Abstract
Cell function is tightly regulated by surface receptors. Earlier reports showed that herpes simplex virus 1 regulates by diverse mechanisms the presentation of antigenic peptides, downregulates the signaling pathways associated with receptor tyrosine kinases, and posttranslationally modifies members of the Src family of protein kinases. Here we report that the receptor for tumor necrosis factor alpha (TNF-R1) rapidly disappears from both the cell surface and total cell lysates in cells infected with wild-type virus or a variety of mutants but not in cells infected with the mutant ΔUL41, which lacks the UL41 gene, the virion host shutoff gene. The half-life of TNF-R1 appears to be less than 30 min in both mock-infected and infected cells. The disappearance of TNF-R1 correlates with the disappearance of cytoplasmic TNF-R1 mRNA in wild-type-virus-infected cells. The results suggest that by degrading the TNFR1 mRNA, the virus precludes the replenishment of naturally decaying TNF-R1.
The cell surface is a giant antenna that receives signals from and transmits signals to the environment. When cells become infected with a virus, some of the signals emanating from the cells inform the environment of the presence of an intruder. Receptors, responding to the emanating signals, attempt to curtail viral replication or induce the infected cells to commit suicide. It is not surprising that viruses have evolved mechanisms to curtail the outflow of signals, to degrade receptors of signals that could have a deleterious effect on viral replication, or at the very least to block the execution of the signals received by the receptors. In the case of herpes simplex virus 1 (HSV-1), clear examples of interference with signaling are evidence that the virus blocks the presentation of antigenic peptides on the cell surface by major histocompatibility complex class I and II proteins (12, 16, 24, 27, 30). More recent studies indicate that interference with signaling is more pervasive. HSV-1 blocks exogenous interferon from affecting viral replication through degradation of the promyelocytic leukemia protein, the organizer of ND10 nuclear structure, which appears to regulate interferon response genes (3, 5), the acceleration of turnover of JAK1 (33), and degradation of IRF3 (22). At least one HSV-1 product, infected-cell protein 0 (ICP0), has been shown to down regulate the receptor tyrosine kinases using epidermal growth factors as prototypic receptors (19). Given this background, it was of interest to determine the fate of the receptors of tumor necrosis factor alpha (TNF-α).
TNF-α is secreted by a variety of cells, including natural killer cells, T lymphocytes, macrophages, etc. In turn, TNF-α induces a wide variety of genes that play significant roles in immune and inflammatory responses. The protein has two major receptors, TNF-R1 and TNF-R2. TNF-R1 is expressed in most tissues, whereas TNF-R2 is typically found in cells of the immune system. The receptors share sequences in their ectodomains but differ with respect to cytoplasmic domains. Activation of both receptors, however, may lead to activation of antiviral responses and cell death. TNF-α has been shown to inhibit HSV as well as other viruses. Extensive literature has documented both the antiviral effects of TNF-α and the properties of the receptors (32). A central goal of the studies described in this report was to determine the fate of the TNF-R1 receptors present on the surface of several cell lines. In this report, we show that TNF-α receptor disappears very rapidly from infected cells and that the disappearance is related to the function of the virion host shutoff factor encoded by the UL41 open reading frame.
