Abstract
Hemorrhagic fever with renal syndrome and hantavirus pulmonary syndrome are two diseases caused by hantaviruses. Capillary leakage is a hallmark of hantavirus infection. Pathogenic hantaviruses are not cytotoxic, but elevated levels of serum lactate dehydrogenase (LDH), indicative of cellular damage, are observed in patients. We report increased levels of serum perforin, granzyme B, and the epithelial cell apoptosis marker caspase-cleaved cytokeratin-18 during Puumala hantavirus infection. Significant correlation was observed between the levels of LDH and perforin and the levels of LDH and caspase-cleaved cytokeratin-18, suggesting that tissue damage is due to an immune reaction and that epithelial apoptosis contributed significantly to the damage.
Hantaviruses are the causative agents of hemorrhagic fever with renal syndrome (HFRS) and hantavirus pulmonary syndrome (HPS). Capillary leak syndrome, causing edema and hemorrhage, is a hallmark of HFRS/HPS. Endothelial cells and monocytes are the primary cell types infected by hantaviruses, but infection has no direct cytopathic effect on these, or other, cells (8, 12, 25, 26, 30, 33, 35, 38, 39), indicating that direct viral cytotoxicity is not responsible for the pathology observed in humans. However, increased levels of serum lactate dehydrogenase (LDH), aspartate aminotransferase, and alanine aminotransferase are observed in patients (3, 5, 37), showing that the cellular membrane integrity is disturbed during infection. It has been argued that pathogenesis is due to cellular immune responses rather than to the infection (reviewed in reference 11).
Special attention has recently been given to the CD8+-T-cell responses induced during hantavirus infection (10, 13, 21, 22, 23, 24, 34, 36). For example, the magnitude of the Sin Nombre hantavirus-specific CD8+-T-cell responses correlate with the severity of HPS, implying that Sin Nombre hantavirus-specific CD8+ T cells contribute to HPS disease outcome (13).
Antigen-specific CD8+ T cells can induce target cell apoptosis by the release of cytolytic granules containing perforin and granzymes, and virus-infected cells are eliminated mainly via this granule exocytosis pathway (18). During this process, some of the perforin and granzymes find their way into the circulation (29). Elevated levels of extracellular granzyme B, indicative of the activation of CD8+ T cells and natural killer (NK) cells, have been detected in viral, bacterial, and parasitic infections (9, 17, 32), and we have previously detected elevated levels of extracellular perforin in human immunodeficiency virus type 1-infected individuals (15).
In this study, serum samples were collected from patients hospitalized with laboratory-verified Puumala hantavirus infection and with typical clinical symptoms of acute nephropathia epidemica, a milder form of HFRS (31). The levels of perforin (Mabtech, Nacka, Sweden), granzyme B (Euroclone, Pero, Italy), caspase-cleaved cytokeratin-18 (CK18) (Peviva, Bromma, Sweden), and total CK18 (Peviva) were analyzed using enzyme-linked immunosorbent assays according to the manufacturer's instructions. Three individuals out of the 21 Puumala hantavirus-infected patients were found to be positive for human anti-mouse antibodies and were excluded from the study to avoid false-positive results in the specific enzyme-linked immunosorbent assays (15). Acute-phase samples were drawn at the time of hospitalization. The individuals arrived at the hospital 2 to 12 days after initial onset of fever. Convalescent-phase serum was drawn 10 days, 1 month, 2 months, or 3 months after recovery from 1, 2, 1, and 14 individuals, respectively. The project was approved by the Research Ethics Committee of Umeå University.
