Abstract
As critical determinants of growth anisotropy in plants, cortical microtubules are thought to constrain the movement of cellulose synthase complexes and thus align newly deposited cellulose microfibrils. We tested this cellulose synthase constraint model using the temperature-sensitive mor1-1 mutant of Arabidopsis. Contrary to predictions, the disruption of cortical microtubules in mor1-1 root epidermal cells led to left-handed root twisting and radial swelling but did not alter the transverse orientation of cellulose microfibrils. We also found that drug-dependent disassembly or hyperstabilization of cortical microtubules did not alter the parallel order of cellulose microfibrils. By measuring cellulose content in mor1-1 seedlings, we verified that cellulose synthesis is not reduced at the restrictive temperature. The independence of cortical microtubule organization and cellulose microfibril alignment was supported by the observation that double mutants of mor1-1 and rsw1-1, the cellulose-deficient mutant with misaligned microfibrils, had additive phenotypes. Our results suggest that cortical microtubules regulate growth anisotropy by some mechanism other than cellulose microfibril alignment or synthesis.
INTRODUCTION
Anisotropic cell expansion is a fundamental aspect of plant development. It is the basis for both axial growth and the diversity of cell shapes found in plant tissues. Cortical microtubules and cellulose microfibrils have been widely accepted as primary determinants of growth anisotropy in plants (Wasteneys, 2000; Baskin, 2001). Crystalline cellulose has great tensile strength, and its highly ordered arrangement in elongating cells specifies the direction of cell expansion (Taiz, 1984; Carpita and Gibeaut, 1993). Many studies have revealed a strong correlation between the orientations of cortical microtubules and cellulose microfibrils, especially during rapid elongation, when both are generally oriented perpendicular to the major growth axis (Baskin, 2001). Perturbing microtubule dynamics or eliminating microtubules altogether with various drugs impairs growth anisotropy (Baskin et al., 1994). The cellulose synthase constraint model proposes that cortical microtubules constrain the paths taken by cellulose synthase complexes resident in the plasma membrane as they add cellulose microfibrils to the inner layer of the cell wall (Giddings and Staehelin, 1991). Verifying the obligate relationship between the orientations of cortical microtubules and cellulose microfibrils requires careful analysis, as does the relationship between these features and growth anisotropy. Such analysis has used pharmacological perturbation and, more recently, mutational analysis.
Numerous Arabidopsis mutants with cell expansion defects have started to provide insights into the cell wall structures that regulate cell expansion (Williamson et al., 2001). The RADIAL SWELLING1 (RSW1) and PROCUSTE genes encode members of the cellulose synthase gene family, CesA1 and CesA6, respectively (Arioli et al., 1998; Fagard et al., 2000). Mutations in these genes reduce cellulose production and growth anisotropy. Several other proteins involved in cellulose synthesis via less direct mechanisms also have been identified (Nicol et al., 1998; Lane et al., 2001; Sato et al., 2001; Burn et al., 2002; Gillmor et al., 2002; Pagant et al., 2002). Together, these discoveries provide genetic evidence for the crucial role that cellulose plays in growth axiality.
Recent studies demonstrate that cellulose synthesis must be maintained at a certain rate for ordered microfibril alignment. Reduced cellulose production caused by the temperature-sensitive allele of CesA1 in rsw1-1 or by treatment with the cellulose synthesis–inhibiting drug 2,6-dichlorobenzonitrile generates disordered microfibrils in the presence of ordered microtubules (Sugimoto et al., 2001). More recently, disordered cellulose microfibrils also were observed in the cellulose-deficient kobito1 (Pagant et al., 2002) and fra2 (Burk and Ye, 2002) mutants. How cellulose synthesis per se can generate parallel microfibril order is not clear, but a recent model (Emons and Mulder, 1998, 2000; Mulder and Emons, 2001) couples the direction of motion of synthase complexes, and hence the orientation of the cellulose microfibrils being deposited, to the local density of the complexes in the membrane.
Other mutants of Arabidopsis provide genetic evidence for cortical microtubules playing a crucial role in growth anisotropy. We showed previously that mutations in the high-molecular-weight microtubule-associated protein MOR1 cause temperature-dependent disassembly of cortical microtubules and that this impairs anisotropic cell expansion (Whittington et al., 2001). Cortical microtubules fail to establish transverse organization during early stages of cell elongation, and axial growth is impaired constitutively in mutants that carry null alleles of the gene encoding the microtubule-severing protein katanin p60 (Bichet et al., 2001; Burk et al., 2001). Mutations in the FRA1 kinesin-like protein also reduce axial expansion and fiber strength (Zhong et al., 2002), although no defects in cortical microtubules have been described. Various other mutations that compromise or abolish microtubule assembly properties cause severe defects in microtubule-dependent processes, including growth anisotropy (Camilleri et al., 2002; Steinborn et al., 2002; Thitamadee et al., 2002).
Therefore, it is certain that cortical microtubules and cellulose microfibrils both play major roles in controlling growth anisotropy, but it does not necessarily follow that they act as suggested by the cellulose synthase constraint model. Several studies of diffusely expanding cells within multicellular tissues demonstrate that drug-dependent microtubule disassembly does not abolish the oriented deposition of cellulose microfibrils (Itoh, 1976; Itoh and Shimeji, 1976; Srivastava et al., 1976; Takeda and Shibaoka, 1981; Yatsu and Jacks, 1981; Mueller and Brown, 1982; Wilms et al., 1990). In these systems, microfibrils retain a locally parallel arrangement but microfibril orientation varies in different regions of the same wall, suggesting that a microtubule-independent mechanism controls local microfibril parallelism but that microtubules may impose an overall alignment.
An unfortunate tendency in the literature has been to assume that radial swelling is an indicator of disordered cellulose deposition. Very few studies that have correlated perturbed microtubules with radial swelling have examined cellulose microfibril alignment (Baskin, 2001). However, a recent study of two Arabidopsis mutants, rsw4 and rsw7, showed extensive radial swelling despite normal levels of cellulose synthesis and no detectable changes in microfibril or cortical microtubule orientation patterns (Wiedemeier et al., 2002). Coorientation of cortical microtubules and cellulose microfibrils clearly is not sufficient to control growth axialization.
The temperature-sensitive mor1-1 mutant provides a powerful system in which to test the causal relationship between cortical microtubules, cellulose microfibrils, and anisotropic cell expansion because it is possible in this mutant to reversibly change microtubule organization by simply changing the culture temperature by a few degrees (Whittington et al., 2001). In this study, we examined the effects of microtubule disruption in the roots of mor1-1 and wild-type seedlings treated with oryzalin and taxol using the correlative approach we described previously (Sugimoto et al., 2000). Our findings demonstrate that although cortical microtubules are essential for maintaining growth anisotropy, their disordering or complete loss does little to alter the parallel alignment of cellulose microfibrils in expanding cells of Arabidopsis roots. To further examine the possible interplay between microtubules and cellulose alignment, we generated double mutants of mor1-1 and the temperature-sensitive rsw1-1 allele of the cellulose synthase catalytic subunit AtCesA1. We showed previously that cellulose microfibrils become disordered rapidly in the rsw1-1 mutant at temperatures that reduce cellulose synthesis (Sugimoto et al., 2001). The mor1-1 rsw1-1 double mutants had an additive phenotype, providing further support for the concept that microtubules control axial growth by some means other than cellulose microfibril alignment. Finally, we confirmed that cellulose content was unchanged in mor1-1 walls at the restrictive temperature. These findings support the idea that cellulose microfibrils can largely self-align into parallel order in the absence of microtubules and that this process requires adequate levels of cellulose synthesis.
RESULTS
Cortical Microtubules Lose Their Transverse Orientation and Appear Shorter in mor1-1 Root Epidermal Cells at Restrictive Temperatures
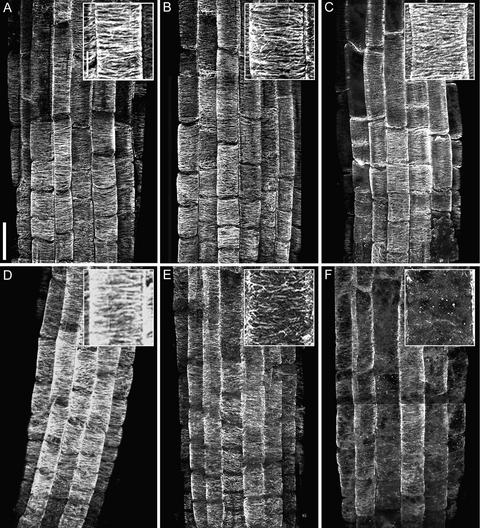
Arabidopsis root cells are ideal for studying morphogenesis because their growth is both consistent and anisotropic (Sugimoto et al., 2000). Here, we first characterized the cortical microtubule patterns in mor1-1 root epidermal cells. Like wild-type cells (Figures 1A to 1C), mor1-1 root epidermal cells have predominantly transverse cortical microtubules throughout most of the elongation phase when grown at 21°C (Sugimoto et al., 2000) (Figure 1D). At 29°C, we found that cortical microtubules in the wild type showed no obvious changes (Figures 1B and 1C), whereas in mor1-1, cortical microtubules appeared to be much shorter than normal and oriented in a variety of directions after 1 h at 29°C (Figure 1E). After 2 h at 29°C, cortical microtubules in mor1-1 were judged to be disrupted maximally: they were short, sparse, and no longer oriented in a predominantly transverse direction (Figure 1F).
Figure 1.
Cortical Microtubule Arrays Become Disrupted in mor1-1 Root Epidermal Cells at 29°C, a Temperature That Does Not Alter Microtubule Arrangement in the Wild Type.
Confocal micrographs of immunofluorescently labeled microtubules in root epidermal cells from 5-day-old seedlings. Insets show higher magnification images. Bar = 50 μm; bar in inset = 10 μm.
(A) Wild type at 21°C.
(B) Wild type after 1 h at 29°C.
(C) Wild type after 2 h at 29°C.
(D) mor1-1 at 21°C.
(E) mor1-1 after 1 h at 29°C.
(F) mor1-1 after 2 h at 29°C.
