamHI; E, EcoRI; H, HindIII; S, SalI; Sc, SacI; X, XbaI.
(B) DNA gel blot hybridization analysis. DNA gel blot analyses were performed with a 32P-labeled cDNA probe for hvnaat-A. Control, control tobacco; naat-A, naat tobacco.
(C) RNA gel blot hybridization analysis. Total RNA isolated from naat tobacco and control tobacco was hybridized with a 32P-labeled probe for naat-A. Ethidium bromide–stained ribosomal bands are shown as a loading control.
(D) NAAT activity in control tobacco and naat tobacco (analysis performed in triplicate). The error bar indicates the standard error of the mean.
Tobacco Plants Overexpressing hvnaat-A Showed Interveinal Chlorosis
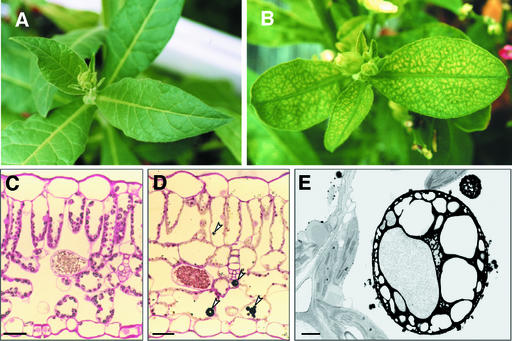
naat tobacco plants grown under nutrient-sufficient conditions showed noticeable interveinal chlorosis in their young leaves (Figure 3B), a finding not observed in control tobacco plants (Figure 3A). This interveinal chlorosis disappeared gradually as the leaves grew older. Chlorotic leaves were examined using light and electron microscopy (Figures 3C to 3E). General leaf anatomy was not modified in naat tobacco, even within chlorotic regions. However, marked differences were observed within the vacuoles. A number of dark-stained, globular structures were present only in the vacuoles of naat tobacco plants (Figure 3D). These structures appeared in epidermal and mesophyll cells and were not restricted to specific tissues. In an effort to understand the detailed structure of these particles, we analyzed the ultrastructure of naat tobacco leaves by electron microscopy (Figure 3E). Unusual electron-dense globular structures were observed in almost all vacuoles in the epidermis and in mesophyll cells. The electron-dense globular structures came in a range of sizes, with the largest reaching ∼10 μm in diameter.
Figure 3.
Control Tobacco and naat Tobacco Leaves.
(A) Normal green color in control tobacco.
(B) Interveinal chlorosis in naat tobacco.
(C) and (D) Light micrographs of longitudinal sections of a control tobacco leaf (C) and a naat tobacco leaf (D). Arrowheads in (D) indicate dark-stained globular structures. Bars = 400 μm.
(E) Electron micrograph of a longitudinal section of a naat tobacco leaf. Bar = 1 μm.
Concentration and Distribution of Metals in Leaves
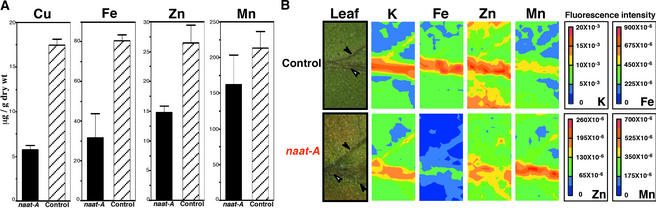
Interveinal chlorosis in young leaves is a common symptom of metal deficiency. Therefore, we measured the concentrations of four metals—Cu, Fe, Zn, and Mn—in the young leaves of control and naat tobacco plants by inductively coupled plasma emission spectrometry. The concentrations of all four metals in young leaves of naat tobacco were lower than those in control tobacco leaves (Figure 4A). The concentrations of Fe and Cu, in particular, were markedly lower in naat tobacco. The percentage decrease in concentration (relative to the control) was of the following order: Mn < Zn < Fe < Cu.
Figure 4.
Concentration and Distribution of Metals in Leaves of Control Tobacco and naat Tobacco.
(A) Metal concentration of control tobacco and naat tobacco leaves (analysis performed in triplicate). Error bars indicate the standard error of the mean. Control; control tobacco, naat-A, naat tobacco.
(B) Distribution of metals in leaves of control tobacco (analysis performed in duplicate) and naat tobacco (analysis performed in quadruplicate). In each micrograph, closed arrowheads indicate the leaf veins and open arrowheads indicate the midrib. The color indicates the fluorescence intensity. Synchrotron radiation–induced x-ray fluorescence spectrometry intensity was normalized to the intensity of the incident x-ray. High intensity indicates high quantities.
Because chlorosis was observed in the interveinal area of young leaves, the distributions of K, Zn, Mn, and Fe were analyzed by synchrotron radiation–induced x-ray fluorescence spectrometry (Figure 4B). There were similar distributions of K, Zn, and Mn in the leaf in naat tobacco and control tobacco; levels were high in the vein and low in the interveinal area in the leaves of both plants. By contrast, there was a marked difference in the distribution of Fe in the leaves of naat tobacco and control tobacco. The Fe concentration in the veins and especially the interveinal areas was much lower in naat tobacco leaves than in control tobacco leaves. Although the concentrations of Zn, Fe, and Cu all were reduced in naat tobacco leaves (Figure 4A), this finding suggests that the interveinal chlorosis (Figure 3B) was attributable to the decreased Fe concentration in the interveinal area.
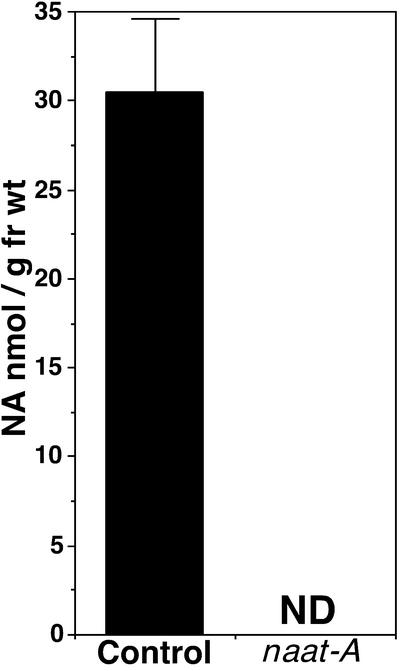
NA Concentration in naat Tobacco
The decrease in metal levels in young naat tobacco leaves indicated that internal metal transport had been altered. Because NA is the substrate of NAAT, it is conceivable that NAAT overproduction consumed NA in situ and that an endogenous NA shortage led to defective internal metal transport in naat tobacco. Therefore, the level of endogenous NA was measured in both plants. As expected, the NA concentration was normal in control plants but could not be detected in naat tobacco (Figure 5). Deoxymugineic acid, which might have been produced, was not detected either (data not shown).
Figure 5.
NA Concentration of Young Leaves of naat Tobacco and Control Tobacco.
Experiments were repeated three times, and each value represents the mean of triplicate experiments. NA was not detected (ND) in naat tobacco. Control, control tobacco; fr wt, fresh weight; naat-A, naat tobacco.
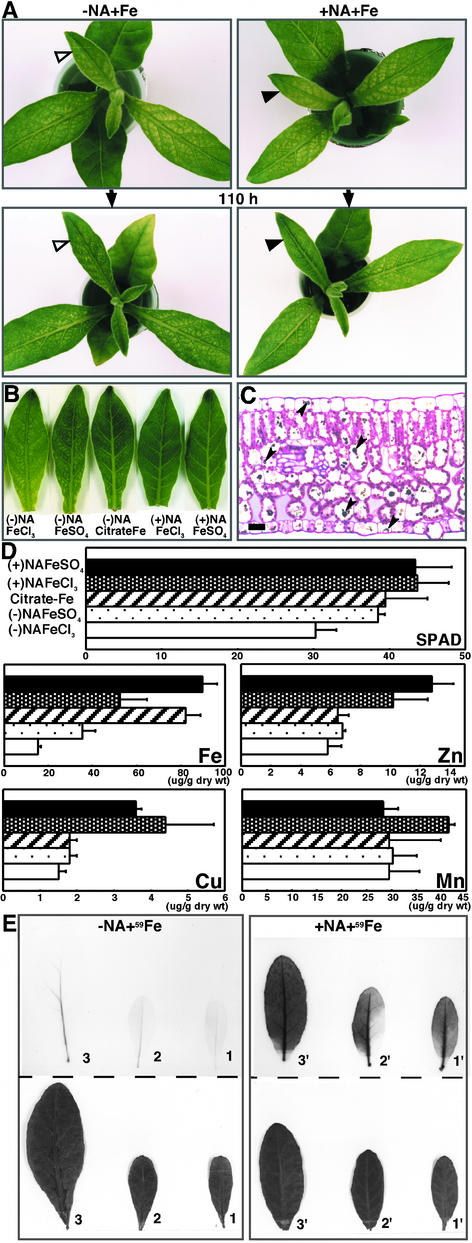
Interveinal Chlorosis Was Reversed after Treatment with NA-Fe
To confirm that interveinal chlorosis resulted from the NA shortage in naat tobacco, Fe was supplied to naat tobacco either with or without NA. Excised naat tobacco axillary buds were treated with one of five solutions: (1) FeCl3, (2) FeSO4, (3) Fe(III) citrate, (4) FeCl3 plus NA, or (5) FeSO4 plus NA. The naat tobacco plants treated with a combination of NA and Fe (solutions 4 and 5) recovered completely from interveinal chlorosis after 110 h (Figures 6A, right, and 6B). Indeed, some recovery was evident within 48 h (data not shown). On the other hand, solutions without NA (solutions 1, 2, and 3) did not induce recovery from chlorosis (Figures 6A, left, and 6B), although Fe(III) citrate enhanced greening to some extent (Figure 6B). These results clearly show that a supply of NA-Fe to young naat tobacco leaves led to recovery from interveinal chlorosis. Interestingly, although the young leaves recovered from chlorosis with the NA-Fe treatments, the electron-dense globular structures observed in the vacuoles of naat tobacco were not eliminated (Figure 6C). Therefore, these unusual structures do not appear to be a direct cause of interveinal chlorosis. In naat tobacco, the 3′′-oxo intermediate (Figure 1) synthesized from NA by NAAT was not converted to deoxymugineic acid. Therefore, the electron-dense globular structures observed in the vacuoles of naat tobacco might be an accumulation of the 3′′-oxo intermediate or overproduced NAAT itself.
