Abstract
Human T-cell leukemia virus type 1 (HTLV-1) persistently infects humans, and the proviral loads that persist in vivo vary widely among individuals. Elevation in the proviral load is associated with serious HTLV-1-mediated diseases, such as adult T-cell leukemia and HTLV-1-associated myelopathy/tropical spastic paraparesis. However, it remains controversial whether HTLV-1-specific T-cell immunity can control HTLV-1 in vivo. We previously reported that orally HTLV-1-infected rats showed insufficient HTLV-1-specific T-cell immunity that coincided with elevated levels of the HTLV-1 proviral load. In the present study, we found that individual HTLV-1 proviral loads established in low-responding hosts could be reduced by the restoration of HTLV-1-specific T-cell responses. Despite the T-cell unresponsiveness for HTLV-1 in orally infected rats, an allogeneic mixed lymphocyte reaction in the splenocytes and a contact hypersensitivity response in the skin of these rats were comparable with those of naive rats. HTLV-1-specific T-cell response in orally HTLV-1-infected rats could be restored by subcutaneous reimmunization with mitomycin C (MMC)-treated syngeneic HTLV-1-transformed cells. The reimmunized rats exhibited lower proviral loads than untreated orally infected rats. We also confirmed that the proviral loads in orally infected rats decreased after reimmunization in the same hosts. Similar T-cell immune conversion could be reproduced in orally HTLV-1-infected rats by subcutaneous inoculation with MMC-treated primary T cells from syngeneic orally HTLV-1-infected rats. The present results indicate that, although HTLV-1-specific T-cell unresponsiveness is an underlying risk factor for the propagation of HTLV-1-infected cells in vivo, the risk may potentially be reduced by reimmunization, for which autologous HTLV-1-infected cells are a candidate immunogen.
Human T-cell leukemia virus type 1 (HTLV-1) is a human retrovirus associated with adult T-cell leukemia (ATL) and a variety of chronic inflammatory diseases including HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) (5, 12, 38, 41). Although a small proportion of HTLV-1-infected individuals develop ATL after a long latency (24, 30, 46), most affected individuals remain asymptomatic during their lifetime. ATL is a highly aggressive CD4+ T-cell leukemia/lymphoma characterized by clonal integration of HTLV-1 in leukemic or lymphoma cells (53). Although the precise mechanism of leukemogenesis in ATL remains unclear, several etiological risk factors have been suggested, including vertical transmission, gender (males more than females), and an increase in the number of abnormal lymphocytes associated with a high HTLV-1 proviral load (13, 14, 37, 44).
In an infected person, the proviral load of HTLV-1 is usually stable over time (32). However, what determines the set point of the proviral load in each person is not well understood. Several studies on HAM/TSP patients and HTLV-1-carriers have indicated that there is a weak positive correlation between the frequency of HTLV-1-specific CD8+ cytotoxic T lymphocytes (CTLs) and the proviral load of HTLV-1 (26, 51). Meanwhile, other studies have reported that circulating CD8+ CTLs from individuals with a low HTLV-1 proviral load express greater levels of genes that encode granzymes and other lytic proteins than the corresponding cells in individuals with a high proviral load (48). A theoretical model has been proposed that the efficacy of CD8+ CTLs determines the level of the set point and that the equilibrium frequency of virus-specific CD8+ CTLs is the same between individuals with lower and higher viral loads (35).
The level of CD8+ CTL activity against HTLV-1 varies widely among HTLV-1-infected individuals. High levels of HTLV-1-specific CTL activity are observed in HAM/TSP patients and some asymptomatic HTLV-1 carriers (16, 21, 40). In contrast, ATL patients are apparently defective for HTLV-specific CTL activity, although it can be sporadically induced during the remission stages or only after mitogenic stimulation with multiple in vitro antigenic stimulations of peripheral blood mononuclear cells (1, 20). HTLV-1-specific CTLs mainly recognize Tax (16, 18), a molecule responsible for T-cell immortalization (17, 52), and CTLs induced in ATL patients in remission are able to lyse autologous tumor cells in vitro (19). These observations suggest that HTLV-1-specific CTLs play a crucial role in host immunosurveillance against ATL cells. In support of this notion, Tax-specific CTLs can eradicate HTLV-1-infected tumors in a rat model of ATL-like HTLV-1-associated lymphoproliferative disease (7).
The reasons for the insufficient HTLV-1-specific CTL responses in ATL patients are not clear. Recent reports have indicated that the phenotype of ATL cells resembles that of regulatory T cells, although their functional properties do not fully match those of regulatory T cells (3). Vertical HTLV-1 transmission, one of the epidemiological risk factors for ATL, may cause insufficiency in the HTLV-1-specific T-cell response. Vertical HTLV-1 transmission mainly occurs through breast-feeding from HTLV-1-carrying mothers (10), since intervention by refraining from breast-feeding was found to block >80% of vertical transmission of HTLV-1 (9). Both oral intake and exposure at a young age may induce immune tolerance against the exposed antigens (47).
We previously reported that the HTLV-1-specific cellular and humoral immunities of orally HTLV-1-infected rats were impaired compared to those of intraperitoneally infected rats (22). In contrast, the HTLV-1 proviral load of orally infected rats was significantly greater than that of intraperitoneally infected rats. These findings indicate that oral HTLV-1 infection induces insufficient host immune conditions that favor viral expansion. Since HTLV-1 is mainly associated with infected cells, an increase in the proviral load implies an increase in the number of infected cells, as a result of cell-to-cell viral transmission in vivo or the proliferation of HTLV-1-infected cells themselves (2, 45). There was a mild inverse correlation between HTLV-1-specific cellular immunity and the proviral load among HTLV-1-infected rats through various routes (8), suggesting that HTLV-1-specific T-cell immunity could actively control the number of HTLV-1-infected cells in this rat model. If this hypothesis is correct, the established equilibrium set point of the HTLV-1 proviral load in an individual showing a low immune response must decrease if the HTLV-1-specific immune response is restored.