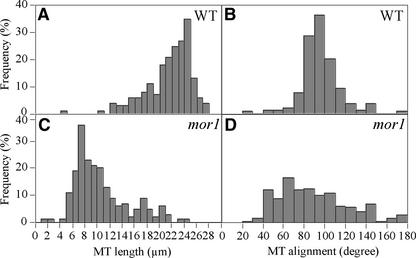
To quantify these changes, we estimated microtubule lengths and orientations from fluorescent images of cells fixed after 4 h at 29°C (Figure 2). In this case, we were able to measure only the lengths of linear fluorescent units, which were more likely to represent overlapped and bundled microtubules rather than single microtubules. Most of the wild-type cortical microtubules were approximately as long as the visible width of the epidermal cells (∼20 μm), although many probably were much longer, because microtubule ends were observed only rarely (Figure 2A). In comparison, the length of the fluorescently labeled microtubule structures in mor1-1 were measured more accurately. They were rarely more than one-third of the cell diameter in length (Figure 2C). In wild-type cells, >80% of microtubules deviated by <20° from the transverse axis (Figure 2B), whereas in mor1-1, microtubule angular deviation was increased greatly (Figure 2D). Microtubule orientation was spread fairly evenly in all angle classes 60° on either side of the transverse axis, although there still were relatively few microtubules oriented in longitudinal directions. Cortical arrays were disorganized in interphase cells in the division zone and throughout the elongation zone. We found no significant differences in microtubule patterns between trichoblasts and atrichoblasts at the restrictive temperature (data not shown). Organization of mor1-1 cortical microtubules did not appear to worsen over the course of longer incubations at 29°C, and we still observed similar short and misaligned cortical microtubules after 48 h (data not shown).
Figure 2.
Quantitative Analysis of Apparent Microtubule Lengths and Orientations in mor1-1 and the Wild Type after 4 h at 29°C.
Measurements were taken from immunofluorescence micrographs in the middle region of the elongation zone. For each treatment, 500 microtubules from epidermal cells in six different roots were measured. Data are displayed as frequency distribution histograms. MT, microtubule; WT, wild type.
(A) Wild-type microtubule length.
(B) Wild-type microtubule orientation.
(C) mor1-1 microtubule length.
(D) mor1-1 microtubule orientation.
mor1-1 Roots Twist and Swell without the Loss of Transverse Microfibril Orientation
We previously described how mor1-1 root tips undergo left-handed twisting and then radial swelling during the first 2 days at restrictive temperature (Whittington et al., 2001). In this study, we investigated whether cellulose microfibril patterns undergo changes similar to those observed for cortical microtubules during the first 24 h at restrictive temperature, when growth converts from strictly longitudinal to left-handed twisting and finally to radial swelling. Using the same correlative procedure described previously (Sugimoto et al., 2000), we determined the relative elemental growth rates along root tips by measuring the displacement of carbon grains over the 1-h period before root tips were fixed for the analysis of cellulose microfibrils.
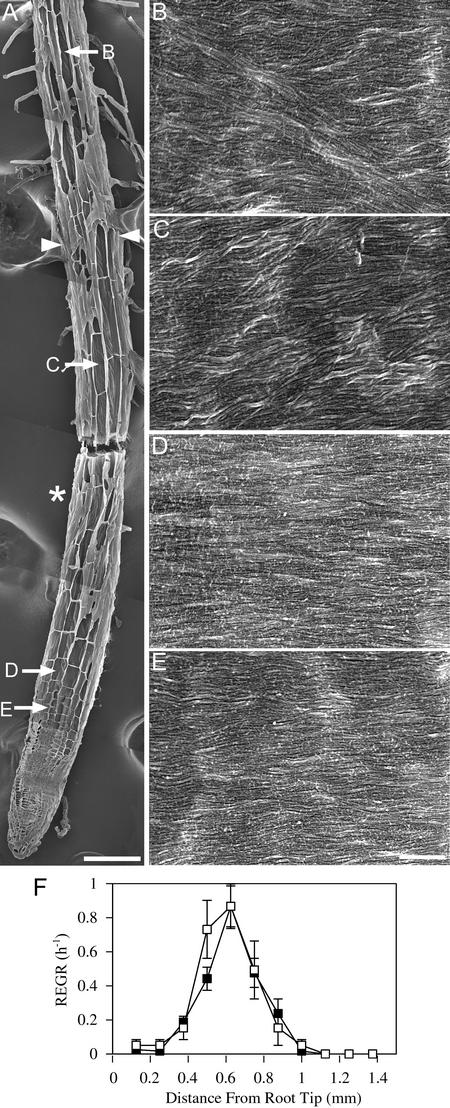
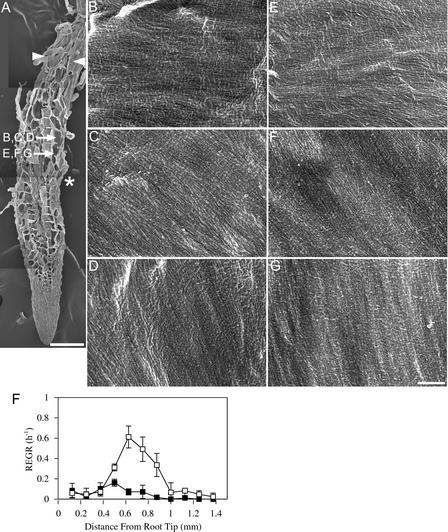
Figures 3, 4, and 5 show mor1-1 root tips at 21°C and after 8 and 24 h at 29°C, respectively. In each case, a low-magnification image of a representative root from each treatment is shown (Figures 3A, 4A, and 5A) together with the locations of the cells whose walls are seen at higher magnifications in Figures 3B to 3E, 4B to 4E, and 5B to 5E. These root tips were prepared for field emission scanning electron microscopy by planing away one surface of the root and then extracting the cytoplasmic contents to expose the most recently deposited layer of cellulose microfibrils. Growth profiles comparing the elongation rates of mor1-1 and the wild type at 21°C and after 8 and 24 h at 29°C also are provided (Figures 3F, 4F, and 5F). As we reported previously (Sugimoto et al., 2000), the most recently deposited cellulose microfibrils of elongating wild-type root epidermal cells were predominantly transverse at 21°C. We found that this also was the case for wild-type roots grown at 29°C (data not shown) and for mor1-1 roots grown at 21°C (Figures 3C to 3E). At 21°C, the elongation zones of mor1-1 and wild-type roots were ∼1 mm long, with the maximum relative elemental growth rate (or strain) of 0.8/h measured at 0.6 mm from the root tip. As with wild-type roots (Sugimoto et al., 2000), we observed some oblique microfibrils, but only in cells that had finished elongating (Figure 3B).
Figure 3.
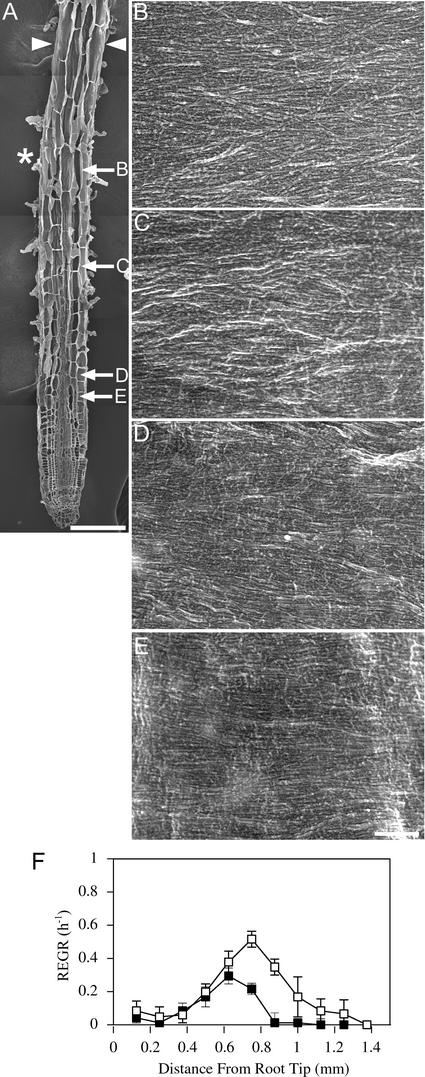
Growth Patterns and Cellulose Microfibril Orientations in mor1-1 Roots Grown at 21°C Are Indistinguishable from Those of the Wild Type.
(A) Field emission scanning electron micrograph of a 5-day-old mor1-1 root tip grown at 21°C. The root tip was cryo-planed to expose the inner tangential and radial walls of many epidermal cells. The asterisk marks the approximate point where the maximum relative elemental growth rate was measured in equivalent roots. Arrowheads show the limits of the elongation zone. The locations of the higher magnification micrographs shown in (B) to (E) are indicated. Bar = 100 μm.
(B) to (E) Higher magnification micrographs showing detailed cellulose microfibril texture from cells in the postelongation zone (B), the late elongation zone (C), the early elongation zone (D), and the cell division zone (E). Bar = 300 nm.
(F) Relative elemental growth rates (REGR) measured over a 1-h period for 5-day-old mor1-1 (closed squares) and wild-type (open squares) root tips show that mor1-1 and the wild type have very similar growth profiles at 21°C.
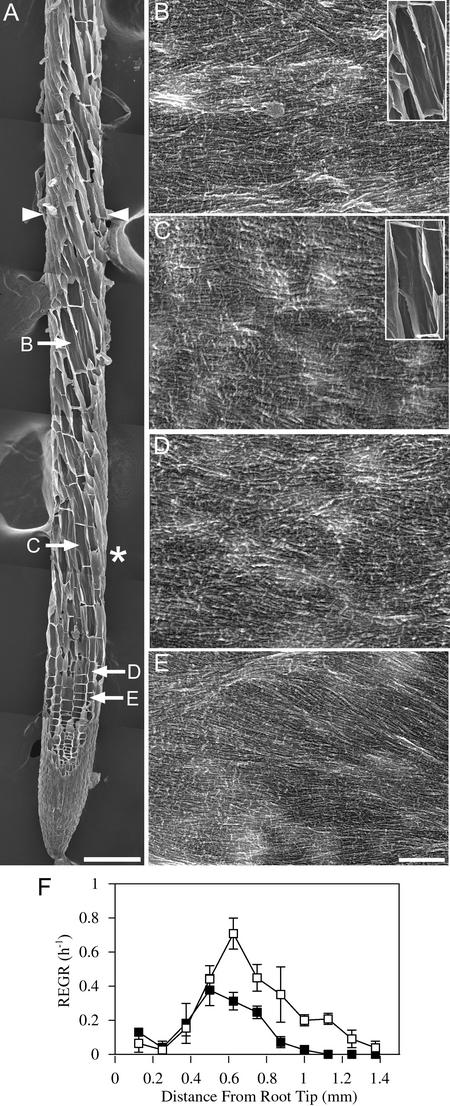
Figure 4.