Figure 6.
naat Tobacco Interveinal Chlorosis Regreening Test.
(A) naat tobacco before (top plant) and 110 h after (bottom plant) treatment with a solution containing Fe without NA (−NA+Fe) or with NA (+NA+Fe). Open and closed arrowheads indicate young leaves that changed markedly.
(B) Young leaves of naat tobacco treated with five solutions: FeCl3, FeSO4, Fe(III) citrate plus NA, FeCl3 plus NA, and FeSO4 plus NA.
(C) Light micrograph of a longitudinal section of a naat tobacco leaf after treatment with solution 5, 100 μM FeSO4 plus 200 μM NA. Arrowheads indicate unusual, dark-stained globular structures. Bar = 400 μm.
(D) SPAD values and concentrations of metals in young naat tobacco leaves treated with the five solutions.
(E) Uptake and distribution of 59Fe in naat tobacco leaves treated without NA (−NA+59Fe) or with NA (+NA+59Fe) for 6 h (analysis performed in duplicate). The top images show autoradiographs of 59Fe and the bottom images show photographs of the leaves. Numbers indicate the same unfolded leaf from the top of the plant.
Figure 6D shows the SPAD values and metal concentrations in young leaves of treated axillary buds (see Methods). The SPAD values of young leaves treated with a solution of NA plus FeSO4 or FeCl3 were higher than those of young leaves treated with Fe(III) citrate and FeSO4 or FeCl3 without NA. This finding was consistent with the reversal of interveinal chlorosis evident in Figure 6B. Young naat tobacco leaves treated with solutions 3, 4, and 5 had higher concentrations of Fe than those treated with solutions 1 and 2. This finding indicates that both NA and citrate promote Fe transport to young leaves. However, the SPAD values of young leaves treated with Fe(III) citrate were the same as those of leaves treated with FeSO4. In addition, the SPAD values of young leaves treated with solutions containing NA were higher than those of young leaves treated with Fe(III) citrate (Figure 6D). These results show that although both NA and citrate promote Fe transport to young leaves, only NA promotes Fe transport to the interveinal area. The similar SPAD values of young leaves treated with Fe(III) citrate and FeSO4, which were significantly higher than those of leaves treated with FeCl3, suggest poor transport of Fe(III) in the absence of a chelate (NA or citrate). Moreover, young leaves treated with a solution containing NA also had high concentrations of Cu and Zn, demonstrating that NA also promotes the transport of these metals.
59Fe Transport in naat Tobacco
Because only NA-Fe solutions reversed chlorosis in young leaves of naat tobacco, 59Fe was used to determine whether NA promotes Fe transport within the leaves. Excised axillary buds of naat tobacco were supplied with NA-59Fe or 59Fe without NA. Autoradiographs of 59Fe in the leaves at 6 h after treatment are shown in Figure 6E.
After treatment with NA-59Fe, a significantly higher level of 59Fe was transported to the veins and interveinal areas in the oldest leaves (Figure 6E, leaf 3′). After only 1 h of treatment with NA-59Fe, 59Fe was distributed throughout the leaves (data not shown). In plants treated with 59Fe alone, however, 59Fe was transported only to the veins and not to the interveinal area (Figure 6E, leaves 1, 2, and 3). Furthermore, treatment with 59Fe alone resulted in no appreciable transport of 59Fe to the veins (Figure 6E, leaf 1). These results demonstrate again that in young naat tobacco leaves, NA promotes Fe transport not only in the veins but also from the veins to the interveinal areas.
Abnormally Shaped Flowers and Sterility
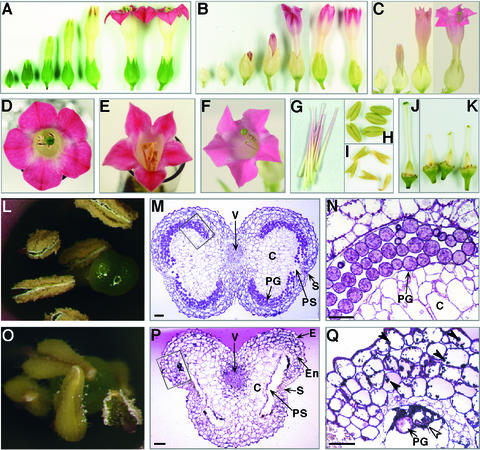
In addition to interveinal chlorosis in young leaves, the naat tobacco inflorescence developed marked morphological abnormalities. The flowers of wild-type and control tobacco both had five petals, and pollen was released before the flower opened (Figures 7A, 8A, 8D, 8H, 8J, and 8L). By contrast, naat tobacco produced two types of abnormally shaped flowers. The first type (type I; Figures 7B to 7T) was formed at an early stage of flowering. The second type (type II; Figures 8B, 8E, 8G, 8I, and 8K) was formed at a later stage.
Figure 7.
Inflorescences of Wild-Type Tobacco and Type-I naat Tobacco.
(A) Wild-type flowers.
(B) naat tobacco flowers.
(C) Buds of wild-type (WT) and naat tobacco flowers (type I). Of the six naat flower buds, one (left) has large sepals but no petals are visible, and the remaining five are the grossly aberrant first buds of naat tobacco.
(D) Flower with projected petal.
(E) Flower with projected petal. Arrowheads indicate filamentous petals.
(F) Flower with petaloid sepal.
(G) Flower with petaloid sepal.
(H) Dehiscent flower with petaloid sepal and staminoid petal.
(I) Dehiscent flower with petaloid sepal and staminoid petal.
(J) Petaloid stamen of the stamen filament shown in (K) that seems to be a petal.
(K) Petaloid sepal with petaloid stamen.
(L) Petaloid stamen.
(M) Petaloid sepal, petaloid stamen, and supernumerary flower organs.
(N) Petaloid stamen and decreased number of flower organs.
(O) Side view of the flower shown in (M).
(P) Pistils of the flower shown in (O).
(Q) Flower with supernumerary flower organs, petaloid sepal, and staminoid petal (top view).
(R) Side view of the flower shown in (Q).
(S) Dehiscent flower with supernumerary flower organs, petaloid sepal, and staminoid petal.
(T) Fused flower with one sepal.
Figure 8.
Inflorescences of Wild-Type Tobacco and Type-II naat Tobacco.
(A) Buds and flowers after anthesis of wild-type tobacco (side view).
(B) Buds and flowers after anthesis of naat tobacco (side view).
(C) Buds and flowers after anthesis of wild-type tobacco with Fe deficiency (side view).
(D) Wild-type tobacco flower (top view).
(E) naat tobacco flower (top view).
(F) Fe-deficient wild-type tobacco flower (top view).
(G) Stamen filaments of naat tobacco.
(H) Anthers of wild-type tobacco.
(I) Anthers of naat tobacco.
(J) Pistil of wild-type tobacco.
(K) Short pistils with irregularly shaped stigmas of naat tobacco.
(L) Anthers of wild-type tobacco.
(M) Transverse section of a wild-type tobacco anther. C, connective; PG, pollen grain; PS, pollen sac; S, stomium; V, vascular bundle. Bar = 400 μm.
(N) Transverse section of a wild-type tobacco anther at higher magnification showing pollen sacs filled with mature pollen grains. Bar = 200 μm.
(O) Anthers of naat tobacco.
(P) Transverse section of a naat tobacco anther. E, epidermis; En, endothecium. Bar = 400 μm.
(Q) Transverse section of a naat tobacco anther at higher magnification. Almost all of the pollen grains (open arrowhead) were immature and the pollen sacs were empty. Dark-stained globular structures (closed arrowheads) were evident in the vacuoles of cells in the epidermis and the endothecium. Bar = 200 μm.
Type-I flower buds had an aberrant shape (Figure 7C), the first bud of naat tobacco being the most aberrant, and type-I flowers had bigger and thicker sepals than those of the wild type. Type-I flowers were classified into the following five groups, some flowers having a combination of several elements. (1) Projected petal. Projected petals were observed (Figures 7D and 7E) and filamentous petals often were observed (Figure 7E). (2) Chimeric flower organ. Petaloid sepals (Figures 7F, 7G, 7H, 7K, 7M, 7O, 7R, and 7S) and petaloid filaments (Figures 7J, 7L, and 7N) were observed; staminoid petals, such as those with the stamen fused to the petal, were common (Figures 7H, 7Q, and 7S). (3) Dehiscent flower. Dehiscent flowers were observed (Figures 7H, 7I, and 7S), with the corolla split open. (4) Abnormal number of flower organs. This type of flower showed supernumerary stamens and petals with multiple carpels (Figures 7M to 7S) or a decreased number of petals and stamens (Figure 7N). (5) Fused flower. This type of flower appeared as if two flowers had fused but had only one set of sepals (Figure 7T). Flowers with supernumerary flower organs could belong to this group (Figures 7M to 7S).
The type-II flowers that developed in naat tobacco (Figures 8B and 8E) were distinct from type-I flowers (Figures 7B to 7T), although there were some common features. Also, type-II flowers had some similarities with flowers of wild-type tobacco under severe Fe deficiency (Figures 8C and 8F). The sepals, like those of type-I flowers, were large and thick but had a normal shape, even those that developed during the transition from type-I to type-II flowers. The sepals, stamen filaments, and petals were similar in color, and the anthers produced little pollen.