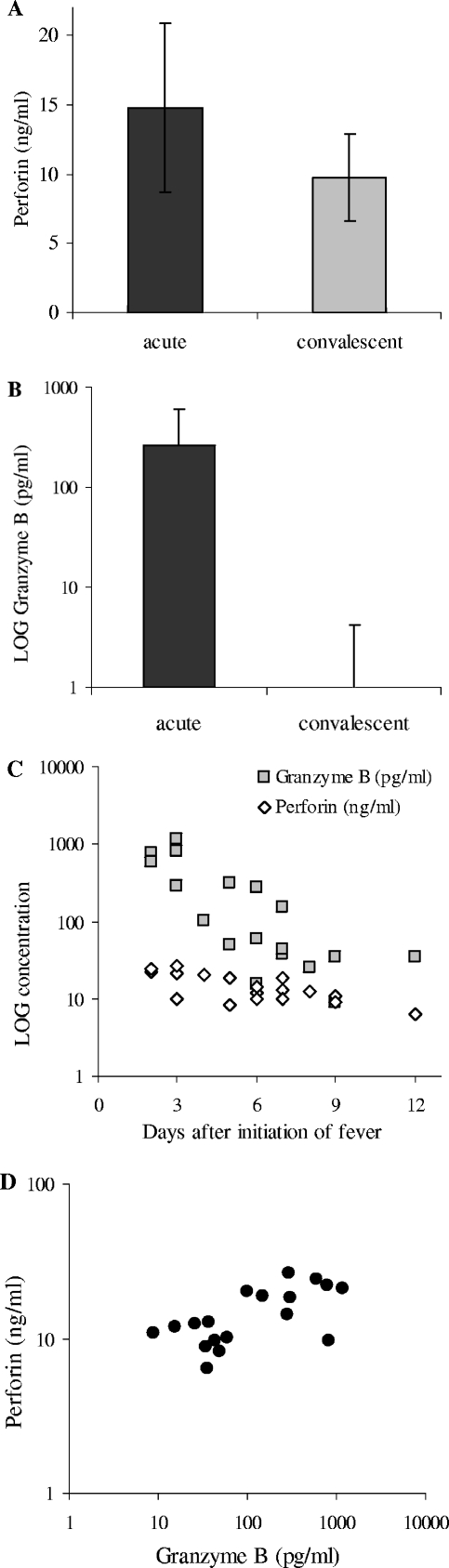
The mean concentrations of serum perforin (Fig. 1A) and granzyme B (Fig. 1B) were significantly higher in the acute phase than in the convalescent phase (P < 0.0001 for perforin and granzyme B by Wilcoxon signed-rank test). All patients except one showed higher levels of extracellular perforin and all patients showed higher levels of granzyme B during the acute phase than during the convalescent phase.
FIG. 1.
Elevated levels of perforin and granzyme B in serum during the acute phase of hantavirus infection. Mean levels of extracellular perforin (A) and granzyme B (B) in serum at the acute (14.8 ± 6.0 ng perforin/ml, ranging from 6.5 to 26.2 ng/ml, and 265.0 ± 341.6 pg granzyme B/ml, ranging from 8.9 to 1,153.3 pg/ml, respectively) and convalescent (9.8 ± 3.1 ng perforin/ml, ranging from 5.4 to 15.0 ng/ml) phases of HFRS are shown. All but two samples were negative for granzyme B during the convalescent phase; the two positive samples had 4.4 and 13.3 pg granzyme B/ml, respectively. Data represent means ± standard deviations (n = 18). (C) Levels of perforin and granzyme B in serum for the acute-phase samples plotted against days after initial fever for the individual patients. (D) Levels of perforin plotted against the level of granzyme B in acute-phase serum from the individual patients. The serum levels of perforin and granzyme B in healthy subjects has been reported to be 8.0 ± 2.79 ng perforin/ml (15) and 11.5 pg granzyme B/ml (32), respectively.
A correlation was observed between the time after onset of fever and the concentrations of perforin (Spearman R = −0.64; P = 0.0045) and granzyme B (Spearman R = −0.84; P = 0.000016), showing that the highest levels of perforin and granzyme B were detected early after the onset of fever (Fig. 1C). The levels of perforin and granzyme B in the individual patients during the acute phase also correlated (Spearman R = 0.56; P = 0.015) (Fig. 1D).