In the present study, we demonstrate that reimmunization of orally HTLV-1-infected rats with an HTLV-1-infected cell line or primary T cells results in a reduction in the HTLV-1 proviral load, indicating that HTLV-1-specific T-cell immunity is capable of controlling the number of HTLV-1-infected cells in vivo. These findings also imply that the risk of ATL may potentially be diminished by reimmunization.
MATERIALS AND METHODS
Animals.
Three-week-old female F344/N Jcl-rnu/+ (F344 n/+) and ACI/NJcl rats were purchased from Clea Japan, Inc. (Tokyo, Japan). The rats were maintained at the experimental animal facilities of Tokyo Medical and Dental University and treated in accordance with the regulations and guidelines of the Animal Care Committee of the university.
Cell lines.
An HTLV-1-producing human T-cell line, MT-2, and an HTLV-1-infected rat T-cell line, FPM1 (25), derived from an F344 n/+ rat were cultured in RPMI1640 medium containing 10% heat-inactivated fetal calf serum (FCS; BioWhittaker, Walkersville, MD), 100 IU of penicillin/ml, 100 μg of streptomycin/ml, and 2 mg of sodium bicarbonate/ml. G14 (36), an interleukin-2-dependent HTLV-1-negative CD8+ T-cell line established from an F344 n/+ rat, and G14-Tax (36), a stable transfectant of G14 containing HTLV-1 Tax-expressing plasmids, were also used. G14 and G14-Tax cells were maintained in a RPMI 1640 medium containing 10−5 M 2-mercaptoethanol and 10 U of recombinant human interleukin-2 (Shionogi Pharmaceutical Co., Osaka, Japan)/ml.
Infection of rats with HTLV-1.
A total of 2 × 107 to 5 × 107 MT-2 cells were treated with 50 μg of mitomycin C (MMC)/ml at 37°C for 30 min, washed, and administered to 3- to 6-week-old female rats either orally or intraperitoneally. For oral infection, MMC-treated MT-2 cells in 0.5 ml of phosphate-buffered saline were directly administered into the esophagus through a feeder tube. For intraperitoneal infection, similarly treated MT-2 cells were injected percutaneously into the abdominal cavity.
Splenectomy.
A total splenectomy was performed at necropsy. A half-splenectomy was performed under anesthesia by intraperitoneal injection of ketamine (75 mg/kg) and xylazine (10 mg/kg). Splenocytes from the excised spleen halves were enriched for T cells by using a nylon-wool column and cryopreserved at −80°C. At 1 week after the operation, the rats were inoculated with 2 × 107 MMC-treated FPM1 cells. After a further 4 weeks, the rats were sacrificed, and T cells isolated from their residual spleens were cryopreserved at −80°C in the same manner as preimmunized splenocytes.
Quantification of the HTLV-1 proviral load.
Genomic DNA samples (approximately 500 ng) were prepared from spleen tissue by digestion with sodium dodecyl sulfate-proteinase K, followed by phenol-chloroform extraction. The samples were then subjected to real-time PCR in a LightCycler PCR system (Roche Diagnostics, Mannheim, Germany) using Tax-specific primers, pX2 (5′-ATA CCC AGT CTA CGT GTT TGG AGA CTG T-3′) and pX3 (5′-CCG ATA ACG CGT CCA TCG ATG GGG TCC-3′), and a QuantiTect SYBR Green PCR kit (QIAGEN, Tokyo, Japan) in accordance with the manufacturer's instructions as described previously (8). The relative HTLV-1 provirus copy numbers were calculated by dividing the raw values by the amount of GAPDH (glyceraldehyde-3-phosphate dehydrogenase) in the same sample. In some experiments, genomic DNA was also amplified by 35 cycles of PCR with the pX2/pX3 primer set, and the PCR products were directly visualized by ethidium bromide staining after 2% agarose gel electrophoresis.
T-cell proliferation assay.
Rat T cells were enriched from spleen cells by passage through a nylon-wool column and used as responder cells. G14 and G14-Tax cells were treated with 1% formalin in phosphate-buffered saline for 30 min, washed, and used as stimulator cells. Responder cells (105 cells/well) and stimulator cells (5 × 104 cells/well) were cultured in medium containing 10% FCS in a 96-well round-bottom culture plate at 37°C for 72 h and then pulsed with [3H]thymidine (37 kBq/well) for 16 h to examine T-cell proliferation. The cells were then harvested by using a Micro 96 Harvester (Skatron, Lier, Norway), and their [3H]thymidine incorporations were measured in a microplate beta counter (Micro Beta Plus; Wallac, Turku, Finland). A proliferation index was calculated as the counts per minute (cpm) of the sample wells divided by the cpm of control wells containing naive splenic T cells with G14-Tax cells as stimulator cells in the same experiment.
Mixed lymphocyte reaction (MLR).
Rat spleen T cells served as responder cells. Whole splenocytes from ACI rats treated with MMC were used as stimulator cells. Responder cells (2 × 105 cells/well) and various numbers of stimulator cells were cultured in RPMI 1640 medium containing 10% FCS in a 96-well round-bottom culture plate at 37°C for 5 days, and the [3H]thymidine incorporation during the last 16 h of the incubation was measured.
IFN-γ production assay.
Rat spleen T cells (105 cells/well) were cultured without or with formalin-fixed G14 or G14-Tax cells (5 × 104 cells/well) in a microtiter plate in 200 μl of medium containing 10% FCS/well for 3 days. Next, the concentrations of gamma interferon (IFN-γ) in the supernatants were measured by enzyme-linked immunosorbent assay (ELISA) using Cytoscreen Rat IFN-γ ELISA kits (BioSource International, Inc., Camarillo, CA).
Induction of a contact hypersensitivity response.
Rats were sensitized and challenged to elicit a contact hypersensitivity response to 2,4-dinitrofluorobenzene (DNFB) (42, 49). The rats were sensitized by painting their shaved back with 500 μl of 1% DNFB in acetone-olive oil (4:1) on days 0 and 1. On day 6, after measurement of the ear thickness using a dial thickness gauge, each rat was challenged by applying 100 μl of 0.5% DNFB to the right side of the ear. The ear thickness was measured again at 24 h after the challenge. The extent of ear swelling was determined by the following calculation: (right ear lobe thickness at 24 h after the challenge − right ear lobe thickness before the challenge) − (left ear lobe thickness at 24 h after the challenge − left ear lobe thickness before the challenge).