Wild-type flowers under Fe deficiency did not have any type-I flowers. In addition, axillary buds exposed to Fe- or Zn-deficient conditions did not produce flowers that were the same as type-I flowers (data not shown). In naat tobacco, both types of flowers produced only small quantities of pollen, which was released several days after the flowers opened. Pollen grains were not filled in the anthers, which were shaped like arrowheads (Figure 8I), and the stamen filament was pink instead of white. Transverse sectioning of an anther in a naat tobacco bud revealed a small quantity of pollen that was poorly developed (Figures 8P and 8Q) compared with wild-type pollen (Figures 8M and 8N). In addition, aberrant pistils that were either too long or too short, and an irregularly shaped stigma, were observed frequently (Figure 8K). All naat tobacco plants with leaves exhibiting interveinal chlorosis were sterile.
Wild-type tobacco and naat tobacco were crossed to determine which reproductive organ was responsible for the sterility. Wild-type pollen was applied to the stigmas of naat tobacco, or naat tobacco pollen was applied to the stigmas of wild-type tobacco (Table 1). Seeds matured in all seed pods produced by crossing naat tobacco pollen and wild-type pistils, although relatively few seeds were produced. By contrast, wild-type pollen placed on naat tobacco pistils produced a few seeds in only 1 of 11 seed pods. The seeds were small and light brown in color. This finding indicates that both the pollen and pistils of naat tobacco have the ability to complete fertilization. However, the number of pollen grains in naat tobacco was not sufficient for complete fertility, and the pistil of naat tobacco could not produce mature seeds because of the shortage of NA metal supply. Therefore, the sterility of naat tobacco appeared to be the result of (1) poor pollen production, (2) late anther dehiscence, and (3) poor ovary maturation after fertilization.
Table 1.
Weight of a Seed Pod of Selfed or Crossed Tobacco
| WT (selfed) | 0.22 ± 0.10 |
|---|---|
| nas (selfed) | 0.24 ± 0.03 |
| naat-A (selfed) | 0.03 ± 0.01 |
| naat-A pollen × WT pistil | 0.10 ± 0.06 |
| WT pollen × naat-A pistil | 0.04 ± 0.01 |
Values shown are g dry weight ± se. WT, wild type; nas, hvnas1 tobacco; naat-A, hvnaat-A tobacco.
The Abnormal Morphology of Reproductive Organs Was Reversed by NA Treatment
To determine whether the endogenous NA shortage caused the morphological abnormalities in the inflorescence, NA was supplied to naat tobacco using the same method described for Figure 6A, except that decreased concentrations of Fe and NA were used. Excised naat tobacco axillary buds were treated with one of five solutions (Table 2). Only a solution of 100 μM NA with 20 μM Fe(III) citrate reversed the morphological abnormalities (Table 2). After NA treatment, both the chlorosis in young leaves and the morphological abnormalities in flowers were reversed (Figure 9D), and plenty of pollen was produced (Figure 9B). A solution of 20 μM Fe(III) citrate without NA did not reverse the chlorosis in young leaves, nor did it reverse the flower abnormalities (Figures 9A and 9C). A solution of 20 μM NA was not sufficient to reverse the morphological abnormalities in flowers, and a solution of 20 μM NA with 20 μM FeCl3 reversed neither the chlorosis nor the morphological abnormalities. Although a solution of 20 μM NA with 20 μM FeSO4 reversed the chlorosis in young leaves, it did not reverse the abnormalities in flowers at all. A solution of 20 μM NA with 20 μM Fe(III) citrate restored 43% of the flowers from the excised axillary buds. These results indicate that NA and Fe are required for normal flower development. However, we cannot exclude the possibility that other metals transported by NA were responsible for the restoration of normal flower development.
Table 2.
Recovery of Flower Abnormalities of naat Tobacco by NA Treatment
| Treatment
|
|||||
|---|---|---|---|---|---|
| Tissue | Fe(III) Citrate | 20 μM NA + FeCl3 | 20 μM NA + FeSO4 | 20 μM NA + Fe(III) Citrate | 100 μM NA + Fe(III) Citrate |
| Restored young leaf | 0 | 0 | 100 | 100 | 100 |
| Restored flower | 0 | 0 | 0 | 43 | 100 |
Percentage recovery was calculated as follows: restored axillary buds/treated axillary buds × 100.
Figure 9.
Recovery from Morphological Inflorescence Abnormalities by NA Treatment.
(A) naat tobacco flower treated with 20 μM Fe(III) citrate (top view).
(B) naat tobacco flower treated with 100 μM NA and 20 μM Fe(III) citrate (top view).
(C) Side view of the flower shown in (A).
(D) Side view of the flower shown in (B).
Fertility Was Restored by Grafting
To examine whether the endogenous NA shortage caused sterility, naat tobacco was grafted onto either wild-type tobacco or transgenic tobacco overexpressing the barley nicotianamine synthase gene (HvNAS1) (nas tobacco) (Figure 10A). Fertility was not restored after grafting onto wild-type tobacco, despite the recovery from interveinal chlorosis of the young leaves. Most anthers remained abnormal, and pollen did not develop (data not shown). Although a few anthers appeared partially normal, the seeds that were produced did not mature.
Figure 10.
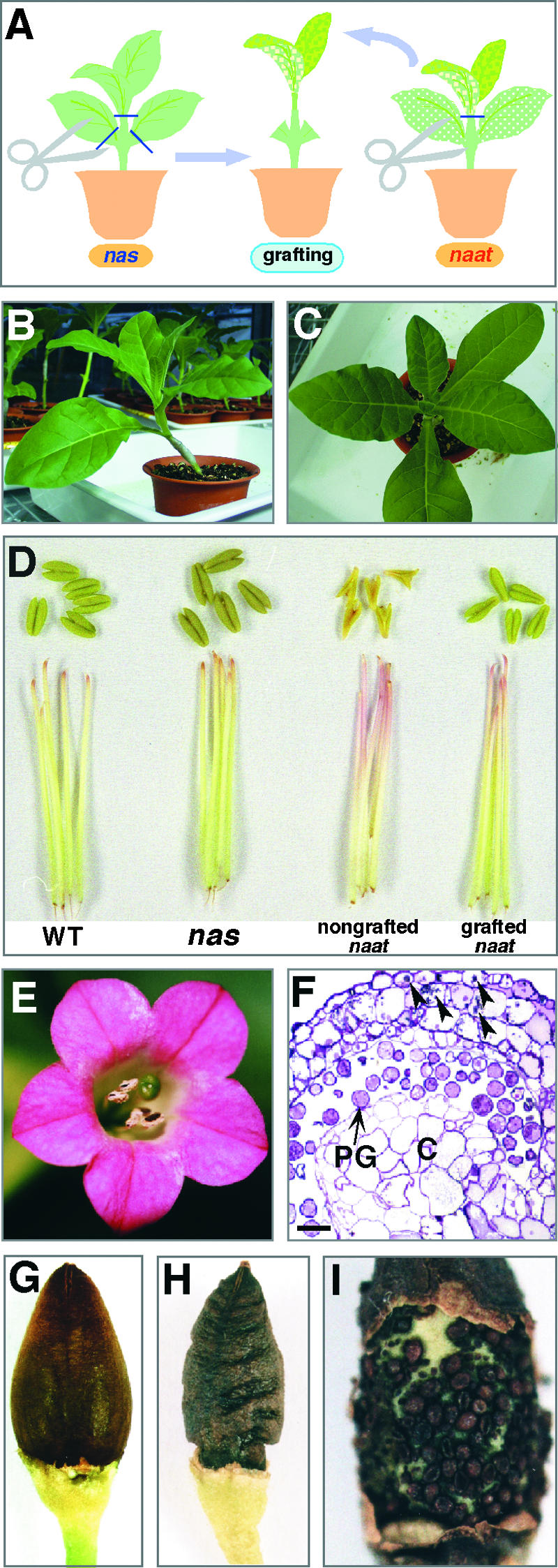
Recovery from Sterility by Grafting.
(A) Scheme of long-term NA supply to naat tobacco (naat) grafted onto nas tobacco (nas) (leaves removed), which overproduces NA.
(B) naat tobacco grafted onto nas tobacco (side view).
(C) Top view of the plant shown in (B).
(D) Anthers and anther filaments of wild-type tobacco (WT), nas tobacco, nongrafted naat tobacco, and grafted naat tobacco.
(E) A flower of naat tobacco grafted onto nas tobacco showing that flower shape was almost normal.
(F) Transverse section of an anther of naat tobacco grafted onto nas tobacco. Many mature pollen grains (PG) were observed. Unusual dark-stained globular structures (arrowheads) were observed in the vacuoles of the epidermis and the endothecium, even after the reversal of pollen immaturity. C, connective. Bar = 200 μm.
(G) Seed pod (sepal removed) of wild-type tobacco filled with mature seeds.
(H) Seed pod (sepal removed) of naat tobacco grafted onto nas tobacco.
(I) Seeds in the pod of naat tobacco grafted onto nas tobacco.
By contrast, grafting onto nas tobacco restored fertility to naat tobacco, and the leaves did not develop chlorosis (Figures 10B and 10C). In addition, most anthers were normal, the pinkish color of the stamen filament reverted to white (Figure 10D), pollen grains developed fully (Figures 10E and 10F), and seeds matured. However, many seeds did not develop fully, leading to shriveled seed pods (Figure 10H) and a reduced number of mature seeds (Figure 10I). Mature seeds obtained from grafted plants germinated normally, but the leaves of the seedlings had interveinal chlorosis and the inflorescences were morphologically abnormal and sterile (data not shown). The SPAD values of young leaves from nongrafted naat tobacco, grafted naat tobacco, and nas tobacco were as follows: 25.4 ± 2.4, 28.6 ± 6.0, and 29.2 ± 3.4, indicating the highest chlorophyll concentration in the nas tobacco.