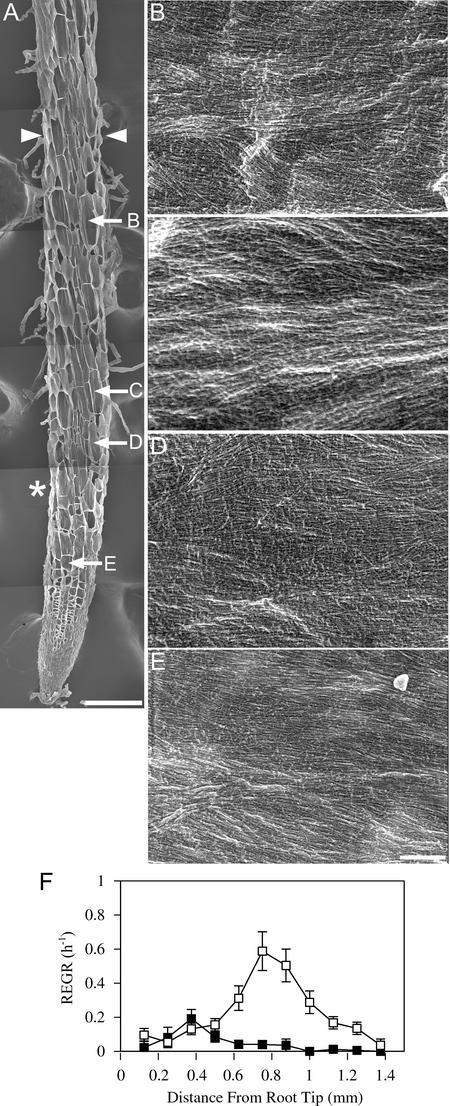
After 8 h at 29°C, Cellulose Microfibrils in mor1-1 Roots Remain Predominantly Transverse to the Root Long Axis, Despite Left-Handed Twisting and Reduced Elongation Rates.
(A) Field emission scanning electron micrograph of a 5-day-old mor1-1 root tip after 8 h of growth at 29°C. The boundary of the elongation zone (arrowheads) and the points from which the higher magnification images ([B] to [E]) were taken are indicated. Left-handed cell file twisting initiates in the late elongation zone, after the point at which maximum relative elemental growth rates were recorded (asterisk). Bar = 100 μm.
(B) to (E) Higher magnification micrographs showing detailed cellulose microfibril arrangement in cells from the late elongation zone (B), near the point of maximum relative elemental growth rate (C), in the early elongation zone (D), and in the cell division zone (E). Microfibril orientation remains transverse to the root's long axis. Insets in (B) and (C) illustrate how the microfibril orientation is not perpendicular to the long axis of the twisting cell files. Bar = 300 nm.
(F) Relative elemental growth rates (REGR) measured over a 1-h period after 7 h at 29°C for mor1-1 (closed squares) and wild-type (open squares) root tips. Compared with those of the wild type, mor1-1 elongation rates are lower and the elongation zone is shorter.
Figure 5.
After 24 h at 29°C, Cellulose Microfibrils in mor1-1 Roots Remain Predominantly Transverse to the Root Long Axis.
(A) Field emission scanning electron micrograph of a 5-day-old mor1-1 root tip after 24 h of growth at 29°C. The boundary of the elongation zone is indicated by arrowheads, and arrows show the points from which the higher magnification images ([B] to [E]) were taken. Left-handed cell file twisting is still detected in the late elongation zone, but the point of maximum recorded relative elemental growth rate (asterisk) is now closer to the root tip apex. Bar = 100 μm.
(B) to (E) Higher magnification micrographs showing detailed cellulose microfibril texture from cells in the late elongation zone (B), near the point of maximum relative elemental growth rate ([C] and [D]), and in the early elongation zone (E). Microfibril orientation remains transverse to the root's long axis. Bar = 300 nm.
(F) Relative elemental growth rates (REGR) measured over a 1-h period after 23 h at 29°C for mor1-1 (closed squares) and wild-type (open squares) root tips.
Within 8 h of shifting the temperature to 29°C, there were significant changes in both the growth rate and the orientation of cell files of mor1-1 root tips (Figure 4). The mor1-1 elongation zone remained ∼1 mm in length, whereas the wild-type root elongation zone increased to an average of 1.3 mm (Figure 4F). In mor1-1, the maximum relative elemental growth rate of 0.4/h was half of that recorded at the permissive temperature and occurred slightly closer to the root tip. Radial swelling was not yet obvious at the single cell level, although root diameters were slightly enlarged after 6 h and significantly enlarged after 12 h (Figure 11D). Left-handed file twisting, first observed ∼4 h after the temperature shift, was well developed by 8 h. Twisting was evident in the region of the elongation zone farthest from the tip, in which elongation rates were in decline, but it was not detected in the cell division zone or the early elongation zone, where elongation rates were increasing. Despite the severe cortical microtubule disruption that commenced 8 h earlier, cellulose microfibrils remained well ordered and predominantly transverse to the longitudinal axis of the root in the late elongation (Figure 4B), rapid elongation (Figure 4C), early elongation (Figure 4D), and cell division (Figure 4E) phases. Despite the oblique orientation of the cell files in the late elongation zone, microfibrils remained transversely oriented relative to the root's long axis (Figures 4B and 4C). When the directional handedness of the cell files is accounted for, these transverse microfibril patterns were arranged in a left-handed (S-form) helical pattern relative to the major axis of cell expansion.
Figure 11.
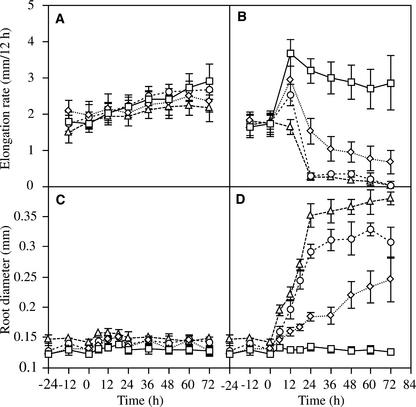
mor1-1 and rsw1-1 Morphological Phenotypes Are Additive.
Root elongation rates ([A] and [B]) and root diameters ([C] and [D]) were measured every 12 h for wild-type (squares), mor1-1 (diamonds), rsw1-1 (circles), and mor1-1 rsw1-1 (triangles) seedlings.
(A) Root elongation rates at 21°C. There is a gradual increase in elongation rates over time for all genotypes.
(B) Root elongation rates after transfer from 21 to 29°C at time 0. In response to the higher culture temperature, there is a transient increase in elongation rates in the wild type and, to a lesser extent, the mor1-1 and rsw1-1 mutants. By 72 h, wild-type elongation rates are similar to those measured in 21°C-grown roots but greatly diminished in mor1-1, rsw1-1, and the double mutant.
(C) Root diameters at 21°C remain constant for all genotypes.
(D) Root diameters after transfer from 21 to 29°C at time 0. Root diameter in the wild type remains unchanged but is increased greatly in all mutants. The increased root diameter of mor1-1 and rsw1-1 is additive in the double mutant.
By 24 h, elongation of mor1-1 cells was reduced significantly and radial swelling was evident (Figures 5A and 5F). Root hairs emerged much closer to the root tip apex from cells that had undergone little elongation during their expansion phase (Figure 5A). Some mature cells were at least as wide as they were long (Figure 5A). Even in such swollen cells, however, cellulose microfibrils were transverse to the long axis of the root, and no random network or even partial disorder was observed (Figures 5B to 5D). Cells in the division zone and cells starting to expand also were examined, but they too had well-aligned cellulose microfibrils (Figure 5E).
Quantitative Analysis of Cellulose Microfibril Alignment
We quantified the angular deviation of cellulose microfibrils mid way along the elongation zones in wild-type and mor1-1 roots at permissive and restrictive temperatures. Figure 6 shows that there were no significant differences in the deviation of cellulose microfibrils from the root long axis. These results demonstrate that the complete loss of cortical microtubules does not quickly cause any major disordering of cellulose microfibrils and that some other events triggered by the loss of microtubules are responsible for inducing twisting and reducing growth anisotropy.
Figure 6.
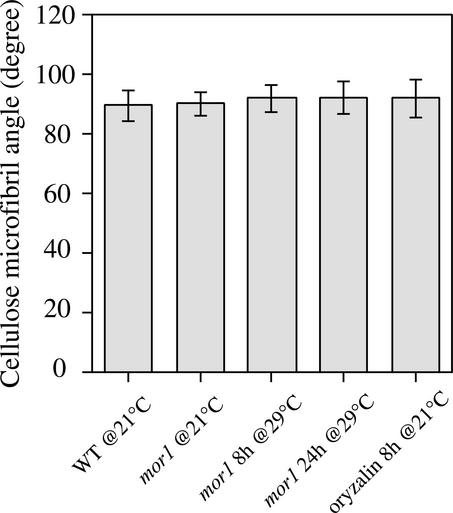
Cellulose Microfibrils Remain Transversely Oriented in the mor1-1 Mutant after 8 h of Treatment with 1 μM Oryzalin.
Quantitative analysis shows the mean orientation of cellulose microfibrils relative to the root long axis. Values for each treatment are means of 300 cellulose microfibrils, measured from the elongation zones of three roots, with standard deviations indicated by error bars. CMF, cellulose microfibril; WT, wild type.