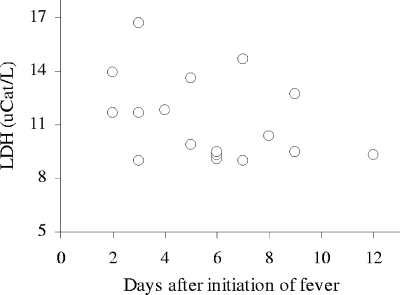
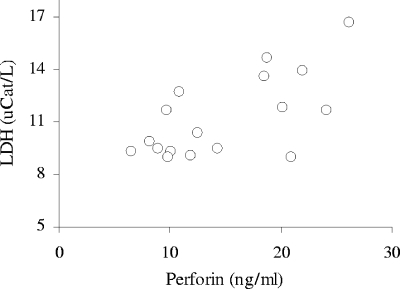
Consistent with previous observations (3, 5, 37), all patients in this study showed increased levels of serum LDH during the acute phase of infection. The LDH levels did not, however, correlate significantly (Spearman R = −0.30; P = 0.24) with time after onset of fever (Fig. 2). The level of LDH correlated significantly with that of perforin (Spearman R = 0.50; P = 0.039) (Fig. 3) but not with the levels of granzyme B (Spearman R = 0.27; P = 0.30).
FIG. 2.
Loss of cell membrane integrity during the acute phase of hantavirus infection. The level of LDH is plotted against time after onset of fever for the individual patients. uCat, units of catalytic activity.
FIG. 3.
Loss of cellular integrity correlates with the level of perforin during the acute phase of hantavirus infection. The individual levels of LDH were plotted against extracellular perforin during the acute phase of infection. uCat, units of catalytic activity.
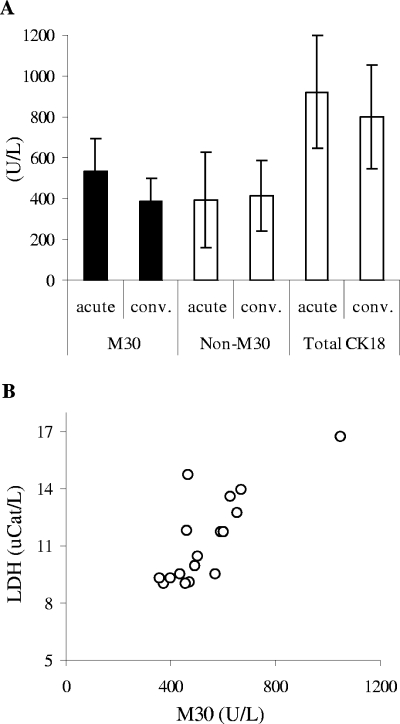
To quantify possible epithelial cell apoptosis in hantavirus-infected patients, we determined the serum levels of a caspase cleavage product of CK18 (1). CK18 is a type I intermediate filament protein that is exclusively expressed by simple epithelial cells, like those of the endothelia. CK18 is cleaved by caspases during apoptosis and is released into serum (1, 2). Increased serum levels of caspase-cleaved CK18 have previously been detected in patients during septic shock (28), in patients with hepatitis (16), and in patients with various carcinomas (19). Interestingly, significantly higher levels of caspase-cleaved CK18 were observed during the acute phase of infection than during the convalescent phase (P = 0.0028 by Wilcoxon signed-rank test) (Fig. 4A). Fourteen of the 18 patients showed higher levels of caspase-cleaved CK18 during the acute phase than during the convalescent phase. Similar to serum LDH, the level of epithelial cell apoptosis showed no significant correlation (Spearman R = −0.45; P = 0.059) with time after onset of fever.
FIG. 4.
Epithelial cell apoptosis is increased and correlates with the loss of cellular integrity during the acute phase of hantavirus infection. (A) Mean levels of the caspase-cleaved part of CK18 (M30) during the acute (530.2 ± 163.1 U/liter of caspase-cleaved CK18, ranging from 375.6 to 1,048.1 U/liter) and convalescent (386.7 ± 112.8 U/liter of caspase-cleaved CK18, ranging from 264.6 to 587.3 U/liter) phases of HFRS. Also shown are the mean levels of the non-caspase-cleaved part of CK18 (Non-M30) during the acute (391.9 ± 231.4 U/liter of non-caspase-cleaved CK18) and convalescent (411.9 ± 174.4 U/liter of non-caspase-cleaved CK18) phases and the total levels of CK18 (including both the caspase-cleaved and the non-caspase-cleaved forms of CK18) during the acute (922.1 ± 278.3 U/liter of total CK18) and convalescent (798.6 ± 253.6 U/liter of total CK18) phases. Data represent means ± standard deviations (n = 18). The mean proportion of caspase-cleaved CK18 out of total CK18 was 59.8% and 49.4% in acute and convalescent phases, respectively. (B) Individual levels of LDH plotted against caspase-cleaved CK18 during the acute phase of infection. The mean level of caspase-cleaved CK18 in a healthy population is 139 ± 82 U/liter (n = 118). uCat, units of catalytic activity.