Metal Concentrations in naat Tobacco Grafted onto nas Tobacco
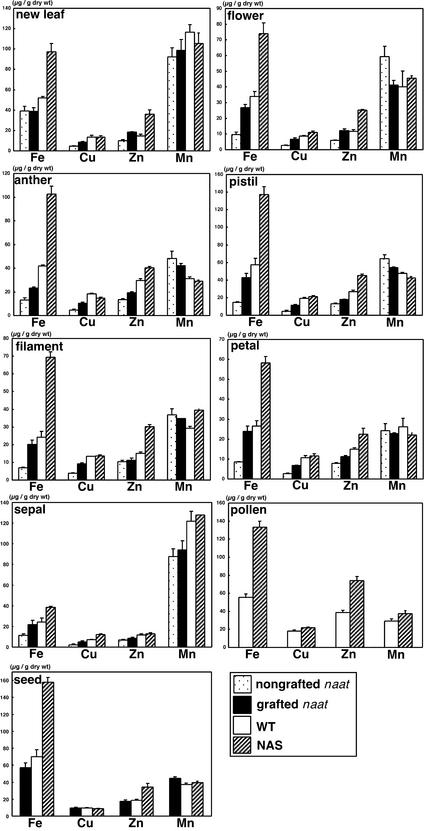
The metal concentrations in grafted tobacco were determined to investigate the effects of grafting naat tobacco onto nas tobacco (Figure 11). After grafting, the concentrations of Cu and Zn in young leaves increased 1.8-fold compared with that in nongrafted naat tobacco. The concentration of Mn increased by 10% and that of Fe increased not at all. However, the concentration of Fe in whole flowers increased 2.8-fold. There also was a marked increase of more than twofold in Cu and Zn concentrations in flowers, but the Mn concentration decreased. The metal concentration in each flower organ also was analyzed (Figure 11). The pattern of change in metal concentration for each flower organ was the same as that for the whole flower except for the sepal. For example, the concentrations of Fe, Cu, and Zn increased, whereas that of Mn decreased, in the anther, pistil, filament, and petal. In the pistil, filament, and petal, the Fe concentration increased the most (≥2.8-fold), that of Cu increased ∼2.5-fold, and that of Zn increased ∼1.3-fold. The Cu concentration increased most in the anther (2.2-fold) compared with that of Fe (1.8-fold) and Zn (1.4-fold). The pattern of change in metal concentration in the sepal was similar to that in the young leaf, except for an increase in Fe. The concentrations of all four metals increased as follows: Cu, 2.3-fold; Fe, 1.9-fold; Zn, 1.2-fold; and Mn, 1.1-fold. These results indicate that providing NA by grafting naat tobacco onto nas tobacco substantially increased the concentrations of Fe, Cu, and Zn in naat tobacco flowers.
Figure 11.
Metal Concentrations in Young Leaves, Whole Flowers, and Flower Organs of Grafted or Nongrafted naat Tobacco, nas Tobacco, and Wild-Type (WT) Tobacco.
Overexpression of HvNAS1 Increased Fe and Zn Concentrations in Young Leaves and Flowers
Metal concentrations in the young leaves and flowers of nas tobacco also were analyzed (Figure 11). In young leaves of nas tobacco, the Zn concentration was 2.5 times higher than that of wild-type tobacco. The Fe concentration also was higher (1.9-fold). In flowers, there was a marked increase in Fe concentration of 2.4-fold in the pistil, 2.5-fold in the anther, 2.4-fold in the pollen, and 2.9-fold in the filament. The Zn concentration also was higher, except in the sepal, by the following amounts: pistil, 1.7-fold; anther, 1.4-fold; pollen, 1.9-fold; filament, 2.0-fold; and petal, 1.5-fold. The Mn concentration in young leaves was lower than that in the wild-type anther, pistil, and petal but higher than that in the whole flower, pollen, filament, and sepal. In contrast to these metals, the Cu concentration was similar to that in the wild type, except for the sepal, in which there was a 1.7-fold increase compared with the wild type. As for other metals in the sepal, the Fe concentration was 1.6-fold higher and the Zn and Mn concentrations were almost the same as in the wild type. Seeds of nas tobacco also had higher Fe and Zn concentrations (Fe, 2.3-fold; and Zn, 1.8-fold), but the Cu concentration was similar to that in the wild type. These results indicate that NA promoted the transport of Fe in particular and also of Zn to young leaves, seeds, and all flower organs except the sepal.
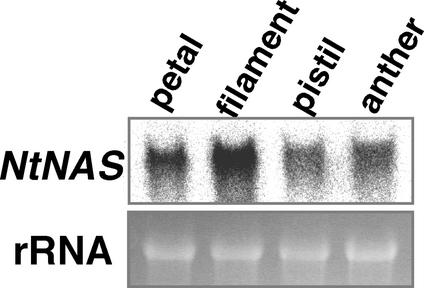
NtNAS Expression
Because our results indicated that NA plays a critical role in tobacco flower development, we investigated NAS expression in tobacco flowers by RNA gel blot analysis (Figure 12). Four flower organs (petal, filament, pistil, and anther) of mature buds were analyzed ∼1 day before anthesis (Figure 8A, fifth bud from the left). NtNAS was expressed in all flower organs, indicating that the NA produced in the inflorescence is required for normal flower development. In particular, NtNAS was highly expressed in the stamen filament, suggesting that NA plays a key role in supplying metals to anthers.
Figure 12.
RNA Gel Blot Analysis of Flower Organs of Wild-Type Tobacco.
DISCUSSION
Transgenic tobacco, constitutively expressing a barley naat-A gene (naat tobacco), showed distinct NAAT activity (Figure 2D) that led to NA consumption in situ. Thus, endogenous NA was not detected in naat tobacco but was present in control plants (Figure 5). This finding suggested that the naat tobacco phenotype is mainly the result of a lack of endogenous NA (Figures 3, 7, and 8).
NA Is Essential for Fe Transport in Veins and from Veins to the Interveinal Area
The concentrations of four metals, particularly Fe and Cu, were lower in naat tobacco leaves than in control tobacco leaves (Figure 4A). Moreover, a marked difference was found in the distribution of Fe in the leaves of naat tobacco and control tobacco (Figure 4B). In control tobacco, Fe was present in both the veins and the interveinal area of young leaves. By contrast, naat tobacco leaves had only a small quantity of Fe in the veins and an extremely low quantity in the interveinal area. These results indicate that the young naat tobacco leaves were Fe deficient. Because the demand for Fe is much higher in young leaves than in older leaves (Mori, 1998), we conclude that the chlorosis in young naat tobacco leaves was caused by insufficient Fe transport to the leaves, particularly to the interveinal area.
Treatment of naat tobacco with Fe alone did not reverse interveinal chlorosis. Fe(III) citrate increased the Fe concentration in young leaves but did not reverse interveinal chlorosis. This result suggests that citrate transported Fe(III) to veins in the young leaves but not into the interveinal area. On the other hand, treatment with Fe in combination with NA increased the Fe concentration and reversed chlorosis (Figures 6A, right, 6B, and 6D, Table 2). In addition, exogenously supplied 59Fe, when given in combination with NA, was transported immediately to the veins and interveinal areas in young naat tobacco leaves (Figure 6E). Thus, NA enhanced Fe transport not only to the veins in young leaves but also to the interveinal areas.
Young leaves treated with a solution containing NA also had a higher concentration of Cu and Zn (Figure 6D), indicating that NA also promotes the transport of these metals to the young leaf. NA chelates the four metals (Fe, Zn, Mn, and Cu) analyzed in this study (Benes et al., 1983; Stephan and Scholz, 1993). The log stability constants of metal-NA complexes are as follows: Mn(II), 8.8; Fe(II), 12.8; Zn(II), 15.4; Cu(II), 18.6; and Fe(III), 20.6 (von Wirén et al., 1999). Interestingly, the concentrations of the four metals in naat tobacco, in both young leaves and flowers, declined in a similar order: Mn < Zn < Fe < Cu. This decrease in the concentrations of metals in naat tobacco probably was attributable to the depletion of endogenous NA.
The interveinal chlorosis phenotype in young leaves also has been reported in the tomato mutant chloronerva, which lacks endogenous NA (Böhme and Scholz, 1960). NAS activity has not been detected in chloronerva (Higuchi et al., 1996). The NAS gene in chloronerva has a single base mutation that results in an amino acid change that is highly conserved in all nas-related genes (Higuchi et al., 1999; Ling et al., 1999; Suzuki et al., 1999). As a result, mutant NAS is inactive and NA is not produced in chloronerva. Consequently, although naat tobacco and chloronerva lack endogenous NA for different reasons, the resulting interveinal chlorosis in young leaves is similar in these two plant types.
NA Is Essential for Reproductive Growth and Fertility
Studies in young leaves showed that a low concentration of NA (20 μM) with Fe(III) citrate or FeSO4 reversed interveinal chlorosis (Table 2) but was not sufficient to completely overcome flower abnormalities (Table 2). Increasing the concentration of NA to 100 μM in combination with Fe(III) citrate, however, overcame the morphological abnormalities in flowers, pollen maturation defects, and late anther dehiscence (Table 2, Figure 9).
If NA was not constitutively consumed by NAAT in situ, a NA concentration of <100 μM would be sufficient to reverse the morphological abnormalities in flowers. It is conceivable, therefore, that NA affects flower morphogenesis at very low concentrations, suggesting a number of important conclusions. (1) A higher NA concentration is required for normal flower development than for normal leaf development. (2) Citrate cannot be substituted for NA at the reproductive stage, nor can Fe supplied as Fe(III) citrate be transported from veins to interveinal areas of young leaves. (3) Both NA and Fe are required for normal flower development, and the combination of NA with Fe(III) citrate is effective for normal flower development. (4) Cu and Zn also participate in normal flower development (Figure 6D).