Complete Depolymerization of Microtubules with Oryzalin Does Not Impair Parallel Cellulose Microfibril Orientation
From the analysis of the mor1-1 phenotype, it is clear that short, misaligned cortical microtubules do not cause equivalent disorder to cellulose microfibrils. To test the possibility that these short microtubule polymers are sufficient to maintain normal microfibril synthesis and orientation, we treated wild-type root tips with oryzalin to completely eliminate cortical microtubules. Unlike in the mor1-1 mutant, in which the preprophase band, spindle, and phragmoplast microtubules appear intact at restrictive temperature (Whittington et al., 2001), oryzalin and other microtubule drugs disassembled all microtubule arrays and quickly arrested cell division. Therefore, drug studies are limited to short-term treatments because, although drugs cause rapid loss of growth anisotropy, they also distort division planes and stop cell production, leading to root growth cessation within 24 h.
We used 1 μM oryzalin to depolymerize all cortical microtubules, a concentration shown previously to remove all microtubules detectable at the light microscope level in roots of Arabidopsis growing in agar culture (Baskin et al., 1994). Within the first hour of drug application, almost all microtubules disappeared from both tangential and radial walls (data not shown), and by 8 h, cell elongation was impaired (Figure 7F). By marking equivalent oryzalin-treated roots with carbon grains to identify the expansion zones, we found that the elongation zone was significantly shorter and the maximum relative elemental elongation rate was reduced considerably after 8 h of treatment (Figure 7F). Anisotropic expansion also was reduced. Cellulose microfibrils in cells at various developmental stages, from mitotic through differentiation, remained predominantly transverse (Figures 7B to 7E). Quantitative analysis of the angular deviation of cellulose microfibrils in 8-h oryzalin-treated cells (Figure 6) showed no significant changes from equivalent wild-type cells.
Figure 7.
Cellulose Microfibril Orientation Is Not Altered in Wild-Type Root Epidermal Cells Treated with Oryzalin for 8 h.
(A) Field emission scanning electron micrograph of a 5-day-old wild-type root tip after 8 h of treatment with 1 μM oryzalin. The root tip was cryo-planed to expose the inner cell walls for examination. The boundary of the elongation zone (arrowheads) and the point of maximum recorded relative elemental growth rate (asterisk) are indicated, as are the points from which the higher magnification images ([B] to [E]) were taken. Bar = 100 μm.
(B) to (E) Cellulose microfibril texture from cells near the point of maximum relative elemental growth rate (B), from the elongation zone (C), and from the early elongation zone ([D] and [E]). Microfibril orientation remains transverse to the root's long axis at all sites. Bar = 300 nm.
(F) Relative elemental growth rates (REGR) measured over a 1-h period at 7 h after the application of 1 μM oryzalin (closed squares) or for equivalent untreated roots (open squares).
After 24 h of oryzalin treatment, radial swelling was more extreme (Figure 8A). Roots grew only slightly in length but expanded extensively in the radial axis, forming nearly isodiametric cells. With aberrant division planes and significant radial swelling, it was difficult to distinguish one axis from another. Cellulose microfibrils remained well ordered locally but showed considerable variation within individual cells, shifting from transverse to oblique to longitudinal within the same cell (Figures 8B to 8G). Microfibril orientation changed gradually, and sudden shifts from one orientation to another were not observed. This patchwork pattern of cellulose microfibrils was observed in cells throughout the root tip.
Figure 8.
Prolonged Disassembly of Cortical Microtubules by Oryzalin Results in the Loss of Overall, but Not Local, Alignment of Cellulose Microfibrils.
(A) Field emission scanning electron micrograph of a 5-day-old wild-type root tip after 24 h of treatment with 1 μM oryzalin. The radially swollen root tip was cryo-planed to expose the inner cell walls for examination. The boundary of the elongation zone (arrowheads) and the point of maximum recorded relative elemental growth rate (asterisk) are indicated, as are the points from which the higher magnification images ([B] to [G]) were taken. Bar = 100 μm.
(B) to (G) Cellulose microfibril texture from two isodiametric cells in the elongation zone. Although locally parallel, microfibrils have variable orientations. Bar = 300 nm.
(F) Relative elemental growth rates (REGR) measured over a 1-h period 23 h after the application of 1 μM oryzalin (closed squares) or for equivalent untreated roots (open squares). Compared with those of wild-type roots, the elongation of oryzalin-treated roots is reduced and the elongation zone is shortened.
Taxol Generates Radial Swelling and Arrests Cell Division, but Cellulose Microfibrils Remain Locally Parallel with Variable Orientations
We also perturbed microtubule function by treating seedlings with taxol. Taxol promotes the polymerization of tubulin and thus stabilizes microtubules (Morejohn and Fosket, 1991; Bokros et al., 1993), and this causes radial swelling in Arabidopsis root tips despite cortical microtubules remaining transversely aligned (Baskin et al., 1994). We found that microfibrils in root epidermal cells treated with 10 μM taxol, like those after prolonged oryzalin treatment, were locally parallel but variably oriented (see supplemental data online).
Cellulose Content Is Normal in mor1-1 Mutants at the Restrictive Temperature
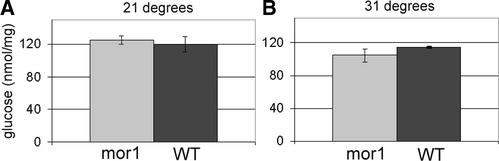
To verify that cellulose synthesis was not reduced in mor1-1, we compared cellulose content in mor1-1 and wild-type seedlings grown at 21 and 31°C. At 31°C, the mor1-1 morphological phenotype was manifested more rapidly than at 29°C. To maximize the amount of cell wall material produced at the restrictive temperature, we grew seedlings for 2 days at 21°C and then for 5 days at 31°C. The amount of cellulose in these seedlings was estimated from the glucose contents in trifluoroacetic acid–insoluble fractions as measured by gas chromatography–mass spectrometry. As shown in Figure 9, mor1-1 seedlings grown at 31°C produced very similar amounts of cellulose as the wild type at 31°C or mor1-1 grown at 21°C. This finding confirms that cellulose synthesis did not stop in mor1-1, so the transverse cellulose microfibrils found at restrictive temperatures were deposited during cortical microtubule disorganization and the radial swelling observed in the mor1-1 mutant was not caused by changes in cellulose synthesis.
Figure 9.
Cellulose Content Is Not Altered in mor1-1 at the Restrictive Temperature.
Cell wall extracts from whole seedlings were fractionated and derivatized, and the glucose content (nmol glucose/mg dry weight) in the trifluoroacetic acid–insoluble cellulose fraction was determined by gas chromatography–mass spectrometry. Data are means from three replicate experiments, with standard deviations indicated by error bars.
(A) Cellulose contents measured in mor1-1 and wild-type (WT) seedlings grown at 21°C for 7 days are not significantly different.
(B) Cellulose content in seedlings grown at 21°C for 2 days followed by 5 days at 31°C. At 31°C, mor1-1 and wild-type seedlings produce similar amounts of cellulose.
The mor1-1 rsw1-1 Double Mutant Phenotype Is Additive
The analysis described thus far demonstrated that the mor1-1 mutant neither disordered cellulose microfibrils nor inhibited cellulose synthesis. We hypothesize that mor1-1 reduces growth anisotropy by a third route. To test this hypothesis, we crossed mor1-1 with the rsw1-1 mutant, which carries a mutant allele of the gene that encodes the cellulose synthase, AtCesA1, and segregated double mutants. rsw1-1 is a temperature-sensitive mutant and, like mor1-1, has a strong phenotype at ∼29°C. At the restrictive temperature, rsw1-1 mutants synthesize less cellulose than normal (Arioli et al., 1998; Peng et al., 2000) and microfibrils lose their orientation after 8 h and their structure after ∼24 h (Sugimoto et al., 2001). Thus, the rsw1-1 mutant probably reduces growth anisotropy both by inhibiting cellulose synthesis and through the loss of cellulose microfibril orientation. Our rationale for this analysis was that if the mor1-1 mutant inhibits growth anisotropy by a third means, then the double mutant phenotype should be additive.
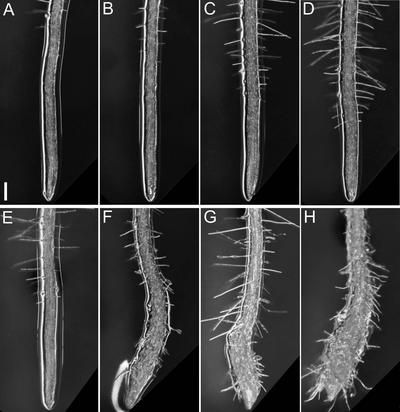
Figure 10 compares root tip morphologies of the wild type (Columbia) and mor1-1, rsw1-1, and mor1-1 rsw1-1 mutants after 7 days at 21°C (Figures 10A to 10D) or 2 days after shifting the culture temperature to 29°C at day 5 (Figures 10E to 10H). We also monitored root elongation and diameter for seedlings grown continuously at 21°C (Figures 11A and 11C) and in seedlings shifted from 21 to 29°C at 5 days after germination (Figures 11B and 11D). At 21°C, growth and morphology were very similar in all genotypes, although elongation rates were slightly lower and diameters were greater in rsw1-1 and the mor1-1 rsw1-1 double mutant (Figures 11A and 11C). In the temperature-shifted seedlings, morphology was unchanged in the wild type (Figure 10E), but the higher temperature stimulated increased elongation rates, with a sharp increase during the first few hours followed by a return to more moderate rates (Figure 11B). The mor1-1 and rsw1-1 single mutants also recorded transient increases in elongation rates (Figure 11B). Carbon grain displacement indicated that the growth surge occurred only during the first few hours, and by 8 h, the elongation rates had subsided (Figure 4). By 24 h, elongation rates in both mutants (Figure 11B) were below the rates recorded at the permissive temperature (Figure 11A).
Figure 10.
Temperature-Dependent Morphological Phenotypes of mor1-1, rsw1-1, and mor1-1 rsw1-1 Double Mutant Root Tips.
(A) Wild type after 7 days at 21°C.
(B) mor1-1 after 7 days at 21°C.
(C) rsw1-1 after 7 days at 21°C.