The level of caspase-cleaved CK18 correlated significantly with that of LDH (Spearman R = 0.74; P = 0.00067) (Fig. 4B). No significant correlation was observed between the levels of perforin (Spearman R = 0.40; P = 0.10) or granzyme B (Spearman R = 0.29; P = 0.24) with those of caspase-cleaved CK18.
The observed elevated levels of caspase-cleaved CK18 show that apoptosis is induced in cells of the epithelial cell lineage during the acute phase of HFRS. Furthermore, the very strong correlation between the levels of epithelial apoptosis and LDH suggests that most of the cell damage observed during hantavirus infections is caused by apoptosis. To our knowledge, this is the first report showing apoptosis of epithelial cells during hantavirus infection in humans.
Significantly higher levels of perforin and granzyme B were observed during the acute phase than during the convalescent phase of infection. Furthermore, the levels of perforin and granzyme B correlated during the acute phase. It could be speculated that the levels of perforin and granzyme B in released cytotoxic granules are roughly proportional to each other during acute infection.
The levels of perforin and LDH correlated significantly, suggesting that hantavirus-specific CD8+ T cells and/or NK cells might be involved in causing the observed cell damage during HFRS/HPS. Interestingly, the levels of granzyme B and LDH did not correlate. This is in line with the proposed functions of perforin and granzyme B during the killing of target cells by cytotoxic cells: although granzyme B induces the apoptosis, perforin is needed for granzyme B to enter the cell (18).
The levels of perforin and caspase-cleaved CK18 did not correlate significantly, and although it could be speculated that the increased vascular permeability observed during HFRS/HPS is due to apoptosis caused by hantavirus-specific CD8+ T cells, this remains to be clearly shown. Apoptosis of other epithelial cells might also contribute to the increased levels of caspase-cleaved CK18. The interaction between the virus and the receptor αvβ3 integrin, tumor necrosis factor, and/or reactive oxygen species might also induce permeability of endothelial cells (4, 6, 7, 14, 20, 27). The levels of perforin and granzyme B observed during the convalescent phase is similar to those previously reported for healthy individuals (15, 32), but the levels of caspase-cleaved CK18 were clearly higher, indicating that epithelial cell apoptosis might be increased for a prolonged time after infection.
We have shown that elevated levels of extracellular perforin, granzyme B, and epithelial cell apoptosis are induced during acute hantavirus infection. The capillary leakage during HFRS/HPS might be due to apoptosis, and the strong hantavirus-specific CD8+-T-cell responses observed might be responsible for the damage.
Acknowledgments
We thank Mats Linderholm for help with collecting patient samples.
This project was supported by grants from the Swedish Medical Research Council (projects 12177 and 12642), the Swedish Society of Medicine, the Medical Faculty of Umeå University, Cancerföreningen i Stockholm, and the European Community (contract no. QLK2-CT-1999-01119 and QLK2-CT-2002-01358). This publication has been partially funded under the EU 6th Framework Program (GOCE-CT-2003-010284 EDEN) and is officially catalogued by the EDEN Steering Committee as EDEN0018.