Grafting of naat tobacco onto wild-type tobacco reversed interveinal chlorosis and aberrant flower shape but did not reverse sterility. Indeed, many anthers remained abnormal, and even flowers with restored anthers did not have any mature seeds. On the other hand, grafting of naat tobacco onto nas tobacco reversed interveinal chlorosis in young leaves, aberrant flower shape, pollen maturation defects, late anther dehiscence, and sterility. Mature seeds of naat tobacco grafted onto wild-type tobacco were produced, but they were few in number (data not shown). Because wild-type tobacco produced less NA than nas tobacco (data not shown), it is likely that the amount of NA produced by the wild type was not sufficient to compensate for the consumption of endogenous NA by NAAT, resulting in overall insufficient NA to mature seeds. Grafting onto nas tobacco increased the concentrations of Fe, Cu, and Zn in whole naat tobacco flowers (Figure 11), and the flowers of nas tobacco had higher concentrations of Fe, Cu, and Zn than did wild-type flowers. Interestingly, the Cu concentration was not as high in nas tobacco, suggesting that NA may be involved in Cu homeostasis. The results from the grafting experiment suggest that seed maturation requires more NA than do normal leaf and flower development and that NA affects metal concentrations in flowers. These results suggest that NA is essential for normal inflorescence formation, for the production of normal pollen, and for seed maturation.
Importance of Metals at the Reproductive Stage
Fe, Cu, Zn, and Mn are important for reproductive development because they are contained in many critical proteins during this stage. It has been reported that Cu deficiency causes male sterility (Graham, 1975; Dell, 1981), that Zn deficiency decreases pollen fertility (Sharma et al., 1990), and that Mn deficiency affects pollen productivity and viability (Sharma et al., 1991). Zn also is related to female fertility, because Zn-finger Polycomb group proteins are necessary for proper female gametophyte and seed development (Grossniklaus et al., 1998; Kiyosue et al., 1999; Luo et al., 1999; Ohad et al., 1999; Brive et al., 2001).
Microsporogenesis requires a great deal of energy, which might impose stress on anther development, and many researchers have noted the importance of mitochondria in male gametophyte development (Warmke and Lee, 1978; Hanson, 1991; Hernould et al., 1993, 1998; Schnable and Wise, 1998). Normal mitochondrial functioning requires high quantities of Fe because many mitochondrial enzymes require Fe for their activity (some mitochondrial enzymes require Cu). In fact, Mori et al. (1991) reported that mitochondria in the cortex cells of rice roots were damaged severely by Fe deficiency. Therefore, an increase in the number of mitochondria would seem to predict greater quantities of Fe-containing proteins in flowers. Consequently, it would be expected that flowers require more Fe than do vegetative organs. In addition, Fe deficiency also might result in female sterility. For example, female sterility was evident in transgenic tobacco plants after suppression of a pistil-specific gene coding for the ethylene-forming enzyme 1-aminocyclopropane-1-carboxylate (ACC) oxidase (Martinis and Mariani, 1999). ACC oxidase requires Fe(II) for its activity (Ververidis and John, 1991). Martinis and Mariani (1999) suggested that female sterility was caused by suppressed ethylene formation resulting from ACC oxidase inhibition. Abnormalities in the lengths of pistils in naat tobacco might be a consequence of an ethylene formation disorder caused by Fe deficiency. It has been reported that an Arabidopsis knockout mutant in IRT1 (iron-regulated metal transporter) is sterile and that plants die after 3 or 4 weeks (Vert et al., 2002). These studies have shown the importance of Fe, Zn, and Mn in fertility.
Fe deficiency causes peroxidase activity depression and the accumulation of phenolic compounds (Sijmons et al., 1985). Similarly, Cu deficiency causes a decrease in polyphenol oxidase activity and an accumulation of phenolic compounds (Judel, 1972). Although anthocyanin should be increased by the accumulated phenolic compounds, anthocyanin synthesis might be decreased by the decreased activity of anthocyanidin synthase, because Fe also is required for anthocyanidin synthase activity (De Carolis and De Luca, 1994). The type-II phenotype might result from high phenolic compounds and low anthocyanin accumulation induced by severe Fe and Cu deficiency.
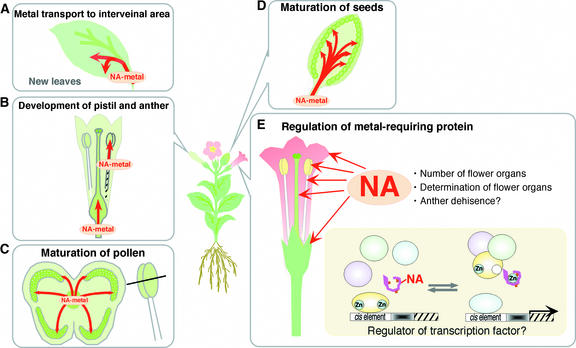
NA Functions at the Reproductive Stage
Figure 13 illustrates NA's roles at the reproductive stage. These include NA acting as a metal carrier and in the regulation of intercellular metals and metal-requiring proteins. These roles are discussed below.
Figure 13.
Model Representing the Multiple Functions of NA at the Reproductive Stage.
NA acts as a metal carrier to young leaves (A), to the developing pistil and anther (B), for the maturation of pollen (C), and for the maturation of seeds (D). NA also is involved in the regulation of metal-requiring proteins (e.g., Zn-finger proteins) (E).
Metal Carrier
NA transports metals to young leaves (Figure 13A), reproductive organs (Figures 13B and 13C), and seeds (Figure 13D). Grafting experiments and metal concentrations in nas tobacco show that NA promotes the transport of Fe, Cu, and Zn to each flower organ and to seeds. NtNAS expression in flower organs also indicates that NA promotes metal transport into the inflorescence. In particular, NtNAS expression in the stamen filament suggests that NA transports metals required for the maturation of pollen in the anther. In young leaf and flower, citrate could not substitute for NA. These results suggest that NA transports metals to cells of the reproductive organs through the specific transporter for NA-metal complexes in vascular bundles of young leaves, inflorescences, and seeds.
In the xylem, Fe is thought to be translocated mainly as Fe(III) citrate (Tiffin, 1966; von Wirén et al., 1999). However, NA must exist in the xylem even if it does not bind with Fe. In growing tissues, phloem elements differentiate earlier than xylem vessels, and the supply of metals to the growing shoot apex is provided by the sieve tubes after transloading from the xylem (Pate, 1975). It has been reported that NA is needed for the normal distribution of metals in young growing tissues, fed via the phloem, because NA prevents their precipitation in the alkaline phloem sap (Stephan and Scholz, 1993). These reports support our data and suggest the following possibilities. In young leaves, the Fe ligand exchange (citrate→NA) could occur during Fe transfer from xylem to phloem in the vein, and NA-Fe could be transported through a NA-metal complex–specific transporter. Fe could be supplied to the inflorescence and seed by NA via the phloem after transloading from the xylem elsewhere (e.g., the stalk). The NA-metal complex–specific transporter also could transport NA-Fe to each flower organ and seed. Recently, Vert et al. (2002) reported that the IRT1 promoter–GUS fusion showed GUS staining exclusively in the anther filament. The Fe and Zn that are not complexed by NA (i.e., free ions) also could be transported to flower organs by IRT1.
Regulation of Intercellular Metals and Metal-Requiring Proteins
NA regulation of metal-requiring proteins is illustrated in Figure 13E. As described above, Fe, Cu, Zn, and Mn are important for reproductive development because they participate in many critical proteins at the reproductive stage. How do these proteins acquire the metals they need? As free ions, they are toxic. Recent work indicates that in yeast and bacterial cells, virtually all cellular Cu and Zn, respectively, are bound to ligands and that any movement within the cell involves exchange reactions between ligands (Rae et al., 1999; Outten and O'Halloran, 2001). Some of these metallochaperones have been identified in yeast, mammals, and plants for Cu (O'Halloran and Culotta, 2000) but not yet for Zn (Clemens et al., 2002). NA could be involved in the regulation of metal transfer within the cell. We present some examples to support our hypothesis below.
Zn Finger: Morphogenesis and Fertility
naat tobacco developed leaves that were long and narrow and various aberrant flowers that were sterile. Furthermore, the concentrations of Cu, Fe, and Zn were low in naat tobacco flowers. Axillary buds that were Fe or Zn deficient (data not shown) did not produce flowers that were the same as type-I naat tobacco flowers, but Fe deficiency resulted in flowers similar to naat tobacco type-II flowers (Figures 8C and 8F). Grafting reversed the morphological abnormality of both flower types, reversed sterility, and restored leaf shape (Figures 10C to 10F). All naat tobacco flowers had larger sepals than wild-type flowers. By contrast, nas tobacco sepals were slightly smaller than wild-type sepals, whereas the pistils were slightly larger. Moreover, NtNAS expression in flower organs suggests that NA is not only a metal carrier in long-distance transport but also a regulator of metals within the cell.
Zn plays an important role in key structural motifs in transcriptional regulatory proteins, including the Zn finger, Zn cluster, and RING finger domains. Furthermore, the transcriptional factors that participate in flower development include many Zn finger proteins (Takatsuji, 1999). Recently, Kapoor et al. (2002) reported that silencing of TAZ1 (TAPETUM DEVELOPMENT ZINC FINGER PROTEIN1) caused premature degeneration of tapetum and pollen abortion in petunia. The Arabidopsis MS1 (MALE STERILITY1) gene that is homologous with the PHD-finger motifs (C4HC3 Zn-finger–like motifs) was reported to be a critical sporophyte-controlling factor for anther and pollen development (Wilson et al., 2001).
The sterility of naat tobacco may be related to these Zn-finger proteins, because it is conceivable that Zn deficiency in the cell, resulting from the shortage of NA, affects the functions of Zn-finger proteins, leading to sterility. Moreover, if NA functions as an intercellular metal carrier, the absence of NA may inhibit the accurate transfer of Zn to the proteins or the removal of Zn from the proteins.