Statistical analysis.
Dunnett's t test was used for evaluating antigen-specificity in T-cell proliferation assays. A Student t test was used for evaluating differences between two groups of samples. P values of <0.05 were considered to be statistically significant.
RESULTS
HTLV-1-specific T-cell unresponsiveness in oral HTLV-1 infection.
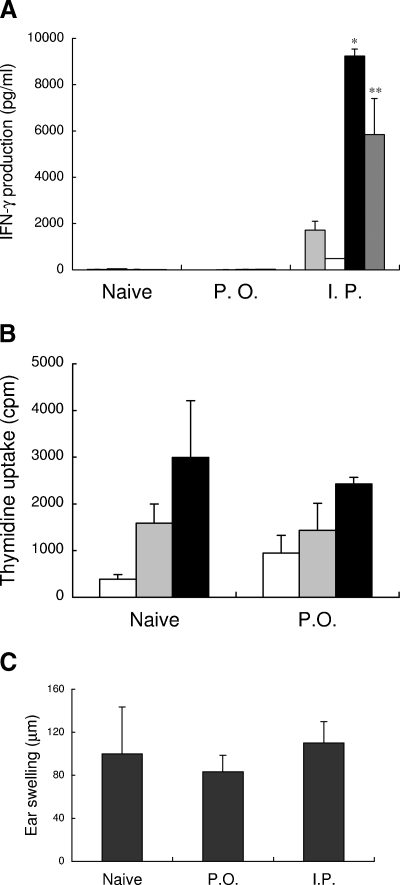
T cells from orally HTLV-1-infected rats are known to show an insufficient response to HTLV-1 antigens (22). First, we assessed whether this T-cell unresponsiveness in orally HTLV-1-infected rats is specific for HTLV-1. Representative Tax-specific T-cell responses for naive, orally infected, and intraperitoneally infected rats are shown in Fig. 1A. Spleen T cells from intraperitoneally infected rats produced significant levels of IFN-γ in response to syngeneic Tax-presenting Tax-G14 or HTLV-1-infected FPM1 cells compared to those against Tax-negative G14 cells or medium controls. In contrast, IFN-γ production in orally infected rats was as low as those in uninfected rats. Similar results were obtained from all of the four sets of orally and intraperitoneally infected rats tested.
FIG. 1.
HTLV-1 specificity of the T-cell unresponsiveness in orally HTLV-1-infected rats. (A) IFN-γ production in spleen T cells isolated from uninfected rats (naive) and orally (P.O.) or intraperitoneally (I.P.) HTLV-1-infected rats at 20 to 21 weeks after infection were examined by ELISA after 3 days of coculture without (░⃞) or with formalin-treated various syngeneic T-cell line cells, including G14 cells (□) negative for Tax, Tax-G14 cells (▪) expressing Tax, and FPM1 cells (▨) infected with HTLV-1. The results represent the mean ± the standard deviation (SD). Similar results were obtained in three other sets of orally or intraperitoneally HTLV-1-infected rats. Asterisks denote statistical significance compared to values without stimulator cells: ✽, P < 0.01; ✽✽, P < 0.05. (B) The alloreactivities of spleen T cells (2 × 105/well) from uninfected (Naive) and orally HTLV-1-infected (P.O.) rats at 17 weeks after infection were examined by MLRs after culture without (□) or with 5 × 104/well (░⃞) or 1 × 105/well (▪) of MMC-treated ACI rat splenocytes in a 96-well plate for 5 days and evaluated by measuring [3H]thymidine incorporation during the last 16 h of culture. (C) The contact hypersensitivity responses in the skin of uninfected (Naive) and orally (P.O.) or intraperitoneally (I.P.) HTLV-1-infected rats were evaluated at 5 weeks after infection by ear swelling for 24 h after DNFB challenge, following sensitization with DNFB in their backs 1 week previously. The ear swelling was calculated as described in Materials and Methods. The results represent the mean ± the SD for three rats in each group.
However, T cells from orally infected rats proliferated well in a set of MLR assays with allogeneic rat splenocytes (Fig. 1B). The level of T-cell proliferation in orally HTLV-1-infected rats against ACI rat spleen cells was comparable to that of naive T cells. The IFN-γ levels in the MLR supernatants were also comparable in naive and orally infected rats (data not shown).
Next, we examined the contact hypersensitivity responses, which are mainly CD8+ T-cell-mediated responses at the effector phase (23), in uninfected and orally infected rats that had been sensitized by DNFB application to their backs. At 1 week after the sensitization, we challenged the rats by applying DNFB to one of their ears and then measured the ear swelling at 24 h after the challenge. As shown in Fig. 1C, the orally infected rats showed levels of ear swelling similar to the uninfected rats. Thus, the T-cell responses were only insufficient against HTLV-1 and not against allogeneic or contact hypersensitivity antigens in orally HTLV-1-infected rats.
Effects of HTLV-1 reimmunization of orally HTLV-1-infected rats on HTLV-1-specific T-cell responses and the provirus load.