(D) mor1-1 rsw1-1 double homozygote after 7 days at 21°C.
(E) Wild type grown at 21°C for 5 days followed by 2 days at 29°C.
(F) mor1-1 grown at 21°C for 5 days followed by 2 days at 29°C.
(G) rsw1-1 grown at 21°C for 5 days followed by 2 days at 29°C.
(H) mor1-1 rsw1-1 double homozygote grown at 21°C for 5 days followed by 2 days at 29°C.
Bar = 100 μm.
As shown in Figures 10D and 10H, the mor1-1 rsw1-1 double mutant roots had normal morphology at 21°C but showed pronounced radial swelling at 29°C. The double mutant combined morphological and growth features of both single mutants. Like mor1-1 (Figure 10F), the mor1-1 rsw1-1 roots bent to the right against the hard agar plate (Figure 10H). Viewed from above, this appears as a bending to the left side of the plate. Root hairs also were noticeably short and crooked in both the mor1-1 and double mutants. Like rsw1-1 (Figure 10G), the double mutant (Figure 10H) had a more dramatic decline in elongation and a more rapid onset of radial swelling than mor1-1 (Figures 11B and 11D). Twenty-four hours after seedlings were transferred to 29°C, the double mutant root diameter had increased to 0.35 ± 0.02 mm, 2.5 times greater than the wild-type root diameter (0.14 ± 0.004 mm). This increase of ∼0.21 mm was approximately additive for the diameter increases measured for mor1-1 (0.05 mm) and rsw1-1 (0.15 mm) (Figure 11D). The additive nature of the mor1-1 rsw1-1 phenotype suggests that the mor1-1 and rsw1-1 phenotypes are generated by independent mechanisms.
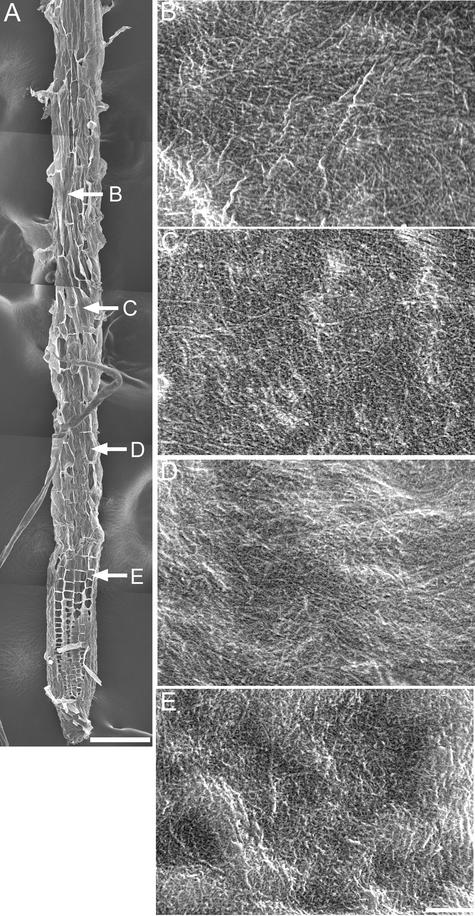
We found that cortical microtubules in the mor1-1 rsw1-1 double mutant resembled those in mor1-1 at the restrictive temperature (see supplemental data online) and that the disordered cellulose microfibrils seen after 8 h at 29°C (Figure 12) closely resembled those observed in the rsw1-1 single mutant after the same treatment (Sugimoto et al., 2001).
Figure 12.
Cellulose Microfibrils in the mor1-1 rsw1-1 Double Mutant Are Disordered after 8 h at 29°C.
(A) Field emission scanning electron micrograph of a 5-day-old mor1-1 rsw1-1 root tip after 8 h of growth at 29°C. The root tip was cryo-planed to expose the inner wall surface for detailed examination at higher magnification at the points indicated by the arrows ([B] to [E]). Bar = 100 μm.
(B) to (E) Higher magnification micrographs showing detailed cellulose microfibril texture. At all points along the root tip, microfibrils show no preferred orientation. Bar = 300 nm.
DISCUSSION
In this study, we demonstrated that cortical microtubules in the mor1-1 mutant became disorganized several hours before root twisting and radial swelling, consistent with a causal relationship. Nevertheless, microtubule disruption did not cause the misalignment of cellulose microfibrils or reduce cellulose synthesis levels. Similarly, drug treatments that either eliminated or stabilized cortical microtubules did not alter the parallel order of cellulose microfibrils. These findings are in contrast to the cellulose synthase constraint model, which predicts that changes in microtubule orientation should alter cellulose microfibril alignment. Our results suggest that cortical microtubules control growth anisotropy by some mechanism other than the alignment or synthesis of cellulose microfibrils, although they do not exclude the possibility that cortical microtubules have some function in microfibril alignment not seen under these conditions. The results also provide further evidence that the alignment of cellulose microfibrils in primary walls is controlled by a self-ordering mechanism that is largely dependent on the rate of cellulose synthesis, in agreement with our previous work (Sugimoto et al., 2001). The ensuing discussion assesses the implications of our results for the mechanisms by which cortical microtubules regulate growth anisotropy.
How Do Microtubules Control Growth Anisotropy?
The cellulose synthase constraint model suggests that cortical microtubules direct the orientation of newly deposited cellulose microfibrils and thus determine the direction of cell expansion (Giddings and Staehelin, 1991; Wasteneys, 2000; Baskin, 2001). Unfortunately, this model has gained popularity without rigorous testing of both microtubule and cellulose microfibril patterns in the same or directly equivalent cells. Sporadic research articles have suggested that microtubule orientation does not always predict the alignment of cellulose microfibrils (Okuda and Mizuta, 1987; Wilms et al., 1990; reviewed extensively by Baskin, 2001) or that microtubules can receive cues from cellulose microfibrils to orient transversely in elongating cells (Fisher and Cyr, 1998). We showed previously that in the Arabidopsis root epidermis, precise transverse alignment of both cortical microtubules and cellulose microfibrils exists during the most rapid phase of elongation (Sugimoto et al., 2000). Cellulose microfibrils already are aligned transversely in the cell division zone, where cortical microtubules are less well aligned, and they remain transverse throughout the elongation zone, suggesting that cellulose microfibrils may establish transverse patterns before microtubules become tightly transverse in rapidly elongating cells (Sugimoto et al., 2000).
Taking advantage of mor1-1's temperature-inducible phenotype, we set out to determine if the loss of organized cortical microtubules causes equivalent misalignment of cellulose microfibrils before detectable morphological changes occur. We concentrated on early events that lead to radial swelling and reduced elongation and found that the cellulose microfibrils remained predominantly transverse in mor1-1 after 24 h at 29°C and in wild-type cells treated with oryzalin for 8 h. We can discount the possibility that what we found were transverse cellulose microfibrils deposited before microtubule disruption in mor1-1 because misaligned microfibrils are deposited in the rsw1 mutant after 8 h at its restrictive temperature (Sugimoto et al., 2001), even though rsw1 shows strongly reduced cellulose synthesis (Arioli et al., 1998; Peng et al., 2000). Cellulose continued to be synthesized at normal levels in the mor1-1 mutant. Earlier studies using colchicine and other microtubule-depolymerizing agents have demonstrated that the complete loss of cortical microtubules also does not affect cellulose synthesis (Marx-Figini, 1970; Srivastava et al., 1976; Shibaoka and Hogetsu, 1977).
It was reported recently that the fra2 mutation affects cortical microtubule organization (Burk et al., 2001) and that this leads to the misorientation of microfibrils in a variety of organs, including hypocotyls, petioles, and stems (Burk and Ye, 2002). The authors of that study concluded that their results strongly support the hypothesis that cortical microtubules regulate the oriented deposition of cellulose microfibrils. Several aspects of the fra2 phenotype, however, need to be taken into account before this conclusion can be justified. First, because the fra2 and other katanin p60 mutants are likely to be null alleles and generate strong constitutive and pleiotropic phenotypes, it is difficult to assign causal relationships between cortical microtubules, cellulose microfibrils, and growth anisotropy after long periods with disrupted microtubules. Second, unlike the mor1-1 mutant, fra2 mutants have a 20% reduction in cellulose as well as a 20 to 30% reduction in rhamnose, arabinose, and xylose levels (Burk et al., 2001). Altered wall composition is correlated similarly with disordered cellulose microfibrils in rsw1 (Sugimoto et al., 2001) and kobito1 (Pagant et al., 2002) without any changes to microtubules. Thus, it remains possible that microfibril misalignment in fra2 is caused by altered wall biosynthesis.
Our results also suggest but do not prove that the well-ordered cellulose microfibrils produced after microtubule disruption did not use the preexisting transverse microfibrils as a template (Baskin, 2001). We estimated previously that it takes a cell ∼8 h to transit the elongation zone (Sugimoto et al., 2000). All cells in the elongation zone after 24 h of microtubule disruption would have been in the cell division zone when cortical microtubule disruption occurred. It seems unlikely that the setting up of transverse microfibril templates at such an early stage of cell development would be sufficient to sustain transverse microfibril deposition through successive cell divisions and expansion. Recent results (R. Himmelspach, R.E. Williamson, and G.O. Wasteneys, unpublished data) suggest that templated incorporation, as postulated by Baskin (2001), does not explain the long-term maintenance of cellulose microfibril alignment.
Transverse Cellulose Microfibrils Are Necessary but Not Sufficient to Maintain Growth Anisotropy
Despite the fact that microfibrils were maintained in the transverse orientation in mor1-1, morphological defects developed, including root twisting and swelling. This finding suggests that although maintaining transverse microfibrils is necessary, it is not sufficient to support the highly anisotropic growth of Arabidopsis root cells. It has been demonstrated that ordered cellulose microfibrils do not always determine the direction of cell expansion (Baskin, 2001). Wiedemeier et al. (2002) also recently reported that two Arabidopsis mutants, rsw4 and rsw7, exhibit severe root radial swelling, although no obvious changes in cortical microtubule or cellulose microfibril patterns were seen. Hence, even when microfibrils remain transversely aligned, defects in other wall components can reduce growth anisotropy. The cellulose-hemicellulose network is the major load-bearing element that dominates the mechanical strength of the wall (Whitney et al., 1999), but recent work suggests that the pectin network also is mechanically important, possibly modifying the hydration status of cellulose (Wilson et al., 2000).