REFERENCES
- 1.Bivén, K., H. Erdal, M. Hägg, T. Ueno, R. Zhou, M. Lynch, B. Rowley, J. Wood, C. Zhang, M. Toi, M. C. Shoshan, and S. Linder. 2003. A novel assay for discovery and characterization of pro-apoptotic drugs and for monitoring apoptosis in patient sera. Apoptosis 8:263-268. [DOI] [PubMed] [Google Scholar]
- 2.Caulin, C., G. S. Salvesen, and R. G. Oshima. 1997. Caspase cleavage of keratin 18 and reorganization of intermediate filaments during epithelial cell apoptosis. J. Cell Biol. 138:1379-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Courouble, P., D. Vanpee, E. Delgrange, J. Donckier, J. M. Pochet, and J. B. Gillet. 2001. Hantavirus infections: clinical presentation in the emergency room. Eur. J. Emerg. Med. 8:17-20. [DOI] [PubMed] [Google Scholar]
- 4.Davis, I. C., A. J. Zajac, K. B. Nolte, J. Botten, B. Hjelle, and S. Matalon. 2002. Elevated generation of reactive oxygen/nitrogen species in hantavirus cardiopulmonary syndrome. J. Virol. 76:8347-8359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duchin, J. S., F. T. Koster, C. J. Peters, G. L. Simpson, B. Tempest, S. R. Zaki, T. G. Ksiazek, P. E. Rollin, S. Nichol, E. T. Umland, R. L. Moolenaar, S. E. Reef, K. B. Nolte, M. M. Gallaher, J. C. Butler, and R. F. Breiman. 1994. Hantavirus pulmonary syndrome: a clinical description of 17 patients with a newly recognized disease. N. Engl. J. Med. 330:949-955. [DOI] [PubMed] [Google Scholar]
- 6.Gavrilovskaya, I. N., M. Shepley, R. Shaw, M. H. Ginsberg, and E. R. Mackow. 1998. β3 integrins mediate the cellular entry of hantaviruses that cause respiratory failure. Proc. Natl. Acad. Sci. USA 95:7074-7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geimonen, E., S. Neff, T. Raymond, S. S. Kocer, I. N. Gavrilovskaya, and E. R. Mackow. 2002. Pathogenic and nonpathogenic hantaviruses differentially regulate endothelial cell responses. Proc. Natl. Acad. Sci. USA 99:13837-13842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hardestam, J., J. Klingström, K. Mattsson, and Å. Lundkvist. 2005. HFRS causing hantaviruses do not induce apoptosis in confluent Vero E6 and A-549 cells. J. Med. Virol. 76:234-240. [DOI] [PubMed] [Google Scholar]
- 9.Hermsen, C. C., Y. Konijnenberg, L. Mulder, C. Loe, M. van Deuren, J. W. van der Meer, G. J. van Mierlo, W. M. Eling, C. E. Hack, and R. W. Sauerwein. 2003. Circulating concentrations of soluble granzyme A and B increase during natural and experimental Plasmodium falciparum infections. Clin. Exp. Immunol. 132:467-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang, C., B. Jin, M. Wang, E. Li, and C. Sun. 1994. Hemorrhagic fever with renal syndrome: relationship between pathogenesis and cellular immunity. J. Infect. Dis. 169:868-870. [DOI] [PubMed] [Google Scholar]
- 11.Khaiboullina, S. F., and S. C. St. Jeor. 2002. Hantavirus immunology. Viral Immunol. 15:609-625. [DOI] [PubMed] [Google Scholar]
- 12.Khaiboullina, S. F., D. M. Netski, P. Krumpe, and S. C. St. Jeor. 2000. Effects of tumor necrosis factor alpha on Sin Nombre virus infection in vitro. J. Virol. 74:11966-11971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kilpatrick, E. D., M. Terajima, F. T. Koster, M. D. Catalina, J. Cruz, and F. A. Ennis. 2004. Role of specific CD8+ T cells in the severity of a fulminant zoonotic viral hemorrhagic fever, hantavirus pulmonary syndrome. J. Immunol. 172:3297-3304. [DOI] [PubMed] [Google Scholar]