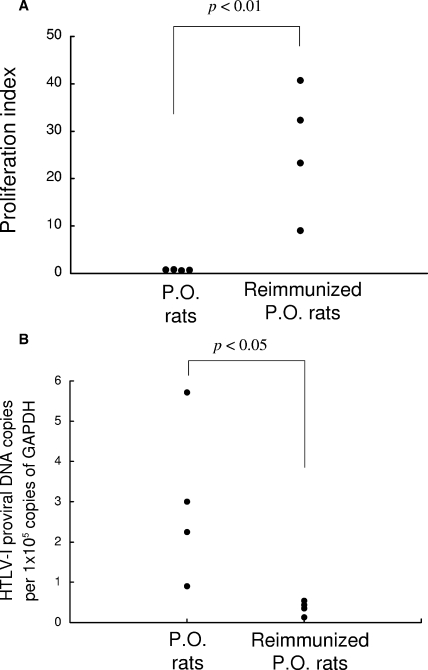
We previously reported that HTLV-1 proviral loads are elevated in orally HTLV-1-infected rats, which may be a consequence of insufficient HTLV-1-specific T-cell responses in these rats (8). Therefore, we next examined whether these conditions in orally HTLV-1-infected rats could be altered by reimmunization with HTLV-1-infected cells. A total of eight rats were orally infected with 5 × 107 MMC-treated MT-2 cells, and then four of the eight rats were reimmunized after 7 weeks with 2 × 107 cells of the MMC-treated syngeneic HTLV-1-infected T-cell line FPM1 by subcutaneous injection. At 4 to 5 weeks after the reimmunization, the HTLV-1-specific T-cell responses and HTLV-1 proviral loads in the spleens were determined, and the results are summarized in Fig. 2A and B, respectively. The Tax-specific T-cell proliferative responses were very low in all four orally HTLV-1-infected rats that were not reimmunized. However, the reimmunized orally HTLV-1-infected rats exhibited significant levels of Tax-specific T-cell proliferation (Fig. 2A). In contrast, real-time PCR assessment of the HTLV-1 provirus loads in the rats revealed results completely opposite to the T-cell responses (Fig. 2B). Although the provirus loads in the untreated orally HTLV-1-infected rats varied among the individual rats, the reimmunized rats showed significantly lower levels of proviral load. A stronger T-cell response coincided with a lower proviral load in the reimmunized rats, suggesting that augmentation of the HTLV-1-specific T-cell response may contribute to reducing the HTLV-1 proviral load.
FIG. 2.
HTLV-1-specific T-cell responses and proviral loads in orally infected and reimmunized rats. (A) A total of eight rats were orally infected with HTLV-1. At 7 weeks after the infection, four of the rats were left untreated (P.O. rats), while the other four rats were subcutaneously administered 2 × 107 MMC-treated HTLV-1-infected syngeneic rat FPM1 cells (Reimmunized P.O. rats). At 4 to 5 weeks after the reimmunization, T-cell-enriched spleen cells from the P.O. or reimmunized P.O. rats were subjected to proliferation assays. The proliferation index of [3H]thymidine incorporation against Tax-G14 cells was calculated as described in Materials and Methods. (B) The HTLV-1 provirus loads in the spleens of the rats in panel A were measured by real-time PCR. The results represent the provirus copy numbers/105 copies of GAPDH.
Reduction in the HTLV-1 provirus load after reimmunization of orally HTLV-1-infected rats.
Since the levels of proviral load in the orally HTLV-1-infected rats varied among individuals, we further examined whether the established proviral load in an orally infected rat could be reduced by HTLV-1 reimmunization. In order to compare the HTLV-1-specific T-cell responses and HTLV-1 provirus loads before and after reimmunization in the same rats, we performed a half-splenectomy in two orally HTLV-1-infected rats to obtain the preimmune splenocytes and then subcutaneously reimmunized these rats with MMC-treated FPM1 cells. At 4 weeks after the reimmunization with FPM1 cells, we harvested the residual spleens and examined the T-cell responses and proviral loads in the splenocytes before and after reimmunization.
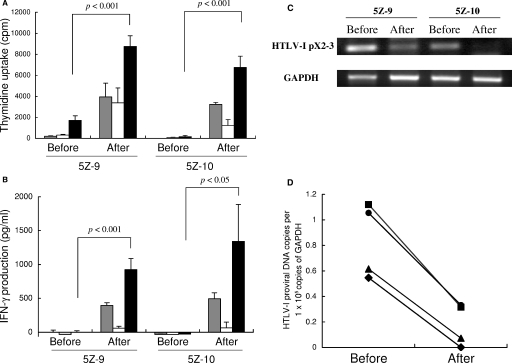
The results for the two rats (5Z-9 and 5Z-10) are shown in Fig. 3. In both rats, the Tax-specific proliferative responses of the splenic T cells were very low before reimmunization but became markedly restored after reimmunization (Fig. 3A). Similar recovery of the Tax-specific T-cell responses after reimmunization in these rats was also observed in IFN-γ production assays (Fig. 3B). HTLV-1 proviruses in the spleen halves harvested from the rats before and after reimmunization were amplified by PCR using Tax-specific primers. The direct staining of PCR products indicated that HTLV-1 proviruses decreased after reimmunization in both rats (Fig. 3C). In rat 5Z-10 in particular, HTLV-1 proviruses became undetectable after reimmunization. Decreases in the provirus copy numbers after reimmunization were confirmed by real-time PCR in these spleen samples and also in spleen halves from two other orally HTLV-1-infected rats, 8H-7 and 8H-9, that were reimmunized with MMC-treated FPM1 cells after half-splenectomy, similarly to the 5Z-9 and 5Z-10 rats (Fig. 3D). These results indicate that the recovery of the T-cell response against HTLV-1 by reimmunization is directly associated with a reduction in the HTLV-1 provirus load in the host.
FIG. 3.
HTLV-1-specific T-cell responses and proviral loads in orally infected rats before and after reimmunization. A half-splenectomy was performed in two orally HTLV-1-infected rats (5Z-9 and 5Z-10) under anesthesia at 47 weeks after infection. The T-cell-enriched fractions of the excised spleen tissues were stored at −80°C. At 1 week after the surgery, 2 × 107 MMC-treated FPM1 cells were administered subcutaneously. The residual spleens were harvested at 4 weeks after the reimmunization. (A and B) The cryopreserved spleen T-cell-enriched fractions before and after the FPM1 cell inoculation were examined for their proliferative (A) and IFN-γ production (B) responses to medium only (░⃞), formalin-treated G14 cells (□), or Tax-G14 cells (▪) by determining the [3H]thymidine incorporation and by ELISA, respectively. The results represent the mean ± the SD of triplicate wells. (C) Comparison of the amounts of HTLV-1 provirus in 5Z-9 and 5Z-10 rats before and after FPM1 cell inoculation. The spleen DNA samples were amplified by 35 cycles of PCR with Tax- and GAPDH-specific primers and visualized with ethidium bromide staining. (D) Quantification of HTLV-1 provirus loads by real-time PCR in the spleens of 5Z-9 (▪), 5Z-10 (⧫), and two additional orally HTLV-1-infected rats, 8H-7 (•) and 8H-9 (▴), that received a half-splenectomy at 20 weeks after infection and were immunized with MMC-treated FPM1 cells similarly to animals 5Z-9 and 5Z10.