Among various pectin molecules, the mechanical importance of rhamnogalactan II was revealed by the rescue of the Arabidopsis fucose biosynthesis mutant mur1 by boron (O'Neill et al., 2001). McCartney et al. (2003) recently demonstrated that pectic (1-4)-β-d-galactan is abundant at the onset of rapid elongation growth in Arabidopsis roots. The root epidermal bulger1/root hair defective1 mutant is mutated in a UDP-d-glucose 4-epimerase–encoding gene, and its phenotype suggests that xyloglucans as well as arabinogalactan proteins help control growth anisotropy (Andeme-Onzighi et al., 2002; Seifert et al., 2002). Although our current study shows that cortical microtubule orientation does not directly control the orientation of cellulose microfibrils, microtubules could influence the deposition, formation, and/or rearrangement of other polysaccharides or influence the secretion or activity of wall proteins. If polysaccharide and protein secretion proves to be microtubule dependent, microtubule coalignment with cellulose microfibrils could be a requirement for such processes to work efficiently.
The Rate of Cellulose Synthesis May Be a Component of a Self-Ordering Mechanism for Parallel Microfibril Alignment
One intriguing finding from this study is that cellulose microfibrils can largely self-align in the absence of functional cortical microtubules in both mor1-1 and drug-treated cells. Even after cortical microtubules were depolymerized completely with oryzalin, cellulose microfibrils remained transverse for at least 8 h and maintained local parallel order for 24 h, when cells were swollen severely. Similarly, cellulose microfibrils retained local parallel order in the presence of taxol, a drug that prevents microtubule disassembly. The incomplete loss of cellulose microfibril alignment by microtubule-targeted drug treatment has been reported during primary wall formation in pine seedlings (Itoh, 1976; Itoh and Shimeji, 1976), lettuce hypocotyls (Srivastava et al., 1976), adzuki bean epicotyls (Takeda and Shibaoka, 1981), cotton hairs (Yatsu and Jacks, 1981), maize seedlings (Mueller and Brown, 1982), and bean suspension culture cells (Weedenburg and Seagull, 1987).
In the absence of constraint by cortical microtubules, what mechanism can account for the parallel alignment of microfibrils? We showed previously that the local parallel order of cellulose microfibrils is lost when cellulose synthesis is inhibited in the rsw1 mutant or in the wild type by application of the herbicide 2,6-dichlorobenzonitrile (Sugimoto et al., 2001). Reduced cellulose levels also are correlated with altered microfibril patterns in fra2 (Burk and Ye, 2002) and kobito1 (Pagant et al., 2002). These results suggest that sufficient cellulose production is required to coalign cellulose microfibrils as they are deposited, consistent with the model of Emons and Mulder (Emons and Mulder, 1998, 2000; Mulder and Emons, 2001). It also is possible that other components of the cell wall, including polysaccharides and proteins, contribute to the organization of cellulose microfibrils and the tensile properties of cells. In vitro work on cellulose films made by the bacterium Acetobacter xylinus have demonstrated that xyloglucans and pectins play some role in aligning cellulose microfibrils and thus may influence wall mechanical properties in plant cells (Whitney et al., 1995, 1999; Chanliaud and Gidley, 1999; Chanliaud et al., 2002). Whether this also applies in vivo remains to be investigated.
mor1-1 Cellulose Microfibrils Form a Left-Handed Helix That Does Not Correspond to the Handedness of Organ Twisting
The first morphological change detected in mor1-1 roots at the restrictive temperature is left-handed helical growth, or twisting (Whittington et al., 2001). Left-handed twisting can be generated in Arabidopsis roots by applying low concentrations of microtubule-specific drugs, including propyzamide and taxol (Furutani et al., 2000), and occurs constitutively in two α-tubulin (lefty) mutants with single amino acid substitutions (Thitamadee et al., 2002). Right-handed organ twisting is observed when the expression of either a green fluorescent protein (GFP)–α-tubulin or microtubule binding domain of mouse MAP4 (MBD-GFP) is driven by the 35S promoter of Cauliflower mosaic virus (Hashimoto, 2002) or in the spr1 and spr2 mutants, whose drug sensitivities suggest some changes in microtubule dynamic properties (Furutani et al., 2000). Collectively, these observations suggest a strong link between microtubule organization, microtubule assembly, and the maintenance of directional organ growth. However, it should be noted that other mutants that alter microtubule organization (Traas et al., 1995; Bichet et al., 2001; Burk et al., 2001; Webb et al., 2002) do not generate organ twisting. Therefore, any connection between microtubule organization and twisting is likely to be complex.
Right-handed helically oriented cortical microtubule arrays are observed in the lefty mutants (Thitamadee et al., 2002) or after treatment with low concentrations of propyzamide (Furutani et al., 2000). Conversely, left-handed helically oriented cortical microtubule arrays have been observed in the spiral1 mutant (Furutani et al., 2000). Hashimoto and coworkers have hypothesized that the handedness of organ twisting could reflect microtubule-dependent skewing of cellulose microfibril orientation (Furutani et al., 2000; Thitamadee et al., 2002). However, this hypothesis does not account for the lack of helically oriented microtubule arrays in the mor1-1 mutant or in taxol-treated or transgenic lines overexpressing the GFP–α-tubulin or MBD-GFP fusion proteins (Hashimoto, 2002). Furthermore, the present data clearly show no link between the disordered cortical microtubules in mor1-1 and the helical handedness of cellulose microfibrils likely to predict the handedness of root twisting. To generate a shift in the growth direction to account for the left-handed twisting in mor1-1, cellulose microfibril patterns would be expected to form a right-handed helical pattern relative to the long axis of the epidermal cells. In fact, we found that the microfibrils were arranged in shallow, left-handed helices. Perhaps more importantly, cortical microtubules remained transverse to the root's long axis, suggesting that the microfibrils may have passively realigned as a result of root twisting.
Organ twisting should involve unbalanced mechanical properties of cell walls at the individual cell level or the tissue level, but this is an area that has not been investigated extensively. Studies of the filamentous alga Chaetomorpha (Frei and Preston, 1961) and the giant internodal cells of the alga Nitella opaca (Probine, 1963) showed that in these cylindrical cell systems, cell twisting is correlated with the stretching of the helix formed by cellulose microfibrils in response to turgor-driven expansion. (Incidentally, Wasteneys and Williamson [1987] found no correlation between the mean cortical microtubule orientation angle and the spiral growth pattern of Nitella cells.) It remains to be determined if this relationship applies to the more complex, multilayered tissue systems found in higher plant organs.
MOR1 and Cortical Microtubule Organization
Mutational analysis of the MOR1 microtubule-associated protein (Whittington et al., 2001) is helping to generate ideas on how transverse cortical microtubule arrays are established and maintained in elongating plant cells (Wasteneys, 2002). The knowledge that single amino acid substitutions in an N-terminal HEAT repeat–encoding region of the MOR1 gene can generate temperature-dependent disorganization of cortical microtubules (Whittington et al., 2001) offers some clues to MOR1's interactions with cortical microtubules. It has been suggested that MOR1 might stabilize microtubules either by modulating the access to microtubules of catastrophe-generating kinesins (Hussey et al., 2002; Wasteneys, 2002) or by selectively stabilizing cortical microtubules that are oriented transversely by linking them to integral plasma membrane proteins (Wasteneys, 2002).
A third plausible mechanism to explain microtubule stabilization is microtubule-microtubule cross-linking by MOR1. A recent in vitro study suggests that the MOR1 homolog from tobacco, isolated from phragmoplasts, can cross-link microtubules (Yasuhara et al., 2002). Is it possible that the loss of MOR1-dependent cross-bridging could account for the apparent reduction in length and increased dispersal about the transverse axis of microtubules in mor1-1 at the restrictive temperature? Paul Green's self-cinching loop hypothesis suggests that the degree of microtubule overlap can regulate cortical microtubule orientation. According to this model, maximizing microtubule overlap will force a microtubule array to encircle a cell in the shortest (i.e., transverse) dimension (Green, 1980). Drugs that prevent tubulin polymerization by binding to tubulin monomers not only shorten cortical microtubules but, at substoichiometric concentrations, also may disturb their transverse orientation (Thitamadee et al., 2002). Thus, ensuring a balanced rate of tubulin polymerization to maintain a minimum microtubule length may be required, along with selective stabilization by cross-linking, to generate a transverse microtubule array. Excessive microtubule turnover could reduce the effectiveness of mechanisms that selectively stabilize transverse cortical microtubules. Ongoing studies by our group will establish the relative stability of cortical microtubules in the mor1-1 mutant at the restrictive temperature.
METHODS
Plant Material and Growth Conditions
Arabidopsis thaliana cv Columbia wild type and the mor1-1 (Whittington et al., 2001) and rsw1 (Arioli et al., 1998) mutants were used in this study. Seedlings were grown on nutrient-solidified agar plates as described (Sugimoto et al., 2000). To induce the conditional phenotypes of mor1-1 and rsw1, 5-day-old seedlings were transferred from a 21°C growth chamber to a 29°C growth chamber with otherwise identical settings. mor1-1 rsw1 double mutant lines were isolated from the F2 population of the mor1-1 × rsw1-1 crosses. The putative double mutant lines were backcrossed to both mor1-1 and rsw1-1 lines, and F1 progeny were tested for the respective parental phenotypes to verify their double homozygous status.
Measurement of Root Growth
Root elongation rate and diameter were measured as described (Sugimoto et al., 2000). At least 30 roots grown on three different plates were measured to calculate mean elongation rates and diameters with standard deviations.
Immunofluorescence Microscopy
Microtubules were labeled for immunofluorescence microscopy as described (Sugimoto et al., 2000). Fluorescent images were recorded with a confocal laser scanning system (MRC-600; Bio-Rad Microscience Division, Hemel Hempstead, UK), which was coupled to a Zeiss Axiovert IM-10 microscope (Jena, Germany). Projections of optical series as well as rotations to analyze microtubule patterns on radial walls used Confocal Assistant 4.02 (written by Todd Clark Brelje, University of Minnesota, Twin Cities) as described (Sugimoto et al., 2000).