In the first series of experiments, we quantified the TNF-R1 in total cell lysates and on the surfaces of infected cells. Replicate HEp-2 cells were mock infected or exposed to 10 PFU of HSV-1(F) per cell and incubated for 6 h at 37°C. To prepare total cell lysates, the cells were harvested by scraping into lysis buffer (1% NP-40, 0.5% sodium deoxycholate, 150 mM NaCl) followed by brief sonication. The proteins were denatured by boiling in sodium dodecyl sulfate (SDS) sample buffer for 5 min and subjected to electrophoresis on a denaturing polyacrylamide gel. For surface protein extraction, the cells were rinsed twice with ice-cold phosphate-buffered saline (PBS) and reacted with 1 mg of sulfo-NHS-LC-biotin reagent (no. 21335; Pierce) per ml at 4°C for 30 min and then rinsed three times with 100 mM glycine in PBS to quench and remove the biotin reagent. The cells were then harvested and lysed by scraping into binding buffer (0.1% SDS, 1% NP-40, 0.5% sodium deoxycholate, fresh protease inhibitor) at 4°C, followed by brief sonication. Immobilized streptavidin agarose beads (no. 53117; Pierce) were rinsed in binding buffer twice and reacted with the lysate overnight at 4°C. The streptavidin-bound complex was collected and rinsed five times with binding buffer. The biotinylated proteins were eluted from the beads with 8 M guanidine HCl. Both total and extracted cell surface proteins were electrophoretically separated on a 10% denaturing polyacrylamide gel, transferred to a nitrocellulose sheet, and reacted with anti-TNF-R1 antibody. As shown in Fig. 1A, both total and cell surface amounts decreased drastically after infection. The decrease in the amount of TNF-R1 on the surface of infected cells mirrored the decrease in the total amount of TNF-R1 accumulating in mock-infected and infected cells.
FIG. 1.
TNF-R1 downregulation in infected HEp-2 cells. (A) HEp-2 cells were mock infected or exposed to 10 PFU of HSV-1(F) per cell. The cells were harvested 6 h after infection and total cell lysate or extracted surface proteins were separated on 10% denaturing polyacrylamide gel, transferred to a nitrocellulose gel and reacted with anti-TNF-R1. (B) Time course of down regulation of total TNF-R1 in infected cells. HEp-2 cells were mock infected or exposed to 5 PFU of HSV-1(F), ΔICP0, ICP0 RING finger mutant ΔR-F, or ΔUL41 per cell. Cells were harvested at 2, 4, and 8 h after infection. Total cell lysates were separated on 10% denaturing polyacrylamide gels and immunoblotted with anti-TNF-R1. A, cross-reacting cellular protein that served as a loading control.
The objective of the second series of experiments was to measure the time course of disappearance of TNF-R1 from wild-type-virus-infected HEp-2 cells. Replicate cultures of HEp-2 cells were mock infected or exposed to 5 PFU of HSV-1(F), ΔICP0, C116G/C156A (ΔR-F), or ΔUL41 per cell. HSV-1(F) is the prototype wild-type strain used in this laboratory (8). ΔICP0 lacks the gene encoding ICP0, and ΔR-F carries amino acid substitutions (C116G/C156A) that disable the RING finger domain of ICP0 (18, 20). ΔUL41 contains the coding sequences of β-galactosidase inserted into the UL41 open reading frame (26). The cultures were harvested at 2, 4, or 8 h after infection, and total cell lysates were processed as above and reacted with TNF-R1 mouse monoclonal antibody. As shown in Fig. 1B, TNF-R1 protein significantly decreased or virtually disappeared from cells at 2 h after infection with all viruses but ΔUL41. In ΔUL41-infected cells, the amount of TNF-R1 protein decreased, but not nearly as rapidly as in cells infected with wild-type virus. Thus, at 8 h after infection the amount of TNF-R1 protein in lysates of ΔUL41-infected cells was higher than that in wild-type-virus-infected cells harvested at 2 h after infection.
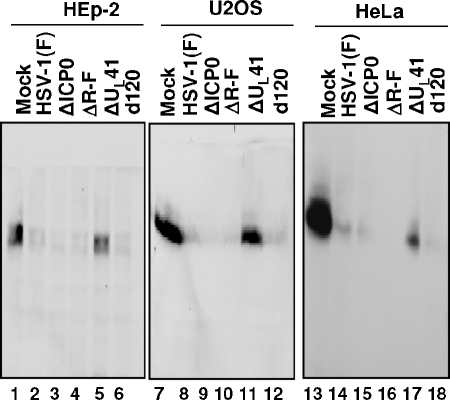
The objective of the third series of experiments was to determine whether the rapid disappearance of TNF-R1 was UL41 dependent in other cell lines. In this series of experiments, replicate cultures of HEp-2 cells, U2OS, or HeLa cells were mock infected or exposed to 10 PFU of HSV-1(F), ΔICP0, ΔR-F, ΔUL41 or d120 per cell. Mutant virus d120 lacks the gene encoding ICP4 (7). The cells were harvested at 6 h after infection, processed as above, and reacted with anti-TNF-R1 antibody. In each cell line tested, TNF-R1 decayed in cells exposed to wild-type or mutant viruses except those exposed to ΔUL41 (Fig. 2). In cells infected with this mutant virus, the amount of TNF-R1 decreased but were significantly higher than in other infected cell cultures. We conclude that the decay in TNF-R1 was UL41 virus dependent but not cell type dependent.