Recovery of the HTLV-1-specific T-cell response by subcutaneous inoculation with autologous primary T cells.
The FPM1 cell line used for the reimmunization is a transformed T-cell line derived from syngeneic rat thymocytes infected with HTLV-1 in vitro, and the cells express a large amount of HTLV-1 Tax (25). However, HTLV-1-infected individuals possess HTLV-1-infected cells among their own T cells in vivo. Finally, therefore, we assessed whether subcutaneous injection of primary T cells isolated from orally HTLV-1-infected rats could abrogate the HTLV-1-specific T-cell unresponsiveness in orally HTLV-1-infected syngeneic rats.
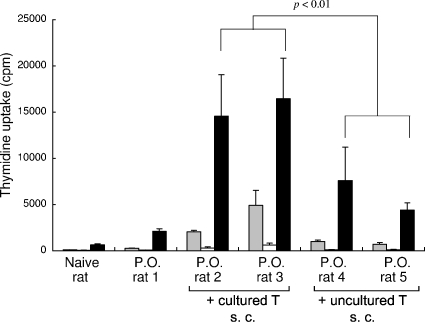
T-cell-enriched splenocytes isolated from orally HTLV-1-infected rats were either uncultured or cultured for 2 days, treated with MMC, and subcutaneously injected into syngeneic rats that had been orally infected with HTLV-1. The T-cell responses in these rats at 5 weeks after the subcutaneous injection are shown in Fig. 4. Both of the rats injected with the MMC-treated cultured primary T cells showed significant levels of Tax-specific T-cell responses. However, the recovery of the T-cell responses in the rats injected with the uncultured primary T cells was less effective.
FIG. 4.
Recovery of Tax-specific T-cell responses in orally HTLV-1-infected rats by subcutaneous (s.c.) inoculation with HTLV-1-infected primary T cells. Rats orally infected with HTLV-1 15 weeks previously were subcutaneously inoculated without (P.O. rat 1) or with (P.O. rats 2 to 5) primary spleen T cells (2 × 107 cells), which had been isolated from other syngeneic orally HTLV-1-infected rats and treated with MMC before the inoculation, either immediately (uncultured T) or after 2 days of culture in RPMI 1640 medium containing 10% FCS (cultured T), as indicated. After 5 weeks, the spleen T cells were harvested from these rats and subjected to proliferation assays against medium only (░⃞), formalin-treated G14 cells (□), or Tax-G14 cells (▪). The results represent the mean [3H]thymidine incorporation ± the SD in triplicate samples.
DISCUSSION
In the present study, we demonstrated that restoration of HTLV-1-specific T-cell immunity was associated with a reduction in the HTLV-1 proviral load in orally HTLV-1-infected rats. Together with our previous finding that orally HTLV-1-infected rats show insufficient HTLV-1-specific T-cell responses with elevated proviral loads (8), the present results strongly suggest that T-cell immunity actively controls the number of HTLV-1-infected cells in vivo.
HTLV-1 in vivo is presumably maintained by cell-to-cell transmission of the virus and multiplication of the infected cells (2, 15). HTLV-1-specific T cells potentially inhibit both pathways but only if the infected cells express target antigens. In the present study, rats were reimmunized at various periods after oral HTLV-1 infection, i.e., in the subacute and chronic phases. Although the efficiency of HTLV-1 transmission is supposed to be much lower in rats than in humans (6), there was an individual variety in the levels of proviral load established. Nevertheless, later recovery of T-cell immunity was able to reduce the viral load, indicating that the infected cells were susceptible to the immune T cells in vivo. As a result, a newly equilibrated proviral load was established.
Although the HTLV-1-specific T-cell response was markedly suppressed in the orally HTLV-1-infected rats, their T-cell responses to other antigens, such as MLR and contact hypersensitivity, were comparable to those of uninfected rats. MLR is a CD4+ T-cell-dominant response to MHC II, whereas contact hypersensitivity induced by DNFB is a CD8+ T-cell-mediated response to cutaneous sensitization and subsequent challenge (23). It is known that measles virus infection reduces contact hypersensitivity in a rodent model (31, 43). In healthy HTLV-1 carriers, suppressed delayed-type hypersensitivity to purified protein derivatives, as been reported in several studies (28, 33, 50), although it remains controversial (34). However, our observed T-cell unresponsiveness in orally infected rats was specific for HTLV-1 and did not merely reflect general immunosuppression. It has been suggested that transforming growth factor β and interleukin-10 produced by regulatory T cells and type 3 helper T cells are involved in oral tolerance to protein antigens (4). The precise mechanism of the HTLV-1-specific T-cell tolerance in orally infected rats remains to be determined.
It is of note that subcutaneous administration of primary spleen T cells from orally HTLV-1-infected rats induced restoration of HTLV-1-specific immune responses in syngeneic orally HTLV-1-infected rats. This is an apparent paradox because similar spleen T cells are already present in the hosts. This phenomenon indicates that the T-cell unresponsiveness in orally infected rats cannot be attributed to the clonal deletion of HTLV-1-specific T cells. We suppose that the HTLV-1-specific T-cell tolerance was abrogated by the subcutaneous administration of HTLV-1-infected cells via the activation of antigen-presenting cells in the skin. The use of cultured splenocytes restored the immune responses more effectively than uncultured splenocytes. This difference may be due to the amount of HTLV-1 antigens expressed in the splenocytes, since HTLV-1 expression is known to be very low in human peripheral blood and spontaneously induced during short-term culture (11, 19). Tax-induced costimulatory molecules in the infected cells may also contribute to the abrogation of immune tolerance by activating both antigen-presenting cells and T-cell responses (27, 29, 39).
In humans, HTLV-1-specific T-cell responses are exhibited by HAM/TSP patients and many asymptomatic HTLV-1 carriers. However, a small proportion of HTLV-1-carriers, including ATL patients, show repression of HTLV-1-specific immune responses. Since a high proviral load has been shown to be one of the risk factors for ATL (14, 37), the reduction in the proviral load after reimmunization demonstrated in the present study implies that restoration of HTLV-1-specific T-cell immunity potentially reduces the risk of ATL in HTLV-1 carriers with low immune responses. Autologous HTLV-1-infected cells in the peripheral blood are a potential candidate for the immunogen.