Field Emission Scanning Electron Microscopy
Cellulose microfibrils were visualized with a Hitachi S4500 field emission scanning electron microscope (Tokyo, Japan) as described (Sugimoto et al., 2000). Scanned images were recorded digitally with Image Slave 2.11 (Meeco, Melbourne, Australia).
Quantitative Analysis of Cortical Microtubule and Cellulose Microfibril Orientation
Cortical microtubule length and angular deviation from the main root growth axis were measured from digital z-series projections using NIH Image 2.2 (National Institutes of Health, Bethesda, MD). Microtubule length was defined as the distance between two visible ends. Thus, the measured maximum length was never greater than the cell width, the upper limit of measurement. In the wild type, however, microtubules often were longer than the measured value, because they extend around the corners of the cell. Therefore, these measurements can compare only relative microtubule lengths in wild-type and mutant cells. For the cortical microtubule angular measurement, transverse orientation was defined as 90° to the long axis plotted along the long axis of the root; microtubules aligned at angles between 0 and 90° (counterclockwise from the top) would form a left-handed helix, whereas those oriented between 90 and 180° would form a right-handed helix. A total of 500 fluorescent linear structures from six different roots were measured, and values are presented as frequencies and percentages. All measurements were taken near the point of maximum relative elemental growth rate, approximately halfway through the elongation zone.
The alignment of cellulose microfibrils was measured from digital images obtained by field emission electron microscopy using NIH Image 2.2. Transverse orientation was defined as described above for cortical microtubules. Measurements were made from wall patches (2.4 μm × 1.5 μm) sampled from several adjacent cells halfway along the elongation zone. Values shown in the figures represent means of 300 microfibrils combined from 30 wall patches from three different roots, with standard deviations indicated as error bars.
Oryzalin and Taxol Treatments
Stock solutions of the microtubule-targeted drugs oryzalin (3,5-dinitro-N4,N4-dipropylsulfanilamide; DowElanco, Indianapolis, IN) and taxol (Paclitaxel; Sigma) were prepared at 10 mM in DMSO, filter-sterilized, and added to freshly autoclaved agar nutrient medium. Five-day-old seedlings were transferred to drug-containing or 0.1% DMSO control plates.
Cellulose Content Assay
For cell wall extractions, whole seedlings were grown on Hoagland medium supplemented with 1% glucose on agar plates for either 7 days at 21°C or 2 days at 21°C followed by 5 days at 31°C. For each sample, three separate batches of 130 seedlings were harvested in liquid nitrogen and freeze-dried. Wall polymer extraction and fractionation were performed according to Lane et al. (2001), and glucose contents (nmol/mg plant dry weight) of the trifluoroacetic acid–insoluble cellulose fraction were measured using gas chromatography–mass spectrometry.
Upon request, all novel materials described in this article will be made available in a timely manner for noncommercial research purposes.
Supplementary Material
Acknowledgments
We thank Nori Hasenbein for expert technical assistance, the staff at the Australian National University (ANU) Electron Microscopy Unit for their assistance with field emission scanning electron microscopy, Charles Hocart (ANU) for his assistance with gas chromatography–mass spectrometry, and Tobias Baskin (University of Missouri) and Brian Gunning (ANU) for helpful advice.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.011593.
Footnotes
Online version contains Web-only data.
References
- Andeme-Onzighi, C., Sivaguru, M., Judy-March, J., Baskin, T.I., and Driouich, A. (2002). The reb1-1 mutation of Arabidopsis alters the morphology of trichoblasts, the expression of arabinogalactan-proteins and the organization of cortical microtubules. Planta 215, 949–958. [DOI] [PubMed] [Google Scholar]
- Arioli, T., et al. (1998). Molecular analysis of cellulose biosynthesis in Arabidopsis. Science 279, 717–720. [DOI] [PubMed] [Google Scholar]
- Baskin, T.I. (2001). On the alignment of cellulose microfibrils by cortical microtubules: A review and a model. Protoplasma 215, 150–171. [DOI] [PubMed] [Google Scholar]
- Baskin, T.I., Wilson, J.E., Cork, A., and Williamson, R.E. (1994). Morphology and microtubule organization in Arabidopsis roots exposed to oryzalin or taxol. Plant Cell Physiol. 35, 935–942. [PubMed] [Google Scholar]
- Bichet, A., Desnos, T., Turner, S., Grandjean, O., and Hofte, H. (2001). BOTERO1 is required for normal orientation of cortical microtubules and anisotropic cell expansion in Arabidopsis. Plant J. 25, 137–148. [DOI] [PubMed] [Google Scholar]
- Bokros, C.L., Hugdahl, J.D., Hanesworth, V.R., Murthy, J.V., and Morejohn, L.C. (1993). Characterization of the reversible taxol-induced polymerization of plant tubulin into microtubules. Biochemistry 32, 3437–3447. [DOI] [PubMed] [Google Scholar]
- Burk, D.H., Liu, B., Zhong, R.Q., Morrison, W.H., and Ye, Z.H. (2001). A katanin-like protein regulates normal cell wall biosynthesis and cell elongation. Plant Cell 13, 807–827. [PMC free article] [PubMed] [Google Scholar]
- Burk, D.H., and Ye, Z.H. (2002). Alteration of oriented deposition of cellulose microfibrils by mutation of a katanin-like microtubule-severing protein. Plant Cell 14, 2145–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burn, J.E., Hocart, C.H., Birch, R.J., Cork, A.C., and Williamson, R.E. (2002). Functional analysis of the cellulose synthase genes CesA1, CesA2, and CesA3 in Arabidopsis. Plant Physiol. 129, 797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri, C., Azimzadeh, J., Pastuglia, M., Bellini, C., Grandjean, O., and Bouchez, D. (2002). The Arabidopsis TONNEAU2 gene encodes a putative novel protein phosphatase 2A regulatory subunit essential for the control of the cortical cytoskeleton. Plant Cell 14, 833–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita, N.C., and Gibeaut, D.M. (1993). Structural models of primary cell walls in flowering plants: Consistency of molecular structure with the physical properties of the walls during growth. Plant J. 3, 1–30. [DOI] [PubMed] [Google Scholar]
- Chanliaud, E., Burrows, K.M., Jeronimidis, G., and Gidley, M.J. (2002). Mechanical properties of primary plant cell wall analogues. Planta 215, 989–996. [DOI] [PubMed] [Google Scholar]
- Chanliaud, E., and Gidley, M.J. (1999). In vitro synthesis and properties of pectin/Acetobacter xylinus cellulose composites. Plant J. 20, 25–35. [DOI] [PubMed] [Google Scholar]
- Emons, A.M.C., and Mulder, B.M. (1998). The making of the architecture of the plant cell wall: How cells exploit geometry. Proc. Natl. Acad. Sci. USA 95, 7215–7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emons, A.M.C., and Mulder, B.M. (2000). How the deposition of cellulose microfibrils builds cell wall architecture. Trends Plant Sci. 5, 35–40. [DOI] [PubMed] [Google Scholar]
- Fagard, M., Desnos, T., Desprez, T., Goubet, F., Refregier, G., Mouille, G., McCann, M., Rayon, C., Vernhettes, S., and Hofte, H. (2000). PROCUSTE1 encodes a cellulose synthase required for normal cell elongation specifically in roots and dark-grown hypocotyls of Arabidopsis. Plant Cell 12, 2409–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher, D.D., and Cyr, R.J. (1998). Extending the microtubule/microfibril paradigm: Cellulose synthesis is required for normal cortical microtubule alignment in elongating cells. Plant Physiol. 116, 1043–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei, E., and Preston, R.D. (1961). Cell wall organization and wall growth in the filamentous green algae Cladophora and Chaetomorpha. II Spiral growth and spiral structure. Proc. R. Soc. Bot. 155, 55–81. [Google Scholar]
- Furutani, I., Watanabe, Y., Prieto, R., Masukawa, M., Suzuki, K., Naoi, K., Thitamadee, S., Shikanai, T., and Hashimoto, T. (2000). The SPIRAL genes are required for directional control of cell elongation in Arabidopsis thaliana. Development 127, 4443–4453. [DOI] [PubMed] [Google Scholar]
- Giddings, T.H., and Staehelin, L.A. (1991). Microtubule-mediated control of microfibril deposition: A re-examination of the hypothesis. In The Cytoskeletal Basis of Plant Growth and Form, C.W. Lloyd, ed (San Diego, CA: Academic Press), pp. 85–100.