FIG. 2.
Downregulation of TNR-R1 is cell type independent. Replicate cultures of HEp-2, U2OS, or HeLa cells were mock infected or exposed to 10 PFU of the indicated virus per cell. The cells were harvested at 6 h after infection, and total cell lysates were separated on 10% denaturing polyacrylamide gels and immunoblotted with anti-TNF-R1 antibody.
The objective of the fourth series of experiments was to determine whether the life spans of TNF-R1 in mock-infected and infected cells were similar. In essence, the question posed was whether TNF-R1 was actively degraded in infected cells in contradistinction to its fate in uninfected cells. Replicate cultures of HEp-2 cells were mock infected or exposed to 10 PFU of HSV-1(F) or ΔUL41 per cell. Cycloheximide (100 μg/ml) was added at the time of mock infection or exposure to virus and maintained until harvest at 0.5, 1.0, 1.4, 2.0 or 2.5 h after infection. The total cell lysates were processed as described above and reacted with monoclonal antibody to TNF-R1 or polyclonal antibody to actin. The salient feature of the results shown in Fig. 3 is that at 0.5 h after exposure to cycloheximide in either infected or mock-infected cells, the amount of TNF-R1 was less than half the amount present at the time of addition of the drug (0 h). The results indicate that the half-life of TNF-R1 is less than 0.5 h in both infected and mock-infected cells. The experiment did not elicit evidence that TNF-R1 protein was actively degraded in infected cells.
FIG. 3.
Half-life of TNF-R1 in infected HEp-2 cells. HEp-2 cells were mock infected or exposed to 10 PFU of HSV-1(F) or ΔUL41 per cell. Cycloheximide (100 μg/ml) was added at the time of exposure of cells to virus (0 h) and maintained during the infection. The cells were harvested at 0, 0.5, 1.0, 1.5, 2.0, or 2.5 h after infection. Total cell lysates were separated on a 10% denaturing polyacrylamide gel and immunoblotted with anti-TNF-R1 antibody or antiactin antibody.
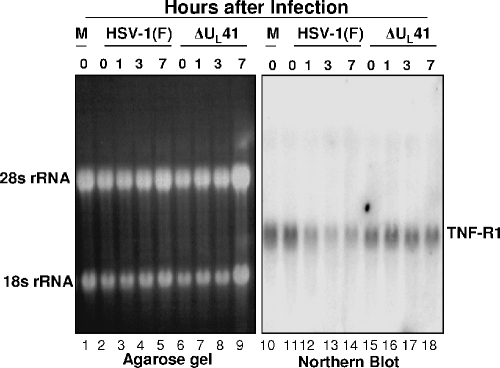
The fundamental property of the virion host shutoff protein encoded by UL41 is that it acts as an endoribonuclease that selectively degrades mRNA (9, 29). If the disappearance of TNF-R1 protein was related to the nucleolytic activity of the UL41 protein, it would be expected that the TNF-R1 mRNA would be degraded in wild-type-virus-infected cells but not in mutant-virus-infected cells. In this series of experiments, replicate cultures of HeLa cells were mock infected or exposed to 5 PFU of HSV-1(F) or ΔUL41 virus per cell. At 0, 1, 3, or 7 h after infection, cytoplasmic RNA was isolated, separated on a 1% agarose gel, and probed with 32P-labeled full-length TNF-R1 cDNA as described elsewhere (9). TNF-R1 fragment was amplified by RT-PCR of cytoplasmic RNA using primers TNFRSF1A forward0 (5′-CATGGGCCTCTCCACCGTGCCTG-3′) and TNFRSF1AB (5′-AGCCTCATCTGAGAAGACTGG-3′). Figure 4 shows a photograph of an ethidium bromide-stained gel of electrophoretically separated RNA: the rRNA serves as a loading control. The autoradiogram shown in Fig. 4 indicates that the mRNA encoding TNF-R rapidly decreased from wild-type-virus-infected cells but remained at a relatively stable level in cells infected with ΔUL41. These results are consistent with and support the hypothesis that UL41 protein acts by degrading the mRNA of TNF-R1.