Acknowledgments
This study was supported by grant from the Ministry of Education, Science, Culture, and Sports of Japan and from the Ministry of Health, Welfare and Labor of Japan.
We thank Kiyoshi Nishioka (Yokohama-Minato Red Cross Hospital, Japan) for valuable advice.
REFERENCES
- 1.Arnulf, B., M. Thorel, Y. Poirot, R. Tamouza, E. Boulanger, A. Jaccard, E. Oksenhendler, O. Hermine, and C. Pique. 2004. Loss of the ex vivo but not the reinducible CD8+ T-cell response to Tax in human T-cell leukemia virus type 1-infected patients with adult T-cell leukemia/lymphoma. Leukemia 18:126-132. [DOI] [PubMed] [Google Scholar]
- 2.Cavrois, M., A. Gessain, S. Wain-Hobson, and E. Wattel. 1996. Proliferation of HTLV-1 infected circulating cells in vivo in all asymptomatic carriers and patients with TSP/HAM. Oncogene 12:2419-2423. [PubMed] [Google Scholar]
- 3.Chen, S., N. Ishii, S. Ine, S. Ikeda, T. Fujimura, L. C. Ndhlovu, P. Soroosh, K. Tada, H. Harigae, J. Kameoka, N. Kasai, T. Sasaki, and K. Sugamura. 2006. Regulatory T cell-like activity of Foxp3+ adult T cell leukemia cells. Int. Immunol. 18:269-277. [DOI] [PubMed] [Google Scholar]
- 4.Faria, A. M., and H. L. Weiner. 2005. Oral tolerance. Immunol. Rev. 206:232-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gessain, A., F. Barin, J. C. Vernant, O. Gout, L. Maurs, A. Calender, and G. de The. 1985. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet ii:407-410. [DOI] [PubMed] [Google Scholar]
- 6.Hakata, Y., M. Yamada, and H. Shida. 2001. Rat CRM1 is responsible for the poor activity of human T-cell leukemia virus type 1 Rex protein in rat cells. J. Virol. 75:11515-11525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanabuchi, S., T. Ohashi, Y. Koya, H. Kato, A. Hasegawa, F. Takemura, T. Masuda, and M. Kannagi. 2001. Regression of human T-cell leukemia virus type I (HTLV-I)-associated lymphomas in a rat model: peptide-induced T-cell immunity. J. Natl. Cancer Inst. 93:1775-1783. [DOI] [PubMed] [Google Scholar]
- 8.Hasegawa, A., T. Ohashi, S. Hanabuchi, H. Kato, F. Takemura, T. Masuda, and M. Kannagi. 2003. Expansion of human T-cell leukemia virus type 1 (HTLV-1) reservoir in orally infected rats: inverse correlation with HTLV-1-specific cellular immune response. J. Virol. 77:2956-2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hino, S., S. Katamine, H. Miyata, Y. Tsuji, T. Yamabe, and T. Miyamoto. 1996. Primary prevention of HTLV-I in Japan. J. Acquir. Immune. Defic. Syndr. Hum. Retrovirol. 13(Suppl. 1):S199-S203. [DOI] [PubMed] [Google Scholar]
- 10.Hino, S., K. Yamaguchi, S. Katamine, H. Sugiyama, T. Amagasaki, K. Kinoshita, Y. Yoshida, H. Doi, Y. Tsuji, and T. Miyamoto. 1985. Mother-to-child transmission of human T-cell leukemia virus type-I. Jpn. J. Cancer Res. 76:474-480. [PubMed] [Google Scholar]
- 11.Hinuma, Y., Y. Gotoh, K. Sugamura, K. Nagata, T. Goto, M. Nakai, N. Kamada, T. Matsumoto, and K. Kinoshita. 1982. A retrovirus associated with human adult T-cell leukemia: in vitro activation. Gann 73:341-344. [PubMed] [Google Scholar]
- 12.Hinuma, Y., K. Nagata, M. Hanaoka, M. Nakai, T. Matsumoto, K. I. Kinoshita, S. Shirakawa, and I. Miyoshi. 1981. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc. Natl. Acad. Sci. USA 78:6476-6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hisada, M., A. Okayama, D. Spiegelman, N. E. Mueller, and S. O. Stuver. 2001. Sex-specific mortality from adult T-cell leukemia among carriers of human T-lymphotropic virus type I. Int. J. Cancer 91:497-499. [DOI] [PubMed] [Google Scholar]
- 14.Hisada, M., A. Okayama, N. Tachibana, S. O. Stuver, D. L. Spiegelman, H. Tsubouchi, and N. E. Mueller. 1998. Predictors of level of circulating abnormal lymphocytes among human T-lymphotropic virus type I carriers in Japan. Int. J. Cancer 77:188-192. [DOI] [PubMed] [Google Scholar]
- 15.Igakura, T., J. C. Stinchcombe, P. K. Goon, G. P. Taylor, J. N. Weber, G. M. Griffiths, Y. Tanaka, M. Osame, and C. R. Bangham. 2003. Spread of HTLV-I between lymphocytes by virus-induced polarization of the cytoskeleton. Science 299:1713-1716. [DOI] [PubMed] [Google Scholar]
- 16.Jacobson, S., H. Shida, D. E. McFarlin, A. S. Fauci, and S. Koenig. 1990. Circulating CD8+ cytotoxic T lymphocytes specific for HTLV-I pX in patients with HTLV-I associated neurological disease. Nature 348:245-248. [DOI] [PubMed] [Google Scholar]
- 17.Jeang, K. T. 2001. Functional activities of the human T-cell leukemia virus type I Tax oncoprotein: cellular signaling through NF-κB. Cytokine Growth Factor Rev. 12:207-217. [DOI] [PubMed] [Google Scholar]
- 18.Kannagi, M., S. Harada, I. Maruyama, H. Inoko, H. Igarashi, G. Kuwashima, S. Sato, M. Morita, M. Kidokoro, M. Sugimoto, et al. 1991. Predominant recognition of human T-cell leukemia virus type I (HTLV-I) pX gene products by human CD8+ cytotoxic T cells directed against HTLV-I-infected cells. Int. Immunol. 3:761-767. [DOI] [PubMed] [Google Scholar]