FIL (FILAMENTOUS FLOWER) is one of the Zn-finger proteins involved in morphogenesis; it has two Zn ions. Its binding to DNA or interaction with other transcription factors is regulated by the release of one Zn ion by EDTA (Kanaya et al., 2001, 2002). FIL is thought to interact with LFY (LEAFY) and APETALA1 (Sawa et al., 1999) or to be required for floral organ formation, determining their correct numbers and positions (Chen et al., 1999). Flowers of the Arabidopsis fil mutant bear several similarities to naat tobacco flowers, including a chimeric flower organ and a decreased number of flower organs and filamentous organs. In addition, 35S:LFY and 35S:NFL (the tobacco homolog of Floricaula and Leafy) tobacco have some similarities. 35S:LFY (class II) tobacco has supernumerary floral organs and chimeric organs that are formed by fusion of the sepal, petal, and stamen. 35S:NFL tobacco exhibits supernumerary stamens and multiple carpels rather than the two fused carpels found normally in wild-type tobacco and a chimeric organ such as a sepaloid petal or a petaloid stamen. Because NA is a chelator of metals, as is EDTA, it is possible that NA regulates some Zn-finger proteins by supplying or removing Zn. This possibility suggests that NA may affect the interaction between transcription factors within the cells.
Fe-Requiring Proteins
NA also may regulate the Fe in Fe-requiring proteins. DME (DEMETER) encodes a large protein with DNA glycosylase and nuclear localization domains. It is expressed primarily in the central cell of the female gametophyte, the progenitor of the endosperm (Choi et al., 2002), and its DNA glycosylate domain holds a (4Fe-4S)2+ in place. DME is required for endosperm gene imprinting and seed viability. Homozygous dme-1 mutant plants produced siliques in which almost all seeds were aborted. These plants sporadically formed individual flowers with reduced or increased petal and sepal numbers, fused stamen filaments, petal-like anthers, two gynoecia, improperly fused carpels, and abnormal leaves and stems.
NA also may affect dehiscence. A flower of an Fe-deficient axillary bud (Figure 8F) dehisced its anthers normally even though there was little pollen. By contrast, in naat tobacco, there was a delay in anther dehiscence, for which jasmonic acid is required. Because allene oxide synthase, a key enzyme for jasmonic acid biosynthesis, has heme-Fe (Kubigsteltig et al., 1999), it might be affected by NA. Alternatively, NA may affect anther wall desiccation directly.
In conclusion, we present here various roles for NA, especially at the reproductive stage. For a long time, it has been supposed that NA is necessary for the redistribution of Fe, Zn, and Mn via the phloem and that it is required for Cu transport in the xylem. This study showed that the role of NA in the transport of metals is required for the normal function of young leaves, for the development of reproductive organs, and for fertility. In addition, we have suggested that NA may be required for the intracellular regulation of metal binding proteins, such as Zn-finger proteins.
METHODS
Binary Vector Construction
hvnaat-A cDNA was isolated from Fe-deficient barley (Hordeum vulgare) roots (Takahashi et al., 1999) and cloned into the SmaI site of the pUC118 vector. pUC118-hvnaat-A was digested with XbaI and SacI and cloned into the pIG121Hm vector (Hiei et al., 1994).
Plant Material and Growth Conditions
The binary vector pIG121Hm-hvnaat-A was introduced into Agrobacterium tumefaciens strain C-58 via triparental mating. Transformation and regeneration were performed in tobacco (Nicotiana tabacum cv SR1) using the standard leaf disc transformation method (Helmer et al., 1984). Seeds from 16 lines of naat tobacco T1 progeny were harvested by grafting axillary buds, excised selectively from >50 lines of naat tobacco T0 progeny, onto nas tobacco. T2 seeds also were harvested by grafting onto nas tobacco. nas tobacco, a transgenic tobacco that had been transformed using pBIGRZ1 vector with the Cauliflower mosaic virus 35S promoter–hvnas1 gene inserted, was confirmed to overexpress nicotianamine synthase (NAS). naat tobacco, nas tobacco, wild-type tobacco, and control tobacco were grown at 25°C (day/night) under natural light (106 μmol·m−2·s−1) in pots filled with vermiculite. The plants were watered every day with 1000-fold diluted Hyponex (Osaka, Japan) (composition: 5.0% N, 10.0% P, 5.0% K, 0.05% Mg, 0.001% Mn, and 0.005% B). Every analysis was performed using more than three naat tobacco lines (T0 and T1 progeny). For the final experiment shown in this study, two lines of naat tobacco T2 progeny were used and analyzed (except for elemental mapping; T0 progeny).
DNA Gel Blot Analysis
Genomic DNA was prepared from the leaves of control and naat tobacco (Murray and Thompson, 1980). DNA samples (20 μg) were digested with HindIII and separated by electrophoresis on 0.8% agarose gels. Gels were blotted with a Hybond-N+ membrane (Amersham Pharmacia Biotech). Hybridization was performed using the internal sequence of naat-A cDNA (∼600 bp) amplified by PCR in the presence of α-32P-ATP. The membrane was hybridized overnight at 65°C with the labeled probe in 0.5 M Church phosphate buffer (Church and Gilbert, 1984), 1 mM EDTA, and 7% (w/v) SDS with 100 μg/mL salmon sperm DNA. After hybridization, the blot was washed twice with 40 mM Church phosphate buffer and 1% (w/v) SDS at 65°C for 5 min (first) and 10 min (second). Radioactivity was detected using a BAS-2000 image analyzer (Fuji, Tokyo, Japan).
RNA Gel Blot Analysis
Total RNA was isolated from young tobacco leaves using RNeasy Plant Mini kits (Qiagen, Valencia, CA). For hvnaat-A expression analysis, RNA (20 μg) was denatured and electrophoresed on 1.2% agarose gels containing 5% (v/v) formaldehyde. It then was blotted on a Hybond-N+ membrane, and the membrane was hybridized with the same probes under the conditions described above. For NtNAS expression analysis, RNA (10 μg) was electrophoresed and blotted as described above. Genomic DNA (3 μg/μL) was prepared from wild-type tobacco leaves (2 g). Using this genomic DNA as a template, PCR was conducted using two primers (5′-GAGAGAGAGATATCATTAGGTCTCATCTCATCAAACTTT-GTGG-3′ and 5′-GAGAGAGAGTCGACGCACGTGCACCATGTGCAC-TTCTAAGC-3′), and the PCR probe was subcloned. Hybridization was performed using this 32P-labeled product (internal sequence of NtNAS1, 530 bp) under the conditions described above.
Nicotianamine Aminotransferase Enzyme Assay
Protein was assayed using a kit from Bio-Rad Laboratories. Aliquots of samples were assayed for nicotianamine aminotransferase activity using nicotianamine (NA) as a substrate, according to the method of Kanazawa et al. (1994). Then, 4 μL of 0.25 M NaBH4 was added to reduce the reaction product to deoxymugineic acid (Ohata et al., 1993). The quantity of deoxymugineic acid was analyzed by HPLC (Mori et al., 1987).
Microscopy
Young leaves (the first unfolded leaf) and anthers in buds at flowering stage 8 were excised and prefixed in 4% paraformaldehyde, 5% glutaraldehyde, 0.1 M CaCl2, and 0.1 M cacodylate buffer, pH 7.2, for 3 h on ice. After washing with the same buffer for 1.5 h, postfixation was performed in 2% osmium tetroxide and 0.1 M cacodylate buffer for 1 h on ice. Samples were dehydrated serially in ethanol and propylene oxide and embedded in Epon-Araldite resin. Semithin sections were cut and stained with toluidine blue and basic fuchsin (epoxy tissue stain; Electron Microscopy Sciences, Fort Washington, PA) for light microscopy. Ultrathin sections were cut and stained with uranium and lead for electron microscopy (1010 EX; JEOL, Akishima, Japan).
Determination of Metal Concentrations
Dry leaf or flower samples weighing 20 to 50 mg were placed with 2 mL of nitric acid in a sealed polytetrafluoroethylene vessel with a stainless steel jacket (Kojima and Iida, 1986). The vessel was heated from room temperature to 150°C in an oven for 60 min and then kept at 150°C for 5 h. After cooling to room temperature, samples were filled to a constant volume and metal concentrations were determined using inductively coupled plasma emission spectrometry (SPS1200 VR; Seiko, Tokyo, Japan).
Elemental Mapping
Synchrotron radiation–induced x-ray fluorescence spectrometry imaging (XRF) was used to locate metals in the leaves, as described by Yoshimura et al. (2000). The first unfolded young leaf was excised, placed between paper towels, and pressed dry using an electric iron. Two-dimensional XRF measurements were made at beam line 4A (Photon Factory, High-Energy Accelerator Research Organization, Tsukuba, Japan) with an energy-dispersive XRF system. The sample was irradiated with a 200 μm × 200 μm parallel x-ray beam of 10.62 keV. Two-dimensional mapping was performed by step-wise scanning of the sample, and the XRF intensities of K, Fe, Zn, and Mn Kα lines were measured with a counting time of 50 s/point. The Kα line is an x-ray having a wavelength due to an electronic transition from K-shell to L-shell in an element.
Extraction and Analysis of NA
NA was extracted and quantified according to the method of Pich et al. (1994). Freshly harvested plant leaves were frozen and stored at −80°C. Tissue was homogenized in a mortar under liquid nitrogen and afterwards thawed by mixing with 20-fold deionized water (w/v). The sample was heated to 80°C and centrifuged at 8500g for 10 min. The supernatant was applied to a column of Amberlite IR-120(H+) (Orugano, Tokyo, Japan). After washing of the column with water, the fraction was eluted with 2 N NH4OH and concentrated in a rotary evaporator at 35°C. HPLC was used for quantitative analysis (Mori and Nishizawa, 1987; Shojima et al., 1989b; Higuchi et al., 2001).
Regreening Test for Interveinal Chlorosis
Excised naat tobacco axillary buds were treated with one of five solutions with a Fe concentration of 100 μM and a NA concentration of 200 μM: (1) FeCl3, (2) FeSO4, (3) Fe(III) citrate plus NA, (4) FeCl3 plus NA, and (5) FeSO4 plus NA. Each solution contained 7 × 10−4 M K2SO4, 1 × 10−4 M KCl, 1 × 10−4 M KH2PO4, 2 × 10−3 M Ca(NO3)2, 5 × 10−4 M MgSO4, 1 × 10−5 M H3BO3, 5 × 10−7 M MnSO4, 5 × 10−7 M ZnSO4, 2 × 10−7 M CuSO4, and 1 × 10−8 M (NH4)6Mo7O24, pH 5.8. Treatment was performed under 24-h light conditions (75 μmol·m−2·s−1) at 25°C (day/night) for 110 h, and the solutions were replaced every day. The degree of chlorosis of the young leaves (the youngest fully expanded leaves) was determined using a SPAD-502 chlorophyll meter (Minolta Co., Tokyo, Japan). This experiment was repeated three times, each time using more than three naat tobacco lines. The first experiment was performed in triplicate, the second used six replicates, and the third used four replicates.