FIG. 4.
Northern blot of TNF-R1 in infected HeLa cells. HeLa cells were mock infected or exposed to 5 PFU of virus per cell. Cells were harvested at 1, 3, or 7 h after infection, and cytoplasmic RNAs were extracted and separated on a 1% agarose gel followed by Northern blot analysis with 32P-labeled full-length TNF-R1 cDNA.
The fundamental conclusion of the studies presented in this report is that two factors account for the disappearance of TNF-R1. First, the receptor has a very short half-life in all of the cell lines tested. Therefore, steady-state levels of TNF-R1 require continuous replenishment by translation of its mRNA. In these studies we have shown that the mRNA levels decrease in a UL41-dependent manner. Hence, the disappearance of TNF-R1 is the result of failure to replenish of the protein because of the degradation of its mRNA. It is of interest that ΔUL41 mutants are avirulent in experimental animal systems. Although the UL41 protein affects many different host mRNAs, there arises the question of whether the inability to block the interaction of TNF-α with its receptor contributes to the virulence of this mutant.
HSV employs a large number of diverse strategies to inactivate host functions inimical to its replication and spread. Some proteins are degraded by the action of E3 ligase activity of ICP0 (4, 6, 11, 14, 19, 21, 25, 31). Others are inactivated by phosphorylation by the viral protein kinases (2, 23, 28), by translocation into inoperative compartments of the cell (10, 13, 17, 34), or by recruitment to perform functions different from those expressed in uninfected cells (1, 15). In the case of TNF-R1, the strategy of HSV-1 is to take advantage of the short natural longevity of the protein.
Acknowledgments
We thank B. Taddeo, A. P. W. Poon, G. Zhou, and H. Gu for advice and useful discussions.
These studies were aided by National Cancer Institute grants CA87661, CA83939, CA71933, CA78766, and CA88860.
REFERENCES
- 1.Advani, S. J., R. R. Weichselbaum, and B. Roizman. 2003. Herpes simplex virus 1 activates cdc2 to recruit topoisomerase II alpha for post-DNA synthesis expression of late genes. Proc. Natl. Acad. Sci. USA 100:4825-4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benetti, L., and B. Roizman. 2006. Protein kinase B/Akt is present in activated form throughout the entire replicative cycle of ΔUS3 mutant virus but only at early times after infection with wild-type herpes simplex virus 1. J. Virol. 80:3341-3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boutell, C., A. Orr, and R. D. Everett. 2003. PML residue lysine 160 is required for the degradation of PML induced by herpes simplex virus type 1 regulatory protein ICP0. J. Virol. 77:8686-8694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boutell, C., S. Sadis, and R. D. Everett. 2002. Herpes simplex virus type 1 immediate-early protein ICP0 and its isolated RING finger domain act as ubiquitin E3 ligases in vitro. J. Virol. 76:841-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chee, A. V., P. Lopez, P. P. Pandolfi, and B. Roizman. 2003. Promyelocytic leukemia protein mediates interferon-based anti-herpes simplex virus 1 effects. J. Virol. 77:7101-7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chelbi-Alix, M. K., and H. de The. 1999. Herpes virus induced proteasome-dependent degradation of the nuclear bodies-associated PML and Sp100 proteins. Oncogene 18:935-941. [DOI] [PubMed] [Google Scholar]