- 14.Klingström, J., A. Plyusnin, A. Vaheri, and Å. Lundkvist. 2002. Wild-type Puumala hantavirus infection induces cytokines, C-reactive protein, creatinine, and nitric oxide in cynomolgus macaques. J. Virol. 76:444-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klingström, J., L. Gudmundsdotter, B. Zuber, J. Hinkula, A. Mörner, B. Wahren, and E. Rollman. 2006. Elevated levels of serum perforin in chronic HIV-1 and acute SIV/SHIV infection. AIDS 20:125-127. [DOI] [PubMed] [Google Scholar]
- 16.Kronenberger, B., M. von Wagner, E. Herrmann, U. Mihm, A. Piiper, C. Sarrazin, and S. Zeuzem. 2005. Apoptotic cytokeratin 18 neoepitopes in serum of patients with chronic hepatitis C. J. Viral. Hepat. 12:307-314. [DOI] [PubMed] [Google Scholar]
- 17.Lauw, F. N., A. J. Simpson, C. E. Hack, J. M. Prins, A. M. Wolbink, S. J. van Deventer, W. Chaowagul, N. J. White, and T. van Der Poll. 2000. Soluble granzymes are released during human endotoxemia and in patients with severe infection due to gram-negative bacteria. J. Infect. Dis. 182:206-213. [DOI] [PubMed] [Google Scholar]
- 18.Lieberman, J. 2003. The ABCs of granule-mediated cytotoxicity: new weapons in the arsenal. Nat. Rev. Immunol. 3:361-370. [DOI] [PubMed] [Google Scholar]
- 19.Linder, S., A. Havelka, T. Ueno, and M. Shoshan. 2004. Determining tumor apoptosis and necrosis in patient serum using cytokeratin 18 as a biomarker. Cancer Lett. 214:1-9. [DOI] [PubMed] [Google Scholar]
- 20.Linderholm, M., C. Ahlm, B. Settergren, A. Waage, and A. Tärnvik. 1996. Elevated plasma levels of tumor necrosis factor (TNF)-alpha, soluble TNF receptors, interleukin (IL)-6, and IL-10 in patients with hemorrhagic fever with renal syndrome. J. Infect. Dis. 173:38-43. [DOI] [PubMed] [Google Scholar]
- 21.Linderholm, M., L. Bjermer, P. Juto, G. Roos, T. Sandström, B. Settergren, and A. Tärnvik. 1993. Local host response in the lower respiratory tract in nephropathia epidemica. Scand. J. Infect. Dis. 25:639-646. [DOI] [PubMed] [Google Scholar]
- 22.Mäkelä, S., J. Mustonen, I. Ala-Houhala, M. Hurme, J. Partanen, O. Vapalahti, A. Vaheri, and A. Pasternack. 2002. Human leukocyte antigen-B8-DR3 is a more important risk factor for severe Puumala hantavirus infection than the tumor necrosis factor-alpha(-308) G/A polymorphism. J. Infect. Dis. 186:843-846. [DOI] [PubMed] [Google Scholar]
- 23.Mustonen, J., H. Helin, K. Pietila, M. Brummer-Korvenkontio, K. Hedman, A. Vaheri, and A. Pasternack. 1994. Renal biopsy findings and clinicopathologic correlations in nephropathia epidemica. Clin. Nephrol. 41:121-126. [PubMed] [Google Scholar]
- 24.Mustonen, J., J. Partanen, M. Kanerva, K. Pietila, O. Vapalahti, A. Pasternack, and A. Vaheri. 1996. Genetic susceptibility to severe course of nephropathia epidemica caused by Puumala hantavirus. Kidney Int. 49:217-221. [DOI] [PubMed] [Google Scholar]
- 25.Nolte, K. B., R. M. Feddersen, K. Foucar, S. R. Zaki, F. T. Koster, D. Madar, T. L. Merlin, P. J. McFeeley, E. T. Umland, and R. E. Zumwalt. 1995. Hantavirus pulmonary syndrome in the United States: a pathological description of a disease caused by a new agent. Hum. Pathol. 26:110-120. [DOI] [PubMed] [Google Scholar]