- Gillmor, C.S., Poindexter, P., Lorieau, J., Palcic, M.M., and Somerville, C. (2002). α-Glucosidase I is required for cellulose biosynthesis and morphogenesis in Arabidopsis. J. Cell Biol. 156, 1003–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, P.B. (1980). Organogenesis, a biophysical view. Annu. Rev. Plant Physiol. 31, 51–82. [Google Scholar]
- Hashimoto, T. (2002). Molecular genetic analysis of left-right handedness in plants. Philos. Trans. R. Soc. Lond. B Biol. Sci. 357, 799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussey, P.J., Hawkins, T.J., Igarashi, H., Kaloriti, D., and Smertenko, A. (2002). The plant cytoskeleton: Recent advances in the study of the plant microtubule-associated proteins MAP-65, MAP-190 and the Xenopus MAP215-like protein, MOR1. Plant Mol. Biol. 50, 915–924. [DOI] [PubMed] [Google Scholar]
- Itoh, T. (1976). Microfibrillar orientation of radially enlarged cells of coumarin- and colchicine-treated pine seedlings. Plant Cell Physiol. 17, 385–398. [Google Scholar]
- Itoh, T., and Shimeji, K. (1976). Orientation of microfibrils and microtubules in cortical parenchyma cells of poplar during elongation growth. Bot. Mag. Tokyo 89, 291–308. [Google Scholar]
- Lane, D.R., et al. (2001). Temperature-sensitive alleles of RSW2 link the KORRIGAN endo-1,4-β-glucanase to cellulose synthesis and cytokinesis in Arabidopsis. Plant Physiol. 126, 278–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx-Figini, M. (1970). Investigation of biosynthesis of cellulose: DPw and yield of cellulose of the alga Valonia in the presence of colchicine. Biochim. Biophys. Acta 237, 75–77. [DOI] [PubMed] [Google Scholar]
- McCartney, L., Steele-King, C.G., Jordan, E., and Knox, J.P. (2003). Cell wall pectic (1→4)-β-d-galactan marks the acceleration of cell elongation in the Arabidopsis seedling root meristem. Plant J. 33, 447–454. [DOI] [PubMed] [Google Scholar]
- Morejohn, L.C., and Fosket, D.E. (1991). The biochemistry of compounds with anti-microtubule activity in plant cells. Pharmacol. Ther. 51, 217–230. [DOI] [PubMed] [Google Scholar]
- Mueller, S.C., and Brown, R.M. (1982). The control of cellulose microfibril deposition in the cell wall of higher plants. II. Freeze-fracture microfibril patterns in maize seedling tissues following experimental alteration with colchicine and ethylene. Planta 154, 501–515. [DOI] [PubMed] [Google Scholar]
- Mulder, B.M., and Emons, A.M.C. (2001). A dynamical model for plant cell wall architecture formation. J. Math. Biol. 42, 261–289. [DOI] [PubMed] [Google Scholar]
- Nicol, F., His, I., Jauneau, A., Vernhettes, S., Canut, H., and Hofte, H. (1998). A plasma membrane-bound putative endo-1,4-β-d-glucanase is required for normal wall assembly and cell elongation in Arabidopsis. EMBO J. 17, 5563–5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda, K., and Mizuta, S. (1987). Modification in cell shape unrelated to cellulose microfibril orientation in growing thallus cells of Chaetomorpha moniligera. Plant Cell Physiol. 28, 461–473. [Google Scholar]
- O'Neill, M.A., Eberhard, S., Albersheim, P., and Darvill, A.G. (2001). Requirement of borate cross-linking of cell wall rhamnogalacturonan II for Arabidopsis growth. Science 294, 846–849. [DOI] [PubMed] [Google Scholar]
- Pagant, S., Bichet, A., Sugimoto, K., Lerouxel, O., Desprez, T., McCann, M., Lerouge, P., Vernhettes, S., and Hofte, H. (2002). KOBITO1 encodes a novel plasma membrane protein necessary for normal synthesis of cellulose during cell expansion in Arabidopsis. Plant Cell 14, 2001–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, L.C., Hocart, C.H., Redmond, J.W., and Williamson, R.E. (2000). Fractionation of carbohydrates in Arabidopsis root cell walls shows that three radial swelling loci are specifically involved in cellulose production. Planta 211, 406–414. [DOI] [PubMed] [Google Scholar]
- Probine, M.C. (1963). Cell growth and the structure and mechanical properties of the wall in internodal cells of Nitella opaca. III. Spiral growth and cell wall structure. J. Exp. Bot. 14, 101–113. [Google Scholar]
- Sato, S., Kato, T., Kakegawa, K., Ishii, T., Liu, Y.G., Awano, T., Takabe, K., Nishiyama, Y., Kuga, S., Nakamura, Y., Tabata, S., and Shibata, D. (2001). Role of the putative membrane-bound endo-1,4-β-glucanase KORRIGAN in cell elongation and cellulose synthesis in Arabidopsis thaliana. Plant Cell Physiol. 42, 251–263. [DOI] [PubMed] [Google Scholar]
- Seifert, G.J., Barber, C., Wells, B., Dolan, L., and Roberts, K. (2002). Galactose biosynthesis in Arabidopsis: Genetic evidence for substrate channeling from UDP-d-galactose into cell wall polymers. Curr. Biol. 12, 1840–1845. [DOI] [PubMed] [Google Scholar]
- Shibaoka, H., and Hogetsu, T. (1977). Effects of ethyl N-phenylcarbamate on wall microtubules and on gibberellin- and kinetin-controlled cell expansion. Bot. Mag. Tokyo 90, 317–321. [Google Scholar]
- Srivastava, L.M., Sawhney, V.K., and Bonnettemaker, M. (1976). Cell growth, wall deposition, and correlated fine structure of colchicine-treated lettuce hypocotyl cells. Can. J. Bot. 55, 902–917. [Google Scholar]
- Steinborn, K., Maulbetsch, C., Priester, B., Trautmann, S., Pacher, T., Geiges, B., Kuttner, F., Lepiniec, L., Stierhof, Y.D., Schwarz, H., Jurgens, G., and Mayer, U. (2002). The Arabidopsis PILZ group genes encode tubulin-folding cofactor orthologs required for cell division but not cell growth. Genes Dev. 16, 959–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto, K., Williamson, R.E., and Wasteneys, G.O. (2000). New techniques enable comparative analysis of microtubule orientation, wall texture, and growth rate in intact roots of Arabidopsis. Plant Physiol. 124, 1493–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto, K., Williamson, R.E., and Wasteneys, G.O. (2001). Wall architecture in the cellulose-deficient rsw1 mutant of Arabidopsis thaliana: Microfibrils but not microtubules lose their transverse alignment before microfibrils become unrecognizable in the mitotic and elongation zones of roots. Protoplasma 215, 172–183. [DOI] [PubMed] [Google Scholar]
- Taiz, L. (1984). Plant-cell expansion: Regulation of cell-wall mechanical properties. Annu. Rev. Plant Physiol. Plant Mol. Biol. 35, 585–657. [Google Scholar]
- Takeda, K., and Shibaoka, H. (1981). Effects of gibberellin and colchicine on microfibril arrangement in epidermal cell walls of Vigna angularis Ohwi et Ohashi epicotyls. Planta 151, 393–398. [DOI] [PubMed] [Google Scholar]
- Thitamadee, S., Tuchihara, K., and Hashimoto, T. (2002). Microtubule basis for left-handed helical growth in Arabidopsis. Nature 417, 193–196. [DOI] [PubMed] [Google Scholar]
- Traas, J., Bellini, C., Nacry, P., Kronenberger, J., Bouchez, D., and Caboche, M. (1995). Normal differentiation patterns in plants lacking microtubular preprophase bands. Nature 375, 676–677. [Google Scholar]
- Wasteneys, G.O. (2000). The cytoskeleton and growth polarity. Curr. Opin. Plant Biol. 3, 503–511. [DOI] [PubMed] [Google Scholar]
- Wasteneys, G.O. (2002). Microtubule organization in the green kingdom: Chaos or self-order? J. Cell Sci. 115, 1345–1354. [DOI] [PubMed] [Google Scholar]
- Wasteneys, G.O., and Williamson, R.E. (1987). Microtubule orientation in developing internodal cells of Nitella: A quantitative analysis. Eur. J. Cell Biol. 43, 14–22. [Google Scholar]
- Webb, M., Jouannic, S., Foreman, J., Linstead, P., and Dolan, L. (2002). Cell specification in the Arabidopsis root epidermis requires the activity of ECTOPIC ROOT HAIR 3, a katanin-p60 protein. Development 129, 123–131. [DOI] [PubMed] [Google Scholar]
- Weedenburg, C.A., and Seagull, R.W. (1987). The effects of taxol and colchicine on microtubule and microfibril arrays in elongating plant cells in culture. Can. J. Bot. 66, 1707–1716. [Google Scholar]
- Whitney, S.E.C., Brigham, J.E., Darke, A.H., Reid, J.S.G., and Gidley, M.J. (1995). In vitro assembly of cellulose/xyloglucan networks: Ultrastructural and molecular aspects. Plant J. 8, 491–504. [Google Scholar]
- Whitney, S.E.C., Gothard, M.G.E., Mitchell, J.T., and Gidley, M.J. (1999). Roles of cellulose and xyloglucan in determining the mechanical properties of primary plant cell walls. Plant Physiol. 121, 657–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington, A.T., Vugrek, O., Wei, K.J., Hasenbein, N.G., Sugimoto, K., Rashbrooke, M.C., and Wasteneys, G.O. (2001). MOR1 is essential for organizing cortical microtubules in plants. Nature 411, 610–613. [DOI] [PubMed] [Google Scholar]
- Wiedemeier, A.M.D., Judy-March, J.E., Hocart, C.H., Wasteneys, G.O., Williamson, R.E., and Baskin, T.I. (2002). Mutant alleles of Arabidopsis RADIALLY SWOLLEN 4 and 7 reduce growth anisotropy without altering the transverse orientation of cortical microtubules or cellulose microfibrils. Development 129, 4821–4830. [DOI] [PubMed] [Google Scholar]
- Williamson, R.E., Burn, J.E., and Hocart, C.H. (2001). Cellulose synthesis: Mutational analysis and genomic perspectives using Arabidopsis thaliana. Cell. Mol. Life Sci. 58, 1475–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilms, F.H.A., Wolters-Arts, A.M.C., and Derksen, J. (1990). Orientation of cellulose microfibrils in cortical cells of tobacco explants. Effects of microtubule-depolymerizing drugs. Planta 182, 1–8. [DOI] [PubMed] [Google Scholar]
- Wilson, R.H., Smith, A.C., Kacurakova, M., Saunders, P.K., Wellner, N., and Waldron, K.W. (2000). The mechanical properties and molecular dynamics of plant cell wall polysaccharides studied by Fourier-transform infrared spectroscopy. Plant Physiol. 124, 397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuhara, H., Muraoka, M., Shogaki, H., Mori, H., and Sonobe, S. (2002). TMBP200, a microtubule bundling polypeptide isolated from telophase tobacco BY-2 cells, is a MOR1 homologue. Plant Cell Physiol. 43, 595–603. [DOI] [PubMed] [Google Scholar]
- Yatsu, L.Y., and Jacks, T.J. (1981). An ultrastructural study of the relationship between microtubules and microfibrils in cotton (Gossypium hirsutum L.) cell-wall reversals. Am. J. Bot. 68, 771–777. [Google Scholar]
- Zhong, R.Q., Burk, D.H., Morrison, W.H., and Ye, Z.H. (2002). A kinesin-like protein is essential for oriented deposition of cellulose microfibrils and cell wall strength. Plant Cell 14, 3101–3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.