- 7.DeLuca, N. A., A. M. McCarthy, and P. A. Schaffer. 1985. Isolation and characterization of deletion mutants of herpes simplex virus type 1 in the gene encoding immediate-early regulatory protein ICP4. J. Virol. 56:558-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ejercito, P. M., E. D. Kieff, and B. Roizman. 1968. Characterization of herpes simplex virus strains differing in their effects on social behavior of infected cells. J. Gen. Virol. 2:357-364. [DOI] [PubMed] [Google Scholar]
- 9.Esclatine, A., B. Taddeo, and B. Roizman. 2004. The UL41 protein of herpes simplex virus mediates selective stabilization or degradation of cellular mRNAs. Proc. Natl. Acad. Sci. USA 101:18165-18170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esclatine, A., B. Taddeo, and B. Roizman. 2004. Herpes simplex virus 1 induces cytoplasmic accumulation of TIA-1/TIAR and both synthesis and cytoplasmic accumulation of tristetraprolin, two cellular proteins that bind and destabilize AU-rich RNAs. J. Virol. 78:8582-8592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Everett, R. D., W. C. Earnshaw, J. Findlay, and P. Lomonte. 1999. Specific destruction of kinetochore protein CENP-C and disruption of cell division by herpes simplex virus immediate-early protein Vmw110. EMBO J. 18:1526-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Früh, K., K. Ahn, H. Djaballah, P. Sempe, P. M. van Endert, R. Tampe, P. A. Peterson, and Y. Yang. 1995. A viral inhibitor of peptide transporters for antigen presentation. Nature 375:415-418. [DOI] [PubMed] [Google Scholar]
- 13.Gu, H., Y. Liang, G. Mandel, and B. Roizman. 2005. Components of the REST/CoREST/histone deacetylase repressor complex are disrupted, modified, and translocated in HSV-1-infected cells. Proc. Natl. Acad. Sci. USA 102:7571-7576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hagglund, R., C. Van Sant, P. Lopez, and B. Roizman. 2002. Herpes simplex virus 1-infected cell protein 0 contains two E3 ubiquitin ligase sites specific for different E2 ubiquitin-conjugating enzymes. Proc. Natl. Acad. Sci. USA 99:631-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He, B., M. Gross, and B. Roizman. 1997. The γ134.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1α to dephosphorylate the α subunit of the eIF-2 translation initiation factor and preclude the shutoff of protein synthesis by double stranded RNA activated protein kinase (PKR). Proc. Natl. Acad. Sci. USA 94:843-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill, A. B., B. C. Barnett, A. J. McMichael, and D. J. McGeoch. 1994. HLA class I molecules are not transported to the cell surface in cells infected with herpes simplex virus types 1 and 2. J. Immunol. 152:2736-2741. [PubMed] [Google Scholar]
- 17.Hill, A., P. Jugovic, I. York, G. Russ, J. Bennink, J. Yewdell, H. Ploegh, and D. Johnson. 1995. Herpes simplex virus turns off the TAP to evade host immunity. Nature 375:411-415. [DOI] [PubMed] [Google Scholar]
- 18.Kawaguchi, Y., C. Van Sant, and B. Roizman. 1997. Herpes simplex virus 1 alpha regulatory protein ICP0 interacts with and stabilizes the cell cycle regulator cyclin D3. J. Virol. 71:7328-7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang, Y., A. Kurakin, and B. Roizman. 2005. Herpes simplex virus 1 infected cell protein 0 forms a complex with CIN85 and Cbl and mediates the degradation of EGF receptor from cell surfaces. Proc. Natl. Acad. Sci. USA 102:5838-5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lium, E. K., and S. Silverstein. 1997. Mutational analysis of the herpes simplex virus type 1 ICP0 C3HC4 zinc ring finger reveals a requirement for ICP0 in the expression of the essential α27 gene. J. Virol. 71:8602-8614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lomonte, P., K. F. Sullivan, and R. D. Everett. 2001. Degradation of nucleosome-associated centromeric histone H3-like protein CENP-A induced by herpes simplex virus type 1 protein ICP0. J. Biol. Chem. 276:5829-5835. [DOI] [PubMed] [Google Scholar]