- 26.Pensiero, M. N., J. B. Sharefkin, C. W. Dieffenbach, and J. Hay. 1992. Hantaan virus infection of human endothelial cells. J. Virol. 66:5929-5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raymond, T., E. Gorbunova, I. N. Gavrilovskaya, and R. E. Mackow. 2005. Pathogenic hantaviruses bind plexin-semaphorin-integrin domains present at the apex of inactive, bent alphavbeta3 integrin conformers. Proc. Natl. Acad. Sci. USA 102:1163-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roth, G. A., C. Krenn, M. Brunner, B. Moser, M. Ploder, A. Spittler, L. Pelinka, T. Sautner, E. Wolner, G. Boltz-Nitulescu, and H. J. Ankersmit. 2004. Elevated serum levels of epithelial cell apoptosis-specific cytokeratin 18 neoepitope m30 in critically ill patients. Shock 22:218-220. [DOI] [PubMed] [Google Scholar]
- 29.Rothstein, T. L., M. Mage, G. Jones, and L. L. McHugh. 1978. Cytotoxic T lymphocyte sequential killing of immobilized allogeneic tumor target cells measured by time-lapse microcinematography. J. Immunol. 121:1652-1656. [PubMed] [Google Scholar]
- 30.Rowe, R. K., and A. Pekosz. 2006. Bidirectional virus secretion and nonciliated cell tropism following Andes virus infection of primary airway epithelial cell cultures. J. Virol. 80:1087-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Settergren, B. 2000. Clinical aspects of nephropathia epidemica (Puumala virus infection) in Europe: a review. Scand. J. Infect. Dis. 32:125-132. [DOI] [PubMed] [Google Scholar]
- 32.Spaeny-Dekking, E. H., W. L. Hanna, A. M. Wolbink, P. C. Wever, A. J. Kummer, A. J. Swaak, J. M. Middeldorp, H. G. Huisman, C. J. Froelich, and C. E. Hack. 1998. Extracellular granzymes A and B in humans: detection of native species during CTL responses in vitro and in vivo. J. Immunol. 160:3610-3616. [PubMed] [Google Scholar]
- 33.Sundstrom, J. B., L. K. McMullan, C. F. Spiropoulou, W. C. Hooper, A. A. Ansari, C. J. Peters, and P. E. Rollin. 2001. Hantavirus infection induces the expression of RANTES and IP-10 without causing increased permeability in human lung microvascular endothelial cells. J. Virol. 75:6070-6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Temonen, M., J. Mustonen, H. Helin, A. Pasternack, A. Vaheri, and H. Holthofer. 1996. Cytokines, adhesion molecules, and cellular infiltration in nephropathia epidemica kidneys: an immunohistochemical study. Clin. Immunol. Immunopathol. 78:47-55. [DOI] [PubMed] [Google Scholar]
- 35.Temonen, M., O. Vapalahti, H. Holthofer, M. Brummer-Korvenkontio, A. Vaheri, and H. Lankinen. 1993. Susceptibility of human cells to Puumala virus infection. J. Gen. Virol. 74:515-518. [DOI] [PubMed] [Google Scholar]
- 36.Van Epps, H. L., M. Terajima, J. Mustonen, T. P. Arstila, E. A. Corey, A. Vaheri, and F. A. Ennis. 2002. Long-lived memory T lymphocyte responses after hantavirus infection. J. Exp. Med. 196:579-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verity, R., E. Prasad, K. Grimsrud, H. Artsob, M. Drebot, L. Miedzinski, and J. Preiksaitis. 2000. Hantavirus pulmonary syndrome in northern Alberta, Canada: clinical and laboratory findings for 19 cases. Clin. Infect. Dis. 31:942-946. [DOI] [PubMed] [Google Scholar]
- 38.Yao, Z. O., W. S. Yang, W. B. Zhang, and X. F. Bai. 1989. The distribution and duration of hantaan virus in the body fluids of patients with hemorrhagic fever with renal syndrome. J. Infect. Dis. 160:218-224. [DOI] [PubMed] [Google Scholar]
- 39.Zaki, S. R., P. W. Greer, L. M. Coffield, C. S. Goldsmith, K. B. Nolte, K. Foucar, R. M. Feddersen, R. E. Zumwalt, G. L. Miller, and A. S. Khan. 1995. Hantavirus pulmonary syndrome. Pathogenesis of an emerging infectious disease. Am. J. Pathol. 146:552-579. [PMC free article] [PubMed] [Google Scholar]