59Fe Uptake
Excised axillary buds of naat tobacco were treated with one of two solutions: (1) +59Fe, +NA, and (2) +59Fe, −NA. Treatment was stopped after 1, 3, or 6 h, and leaves were cut and dried using an electric iron. The distribution of 59Fe was detected on x-ray film to obtain autoradiographs with high spatial resolution (Fuji) (Bughio et al., 1997; Mori, 1998).
Recovery Test for Morphological Flower Abnormality
Excised axillary buds were treated with five solutions: (1) 0 μM NA and 20 μM citrate Fe(III), (2) 20 μM NA and 20 μM FeCl3, (3) 20 μM NA and 20 μM FeSO4, (4) 20 μM NA and 20 μM Fe(III) citrate, and (5) 100 μM NA and 20 μM Fe(III) citrate. Each solution contained a 10% concentration of Murashige and Skoog (1962) medium, pH 5.5. Treatment was performed under 24-h light conditions at 25°C (day/night) for 4 to 5 weeks, and the solutions were replaced every day. There were four to seven replicates using more than three naat tobacco lines.
Grafting of naat Tobacco onto nas Tobacco
Axillary buds of naat tobacco were grafted onto nas tobacco as a rootstock, the leaves of which had been removed (Figure 9A). Grafting experiments were performed twice to obtain a sufficient number of seeds. Finally, 28 axillary buds (two lines of T2 naat tobacco) were grafted and analyzed. Plants were grown under natural light (106 μmol·m−2·s−1) in pots filled with vermiculite at 25°C (day/night) and watered every day with 1000-fold diluted Hyponex (see above).
Upon request, all novel materials described in this article will be made available in a timely manner for noncommercial research purposes.
Accession Number
The accession number for NtNAS1 is AB097697.
Acknowledgments
We thank Kyoko Higuchi (Tokyo University of Agriculture) for providing the nas tobacco seeds, Kenzo Nakamura (Nagoya University) for the pIG121Hm vector, Takeshi Kitahara (University of Tokyo) for NA, Hirotaka Yamaguchi, Takashi Negishi, and Takuya Nakagawa for technical assistance with our experiments, and P. Blamey (University of Tokyo) for assistance with English expression.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.010256.
References
- Benes, I., Schreiber, K., Ripperger, H., and Kircheiss, A. (1983). Metal complex formation by nicotianamine, a possible phytosiderophore. Experientia 39, 261–262. [Google Scholar]
- Böhme, H., and Scholz, G. (1960). Versuche zur Normalisierung des Phänotyps der Mutante Chloronerva von Lycopersicon esculentum Mill. Kulturpflanze 8, 93–109. [Google Scholar]
- Bughio, N., Takahashi, M., Yoshimura, E., Nishizawa, N.K., and Mori, S. (1997). Light-dependent iron transport into isolated barley chloroplasts. Plant Cell Physiol. 38, 101–105. [Google Scholar]
- Brive, A., Sengupta, A.K., Beuchle, D., Larsson, J., Kennison, J.A., Rasmuson-Lestander, A., and Muller, J. (2001). Su(z)12, a novel Drosophila Polycomb group gene that is conserved in vertebrates and plants. Development 128, 3371–3379. [DOI] [PubMed] [Google Scholar]
- Chen, Q., Atkinson, A., Otsuga, D., Christensen, T., Reynolds, L., and Drews, G.N. (1999). The Arabidopsis FILAMENTOUS FLOWER gene is required for flower formation. Development 126, 2715–2726. [DOI] [PubMed] [Google Scholar]
- Choi, Y., Gehring, M., Johnson, L., Hannon, M., and Harada, J.J. (2002). DEMETER, a DNA glycosylase domain protein, is required for endosperm gene imprinting and seed viability in Arabidopsis. Cell 110, 33–42. [DOI] [PubMed] [Google Scholar]
- Church, M., and Gilbert, W. (1984). Genomic sequencing. Proc. Natl. Acad. Sci. USA 81, 1991–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens, S., Palmgren, M.G., and Krämer, U. (2002). A long way ahead: Understanding and engineering plant metal accumulation. Trends Plant Sci. 7, 309–315. [DOI] [PubMed] [Google Scholar]
- De Carolis, E., and De Luca, V. (1994). 2-Oxoglutarate-dependent dioxygenase and related enzymes: Biochemical characterization. Phytochemistry 36, 1093–1107. [DOI] [PubMed] [Google Scholar]
- Dell, B. (1981). Male sterility and outer wall structure in copper-deficient plants. Ann. Bot. 48, 599–608. [Google Scholar]
- Graham, R.D. (1975). Male sterility in wheat plants deficient in copper. Nature 254, 514–515. [Google Scholar]
- Grossniklaus, U., Vielle-Calzada, J.-P., Hoeppner, M.A., and Gagliano, W.B. (1998). Maternal control of embryogenesis by MEDEA, a Polycomb-group gene in Arabidopsis. Science 280, 446–450. [DOI] [PubMed] [Google Scholar]
- Hanson, M.R. (1991). Plant mitochondrial mutations and male sterility. Annu. Rev. Genet. 25, 461–486. [DOI] [PubMed] [Google Scholar]
- Helmer, G., Casadaban, M., Bevan, M., Kayes, L., and Chilton, M.D. (1984). A young chimeric gene as a marker for plant transformation: The expression of Escherichia coli β-galactosidase in sunflower and tobacco cells. Bio/Technology 2, 520–527. [Google Scholar]
- Hernould, M., Suharsono, S., Litvak, S., Araya, A., and Mouras, A. (1993). Male sterility induction in transgenic tobacco plants with an unedited ATP9 mitochondrial gene from wheat. Proc. Natl. Acad. Sci. USA 90, 2370–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernould, M., Suharsono, S., Zabaleta, E., Carde, J.P., Litvak, S., Araya, A., and Mouras, A. (1998). Impairment of tapetum and mitochondria in engineered male-sterile tobacco plants. Plant Mol. Biol. 36, 499–508. [DOI] [PubMed] [Google Scholar]
- Hiei, Y., Ohta, S., Komari, T., and Kumashiro, T. (1994). Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J. 6, 271–282. [DOI] [PubMed] [Google Scholar]
- Higuchi, K., Nishizawa, N., Römheld, V., Marschner, H., and Mori, S. (1996). Absence of nicotianamine synthase activity in the tomato mutant ‘chloronerva.’ J. Plant Nutr. 19, 1235–1239. [Google Scholar]
- Higuchi, K., Nishizawa, N.-K., Yamaguchi, H., Römheld, H., Marschner, H., and Mori, S. (1995). Response of nicotianamine synthase activity to Fe-deficiency in tobacco plants as compared with barley. J. Exp. Bot. 46, 1061–1063. [Google Scholar]
- Higuchi, K., Suzuki, K., Nakanishi, H., Yamaguchi, H., Nishizawa, N.K., and Mori, S. (1999). Cloning of nicotianamine synthase genes, novel genes involved in the biosynthesis of phytosiderophores. Plant Physiol. 119, 471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi, K., Watanabe, S., Takahashi, M., Kawasaki, S., Nakanishi, H., Nishizawa, N.K., and Mori, S. (2001). Nicotianamine synthase gene expression differs in barley and rice under Fe-deficient conditions. Plant J. 25, 159–167. [DOI] [PubMed] [Google Scholar]
- Judel, G.K. (1972). Änderungen in der Aktivitaet der Peroxidase und der Katalase und im Gehalt an Gesamtphenolen in den Blaettern der Sonnenblume unter dem Einfluss von Kupfer- und Stickstoffmangel. Z. Pflanzenernaehr. Bodenkd. 133, 81–92. [Google Scholar]
- Kanaya, E., Nakajima, N., and Okada, K. (2002). Non-sequence-specific DNA binding by the FILAMENTOUS FLOWER protein from Arabidopsis thaliana is reduced by EDTA. J. Biol. Chem. 277, 11957–11964. [DOI] [PubMed] [Google Scholar]
- Kanaya, E., Watanabe, K., Nakajima, N., Okada, K., and Shimura, Y. (2001). Zinc release from the CH2C6 zinc finger domain of FILAMENTOUS FLOWER protein from Arabidopsis thaliana induces self-assembly. J. Biol. Chem. 276, 7383–7390. [DOI] [PubMed] [Google Scholar]
- Kanazawa, K., Higuchi, K., Nishizawa, N.K., Fushiya, S., Chino, M., and Mori, S. (1994). Nicotianamine aminotransferase activities are correlated to the phytosiderophore secretions under Fe-deficient conditions in Gramineae. J. Exp. Bot. 45, 1903–1906. [Google Scholar]
- Kapoor, S., Kobayashi, A., and Takatsuji, H. (2002). Silencing of the tapetum-specific zinc finger gene TAZ1 causes premature degeneration of tapetum and pollen abortion in petunia. Plant Cell 14, 2353–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyosue, T., Ohad, N., Yadegari, R., Hannon, M., Dinneny, J., Wells, D., Katz, A., Margossian, L., Harada, J., Goldberg, R.B., and Fischer, R.L. (1999). Control of fertilization-independent endosperm development by the MEDEA Polycomb gene in Arabidopsis. Proc. Natl. Acad. Sci. USA 96, 4186–4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima, I., and Iida, C. (1986). Vapor phase digestion of botanical samples with acids in sealed polytetrafluoroethylene bomb. Anal. Sci. 2, 567–570. [Google Scholar]
- Kubigsteltig, I., Laudert, D., and Weiler, E.W. (1999). Structure and regulation of the Arabidopsis thaliana allene oxide synthase gene. Planta 208, 463–471. [DOI] [PubMed] [Google Scholar]
- Ling, H.Q., Koch, G., Bäumlein, H., and Ganal, M.W. (1999). Map-based cloning of chloronerva, a gene involved in iron uptake of higher plants encoding nicotianamine synthase. Proc. Natl. Acad. Sci. USA 96, 7098–7103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, M., Bilodeau, P., Koltunow, A., Dennis, E.S., Peacock, W.J., and Chaudhury, A.M. (1999). Genes controlling fertilization-independent seed development in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 96, 296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinis, D.D., and Mariani, C. (1999). Silencing gene expression of ethylene-forming enzyme results in a reversible inhibition of ovule development in transgenic tobacco plants. Plant Cell 11, 1061–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori, S. (1998). Iron transport in graminaceous plants. In Metal Ions in Biological Systems, A. Sigel and H. Sigel, eds (New York: Marcel Decker), pp. 215–238.