- 22.Melroe, G. T., N. A. DeLuca, and D. M. Knipe. 2004. Herpes simplex virus 1 has multiple mechanisms for blocking virus-induced interferon production. J. Virol. 78:8411-8420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munger, J., and B. Roizman. 2001. The US3 protein kinase of herpes simplex virus 1 mediates the posttranslational modification of BAD and prevents BAD-induced programmed cell death in the absence of other viral proteins. Proc. Natl. Acad. Sci. USA 98:10410-10415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neumann, J., A. M. Eis-Hubinger, and N. Koch. 2003. Herpes simplex virus type 1 targets the MHC class II processing pathway for immune evasion. J. Immunol. 171:3075-3083. [DOI] [PubMed] [Google Scholar]
- 25.Parkinson, J., S. P. Lees-Miller, and R. D. Everett. 1999. Herpes simplex virus type 1 immediate-early protein Vmw110 induces the proteasome-dependent degradation of the catalytic subunit of DNA-dependent protein kinase. J. Virol. 73:650-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poon, A. P., and B. Roizman. 1997. Differentiation of the shutoff of protein synthesis by virion host shutoff and mutant γ134.5 genes of herpes simplex virus 1. Virology 229:98-105. [DOI] [PubMed] [Google Scholar]
- 27.Sievers, E., J. Neumann, M. Raftery, G. Schönrich, A. M. Eis-Hubinger, and N. Koch. 2002. Glycoprotein B from strain 17 of herpes simplex virus type I contains an invariant chain homologous sequence that binds to MHC class II molecules. Immunology 107:129-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sloan, D. D., G. Zahariadis, C. M. Posavad, N. T. Pate, S. J. Kussick, and K. R. Jerome. 2003. CTL are inactivated by herpes simplex virus-infected cells expressing a viral protein kinase. J. Immunol. 171:6733-6741. [DOI] [PubMed] [Google Scholar]
- 29.Taddeo, B., W. Zhang, and B. Roizman. 2006. TheUL41 protein of herpes simplex virus 1 degrades RNA by endonucleolytic cleavage in absence of other cellular or viral proteins. Proc. Nat. Acad. Sci. USA 103:2827-2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trgovcich, J., D. Johnson, and B. Roizman. 2002. Cell surface major histocompatibility complex class II proteins are regulated by the products of the γ134.5 and UL41 genes of herpes simplex virus 1. J. Virol. 76:6974-6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Sant, C., R. Hagglund, P. Lopez, and B. Roizman. 2001. The infected cell protein 0 of herpes simplex virus 1 dynamically interacts with proteasomes, binds and activates the cdc34 E2 ubiquitin-conjugating enzyme, and possesses in vitro E3 ubiquitin ligase activity. Proc. Natl. Acad. Sci. USA 98:8815-8820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wajant, H., K. Pfizenmaier, and P. Scheurich. 2003. Tumor necrosis factor signaling. Cell Death Differ. 10:45-65. [DOI] [PubMed] [Google Scholar]
- 33.Yokota, S., N. Yokosawa, T. Kubota, T. Suzutani, I. Yoshida, S. Miura, K. Jimbow, and N. Fujii. 2001. Herpes simplex virus type 1 suppresses the interferon signaling pathway by inhibiting phosphorylation of STATs and Janus kinases during an early infection stage. Virology 286:119-124. [DOI] [PubMed] [Google Scholar]
- 34.York, I. A., C. Roop, D. W. Andrews, S. R. Riddell, F. L. Graham, and D. C. Johnson. 1994. A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8+ T lymphocytes. Cell 77:525-535. [DOI] [PubMed] [Google Scholar]