- 19.Kannagi, M., S. Matsushita, and S. Harada. 1993. Expression of the target antigen for cytotoxic T lymphocytes on adult T-cell-leukemia cells. Int. J. Cancer 54:582-588. [DOI] [PubMed] [Google Scholar]
- 20.Kannagi, M., K. Sugamura, K. Kinoshita, H. Uchino, and Y. Hinuma. 1984. Specific cytolysis of fresh tumor cells by an autologous killer T-cell line derived from an adult T-cell leukemia/lymphoma patient. J. Immunol. 133:1037-1041. [PubMed] [Google Scholar]
- 21.Kannagi, M., K. Sugamura, H. Sato, K. Okochi, H. Uchino, and Y. Hinuma. 1983. Establishment of human cytotoxic T-cell lines specific for human adult T-cell leukemia virus-bearing cells. J. Immunol. 130:2942-2946. [PubMed] [Google Scholar]
- 22.Kato, H., Y. Koya, T. Ohashi, S. Hanabuchi, F. Takemura, M. Fujii, H. Tsujimoto, A. Hasegawa, and M. Kannagi. 1998. Oral administration of human T-cell leukemia virus type 1 induces immune unresponsiveness with persistent infection in adult rats. J. Virol. 72:7289-7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kehren, J., C. Desvignes, M. Krasteva, M. T. Ducluzeau, O. Assossou, F. Horand, M. Hahne, D. Kagi, D. Kaiserlian, and J. F. Nicolas. 1999. Cytotoxicity is mandatory for CD8+ T cell-mediated contact hypersensitivity. J. Exp. Med. 189:779-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kondo, T., H. Kono, N. Miyamoto, R. Yoshida, H. Toki, I. Matsumoto, M. Hara, H. Inoue, A. Inatsuki, T. Funatsu, et al. 1989. Age- and sex-specific cumulative rate and risk of ATLL for HTLV-I carriers. Int. J. Cancer 43:1061-1064. [DOI] [PubMed] [Google Scholar]
- 25.Koya, Y., T. Ohashi, H. Kato, S. Hanabuchi, T. Tsukahara, F. Takemura, K. Etoh, M. Matsuoka, M. Fujii, and M. Kannagi. 1999. Establishment of a seronegative human T-cell leukemia virus type 1 (HTLV-1) carrier state in rats inoculated with a syngeneic HTLV-1-immortalized T-cell line preferentially expressing Tax. J. Virol. 73:6436-6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kubota, R., T. Kawanishi, H. Matsubara, A. Manns, and S. Jacobson. 2000. HTLV-I specific IFN-γ+ CD8+ lymphocytes correlate with the proviral load in peripheral blood of infected individuals. J. Neuroimmunol. 102:208-215. [DOI] [PubMed] [Google Scholar]
- 27.Kurihara, K., N. Harashima, S. Hanabuchi, M. Masuda, A. Utsunomiya, R. Tanosaki, M. Tomonaga, T. Ohashi, A. Hasegawa, T. Masuda, J. Okamura, Y. Tanaka, and M. Kannagi. 2005. Potential immunogenicity of adult T-cell leukemia cells in vivo. Int. J. Cancer 114:257-267. [DOI] [PubMed] [Google Scholar]
- 28.Kuroda, Y., and H. Takashima. 1990. Impairment of cell-mediated immune responses in HTLV-I-associated myelopathy. J. Neurol. Sci. 100:211-216. [DOI] [PubMed] [Google Scholar]
- 29.Lal, R. B., D. L. Rudolph, C. S. Dezzutti, P. S. Linsley, and H. E. Prince. 1996. Costimulatory effects of T cell proliferation during infection with human T lymphotropic virus types I and II are mediated through CD80 and CD86 ligands. J. Immunol. 157:1288-1296. [PubMed] [Google Scholar]
- 30.Manns, A., F. R. Cleghorn, R. T. Falk, B. Hanchard, E. S. Jaffe, C. Bartholomew, P. Hartge, J. Benichou, and W. A. Blattner. 1993. Role of HTLV-I in development of non-Hodgkin lymphoma in Jamaica and Trinidad and Tobago. The HTLV Lymphoma Study Group. Lancet 342:1447-1450. [DOI] [PubMed] [Google Scholar]
- 31.Marie, J. C., J. Kehren, M. C. Trescol-Biemont, A. Evlashev, H. Valentin, T. Walzer, R. Tedone, B. Loveland, J. F. Nicolas, C. Rabourdin-Combe, and B. Horvat. 2001. Mechanism of measles virus-induced suppression of inflammatory immune responses. Immunity 14:69-79. [DOI] [PubMed] [Google Scholar]
- 32.Matsuzaki, T., M. Nakagawa, M. Nagai, K. Usuku, I. Higuchi, K. Arimura, H. Kubota, S. Izumo, S. Akiba, and M. Osame. 2001. HTLV-I proviral load correlates with progression of motor disability in HAM/TSP: analysis of 239 HAM/TSP patients including 64 patients followed up for 10 years. J. Neurovirol. 7:228-234. [DOI] [PubMed] [Google Scholar]
- 33.Murai, K., N. Tachibana, S. Shioiri, E. Shishime, A. Okayama, J. Ishizaki, K. Tsuda, and N. Mueller. 1990. Suppression of delayed-type hypersensitivity to PPD and PHA in elderly HTLV-I carriers. J. Acquir. Immune. Defic. Syndr. 3:1006-1009. [PubMed] [Google Scholar]
- 34.Murphy, E. L., Y. Wu, H. E. Ownby, J. W. Smith, R. K. Ruedy, R. A. Thomson, D. I. Ameti, D. J. Wright, and G. J. Nemo. 2001. Delayed hypersensitivity skin testing to mumps and Candida albicans antigens is normal in middle-aged HTLV-I- and-II-infected U.S. cohorts. AIDS Res. Hum. Retrovir. 17:1273-1277. [DOI] [PubMed] [Google Scholar]
- 35.Nowak, M. A., and C. R. Bangham. 1996. Population dynamics of immune responses to persistent viruses. Science 272:74-79. [DOI] [PubMed] [Google Scholar]