- Mori, S., and Nishizawa, N. (1987). Methionine as a dominant precursor of phytosiderophores in Gramineae plants. Plant Cell Physiol. 28, 1081–1092. [Google Scholar]
- Mori, S., Nishizawa, N.K., Hayashi, M., Chino, M., Yoshimura, E., and Ishihara, J. (1991). Why are young rice plants highly susceptible to iron deficiency? In Iron Nutrition and Interactions in Plants, Y. Chen and Y. Hadar, eds (Dordrecht, The Netherlands: Kluwer Academic Publishers), pp. 175–188.
- Mori, S., Nishizawa, N.K., Kawai, S., Sato, S., and Takagi, S. (1987). Dynamic state of mugineic acid and analogous phytosiderophores in Fe-deficient barley. J. Plant Nutr. 10, 1003–1011. [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15, 473–497. [Google Scholar]
- Murray, M.G., and Thompson, W.F. (1980). Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 8, 4321–4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma, M., and Noguchi, M. (1976). Occurrence of nicotianamine in higher plants. Phytochemistry 15, 1701–1702. [Google Scholar]
- Noma, M., Noguchi, M., and Tamaki, E. (1971). A new amino acid, nicotianamine, from tobacco leaves. Tetrahedron Lett. 22, 2017–2020. [Google Scholar]
- Ohad, N., Yadegari, R., Margossian, L., Hannon, M., Michaeli, D., Harada, J.J., Goldberg, R.B., and Fischer, R.L. (1999). Mutations in FIE, a WD Polycomb group gene, allow endosperm development without fertilization. Plant Cell 11, 407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Halloran, T.V., and Culotta, V.C. (2000). Metallochaperones, an intracellular shuttle service for metal ions. J. Biol. Chem. 275, 25057–25060. [DOI] [PubMed] [Google Scholar]
- Ohata, T., Kanazawa, K., Mihashi, S., Nishizawa, N.-K., Fushiya, S., Nozoe, S., Chino, M., and Mori, S. (1993). Biosynthetic pathway of phytosiderophores in iron-deficient graminaceous plants: Development of assay system for the detection of nicotianamine aminotransferase activity. Soil Sci. Plant Nutr. 39, 745–749. [Google Scholar]
- Outten, C.E., and O'Halloran, T.V. (2001). Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science 292, 2488–2492. [DOI] [PubMed] [Google Scholar]
- Pate, J.S. (1975). Exchange of solutes between phloem and xylem and circulation in the whole plant. In Transport in Plants. I. Phloem Transport, M.H. Zimmerman and J.A. Milburn, eds (Berlin: Springer Verlag), pp. 451–473.
- Pich, A., and Scholz, G. (1996). Translocation of copper and other micronutrients in tomato plants (Lycopersicon esculentum Mill.): Nicotianamine-stimulated copper transport in the xylem. J. Exp. Bot. 294, 41–47. [Google Scholar]
- Pich, A., Scholz, G., and Stephan, U.W. (1994). Iron-dependent changes of heavy metals, nicotianamine, and citrate in different plant organs and in the xylem exudate of two tomato genotypes: Nicotianamine as possible copper translocator. Plant Soil 165, 189–196. [Google Scholar]
- Rae, T.D., Schmidt, P.J., Pufahl, R.A., Culotta, V.C., and O'Halloran, T.V. (1999). Undetectable intracellular free copper: The requirement of a copper chaperone for superoxide dismutase. Science 284, 805–808. [DOI] [PubMed] [Google Scholar]
- Rudolph, A., Becker, R., Scholz, G., Procházka, Z., Toman, J., Macek, T., and Herout, V. (1985). The occurrence of the amino acid nicotianamine in plants and microorganisms: A reinvestigation. Biochem. Physiol. Pflanz. 180, 557–563. [Google Scholar]
- Sawa, S., Ito, T., Shimura, Y., and Okada, K. (1999). FILAMENTOUS FLOWER controls the formation and development of Arabidopsis inflorescences and floral meristems. Plant Cell 11, 69–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnable, P.S., and Wise, R.P. (1998). The molecular basis of cytoplasmic male sterility and fertility restoration. Trends Plant Sci. 3, 175–180. [Google Scholar]
- Sharma, C.P., Sharma, O.N., Chatterjee, C., and Agarwala, S.C. (1991). Manganese deficiency in maize affects pollen viability. Plant Soil 138, 139–142. [Google Scholar]
- Sharma, P.N., Chatterjee, C., Agarwala, S.C., and Sharma, C.P. (1990). Zinc deficiency and pollen fertility in maize (Zea mays). Plant Soil 124, 221–225. [Google Scholar]
- Shojima, S., Nishizawa, N.K., Fushiya, S., Nozoe, S., Irifune, T., and Mori, S. (1990). Biosynthesis of phytosiderophores. Plant Physiol. 93, 1497–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shojima, S., Nishizawa, N.K., Fushiya, S., Nozoe, S., Kumashiro, T., Nagata, T., Ohata, T., and Mori, S. (1989. a). Biosynthesis of nicotianamine in the suspension-cultured cells of tobacco (Nicotiana megalosiphon). Biol. Metals 2, 142–145. [Google Scholar]
- Shojima, S., Nishizawa, N.K., and Mori, S. (1989. b). Establishment of a cell-free system for the biosynthesis of nicotianamine. Plant Cell Physiol. 30, 673–677. [Google Scholar]
- Sijmons, P.C., Kolattukudy, P.E., and Bienfait, H.F. (1985). Iron-deficiency decreases suberization in bean roots through a de-crease in suberin-specific peroxidase activity. Plant Physiol. 78, 115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan, U.W., Schmidke, I., Stephan, V.W., and Scholz, G. (1996). The nicotianamine molecule is made-to-measure for complexation of metal micronutrients in plants. Biometals 9, 84–90. [Google Scholar]
- Stephan, U.W., and Scholz, G. (1993). Nicotianamine: Mediator of transport of iron and heavy metals in the phloem? Physiol. Plant. 88, 522–529. [Google Scholar]
- Suzuki, K., Higuchi, K., Nakanishi, H., Nishizawa, N.K., and Mori, S. (1999). Cloning of nicotianamine synthase gene from Arabidopsis. Soil Sci. Plant Nutr. 45, 993–1002. [Google Scholar]
- Takagi, S. (1976). Naturally occurring iron-chelating compounds in oat- and rice-root washing. I. Activity measurement and preliminary characterization. Soil Sci. Plant Nutr. 22, 423–433. [Google Scholar]
- Takahashi, M., Nakanishi, H., Kawasaki, S., Nishizawa, N.K., and Mori, S. (2001). Enhanced tolerance of rice to low iron availability in alkaline soils using barley nicotianamine aminotransferase genes. Nat. Biotechnol. 19, 466–469. [DOI] [PubMed] [Google Scholar]
- Takahashi, M., Yamaguchi, H., Nakanishi, H., Shioiri, T., Nishizawa, N.K., and Mori, S. (1999). Cloning two genes for nicotianamine aminotransferase, a critical enzyme in iron acquisition (strategy II) in graminaceous plants. Plant Physiol. 121, 947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsuji, H. (1999). Zinc-finger proteins: The classical zinc finger emerges in contemporary plant science. Plant Mol. Biol. 39, 1073–1078. [DOI] [PubMed] [Google Scholar]
- Tiffin, L.O. (1966). Iron translocation. II. Citrate iron ratios in plant stem exudates. Plant Physiol. 41, 515–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vert, G., Grotz, N., Dédaldéchamp, F., Gaymard, F., Guerinot, M.L., Briat, J.-F., and Curie, C. (2002). IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 14, 1223–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ververidis, P., and John, P. (1991). Complete recovery in vitro of ethylene-forming enzyme activity. Phytochemistry 30, 725–727. [Google Scholar]
- von Wirén, N., Klair, S., Bansal, S., Briat, J.F., Khodr, H., Shioiri, T., Leigh, R.A., and Hider, R.C. (1999). Nicotianamine chelates both FeIII and FeII: Implications for metal transport in plants. Plant Physiol. 119, 1107–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warmke, H.E., and Lee, S.J. (1978). Pollen abortion in T cytoplasmic male-sterile corn anthers. J. Hered. 68, 213–222. [DOI] [PubMed] [Google Scholar]
- Wilson, Z.A., Morroll, S.M., Dawson, J., Swarup, R., and Tighe, P.J. (2001). The Arabidopsis MALE STERILITY (MS1) gene is a transcriptional regulator of male gametogenesis, with homology to the PHD-finger family of transcription factors. Plant J. 28, 27–39. [DOI] [PubMed] [Google Scholar]
- Yoshimura, E., Sakaguchi, T., Nakanishi, H., Nishizawa, N.K., Nakai, I., and Mori, S. (2000). Characterization of the chemical state of iron in the leaves of wild-type tomato and of a nicotianamine-free mutant chloronerva by X-ray absorption near-edge structure (XANES). Phytochem. Anal. 11, 160–162. [Google Scholar]