- 36.Ohashi, T., S. Hanabuchi, H. Kato, H. Tateno, F. Takemura, T. Tsukahara, Y. Koya, A. Hasegawa, T. Masuda, and M. Kannagi. 2000. Prevention of adult T-cell leukemia-like lymphoproliferative disease in rats by adoptively transferred T cells from a donor immunized with human T-cell leukemia virus type 1 Tax-coding DNA vaccine. J. Virol. 74:9610-9616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okayama, A., S. Stuver, M. Matsuoka, J. Ishizaki, G. Tanaka, Y. Kubuki, N. Mueller, C. C. Hsieh, N. Tachibana, and H. Tsubouchi. 2004. Role of HTLV-1 proviral DNA load and clonality in the development of adult T-cell leukemia/lymphoma in asymptomatic carriers. Int. J. Cancer 110:621-625. [DOI] [PubMed] [Google Scholar]
- 38.Osame, M., K. Usuku, S. Izumo, N. Ijichi, H. Amitani, A. Igata, M. Matsumoto, and M. Tara. 1986. HTLV-I associated myelopathy, a new clinical entity. Lancet i:1031-1032. [DOI] [PubMed] [Google Scholar]
- 39.Pankow, R., H. Durkop, U. Latza, H. Krause, U. Kunzendorf, T. Pohl, and S. Bulfone-Paus. 2000. The HTLV-I tax protein transcriptionally modulates OX40 antigen expression. J. Immunol. 165:263-270. [DOI] [PubMed] [Google Scholar]
- 40.Parker, C. E., S. Daenke, S. Nightingale, and C. R. Bangham. 1992. Activated, HTLV-1-specific cytotoxic T-lymphocytes are found in healthy seropositives as well as in patients with tropical spastic paraparesis. Virology 188:628-636. [DOI] [PubMed] [Google Scholar]
- 41.Poiesz, B. J., F. W. Ruscetti, A. F. Gazdar, P. A. Bunn, J. D. Minna, and R. C. Gallo. 1980. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 77:7415-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skoglund, C., and A. Scheynius. 1990. Effects of interferon-gamma treatment on the cutaneous DTH reaction in rats. Arch. Dermatol. Res. 282:318-324. [DOI] [PubMed] [Google Scholar]
- 43.Streif, S., K. Pueschel, A. Tietz, J. Blanco, V. T. Meulen, and S. Niewiesk. 2004. Effector CD8+ T cells are suppressed by measles virus infection during delayed type hypersensitivity reaction. Viral. Immunol. 17:604-608. [DOI] [PubMed] [Google Scholar]
- 44.Tajima, K., et al. 1990. The 4th nationwide study of adult T-cell leukemia/lymphoma (ATL) in Japan: estimates of risk of ATL and its geographical and clinical features. Int. J. Cancer 45:237-243. [DOI] [PubMed] [Google Scholar]
- 45.Taylor, G. P., S. E. Hall, S. Navarrete, C. A. Michie, R. Davis, A. D. Witkover, M. Rossor, M. A. Nowak, P. Rudge, E. Matutes, C. R. Bangham, and J. N. Weber. 1999. Effect of lamivudine on human T-cell leukemia virus type 1 (HTLV-1) DNA copy number, T-cell phenotype, and anti-tax cytotoxic T-cell frequency in patients with HTLV-1-associated myelopathy. J. Virol. 73:10289-10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uchiyama, T. 1997. Human T-cell leukemia virus type I (HTLV-I) and human diseases. Annu. Rev. Immunol. 15:15-37. [DOI] [PubMed] [Google Scholar]
- 47.Vaz, N., A. M. Faria, B. A. Verdolin, and C. R. Carvalho. 1997. Immaturity, ageing and oral tolerance. Scand. J. Immunol. 46:225-229. [DOI] [PubMed] [Google Scholar]
- 48.Vine, A. M., A. G. Heaps, L. Kaftantzi, A. Mosley, B. Asquith, A. Witkover, G. Thompson, M. Saito, P. K. Goon, L. Carr, F. Martinez-Murillo, G. P. Taylor, and C. R. Bangham. 2004. The role of CTLs in persistent viral infection: cytolytic gene expression in CD8+ lymphocytes distinguishes between individuals with a high or low proviral load of human T-cell lymphotropic virus type 1. J. Immunol. 173:5121-5129. [DOI] [PubMed] [Google Scholar]
- 49.Walker, D. B., W. C. Williams, C. B. Copeland, and R. J. Smialowicz. 2004. Persistent suppression of contact hypersensitivity, and altered T-cell parameters in F344 rats exposed perinatally to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Toxicology 197:57-66. [DOI] [PubMed] [Google Scholar]
- 50.Welles, S. L., N. Tachibana, A. Okayama, S. Shioiri, S. Ishihara, K. Murai, and N. E. Mueller. 1994. Decreased reactivity to PPD among HTLV-1 carriers in relation to virus and hematologic status. Int. J. Cancer 56:337-340. [DOI] [PubMed] [Google Scholar]
- 51.Wodarz, D., S. E. Hall, K. Usuku, M. Osame, G. S. Ogg, A. J. McMichael, M. A. Nowak, and C. R. Bangham. 2001. Cytotoxic T-cell abundance and virus load in human immunodeficiency virus type 1 and human T-cell leukaemia virus type 1. Proc. Biol. Sci. 268:1215-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoshida, M. 2001. Multiple viral strategies of HTLV-1 for dysregulation of cell growth control. Annu. Rev. Immunol. 19:475-496. [DOI] [PubMed] [Google Scholar]
- 53.Yoshida, M., M. Seiki, K. Yamaguchi, and K. Takatsuki. 1984. Monoclonal integration of human T-cell leukemia provirus in all primary tumors of adult T-cell leukemia suggests causative role of human T-cell leukemia virus in the disease. Proc. Natl. Acad. Sci. USA 81:2534-2537. [DOI] [PMC free article] [PubMed] [Google Scholar]