Abstract
The nonstructural protein NSm of Bunyamwera virus (BUNV), the prototype of the Bunyaviridae family, is encoded by the M segment in a polyprotein precursor, along with the virion glycoproteins, in the order Gn-NSm-Gc. As little is known of its function, we examined the intracellular localization, membrane integrality, and topology of NSm and its role in virus replication. We confirmed that NSm is an integral membrane protein and that it localizes in the Golgi complex, together with Gn and Gc. Coimmunoprecipitation assays and yeast two-hybrid analysis demonstrated that NSm was able to interact with other viral proteins. NSm is predicted to contain three hydrophobic (I, III, and V) and two nonhydrophobic (II and IV) domains. The N-terminal nonhydrophobic domain II was found in the lumen of an intracellular compartment. A novel BUNV assembly assay was developed to monitor the formation of infectious virus-like-particles (VLPs). Using this assay, we showed that deletions of either the complete NSm coding region or domains I, II, and V individually seriously compromised VLP production. Consistently, we were unable to rescue viable viruses by reverse genetics from cDNA constructs that contained the same deletions. However, we could generate mutant BUNV with deletions in NSm domains III and IV and also a recombinant virus with the green fluorescent protein open reading frame inserted into NSm domain IV. The mutant viruses displayed differences in their growth properties. Overall, our data showed that the N-terminal region of NSm, which includes domain I and part of domain II, is required for virus assembly and that the C-terminal hydrophobic domain V may function as an internal signal sequence for the Gc glycoprotein.
The Bunyaviridae family contains more than 300 mainly arthropod-transmitted viruses that are divided among five genera (Orthobunyavirus, Hantavirus, Nairovirus, Phlebovirus, and Tospovirus) (36). Several members of the family are serious human pathogens, such as La Crosse and Oropouche orthobunyaviruses, Hantaan and Sin Nombre hantaviruses, Rift Valley fever phlebovirus, and Crimean-Congo hemorrhagic fever nairovirus, and are recognized as posing an increasing threat to human health (7). Tomato spotted wilt tospovirus infects a wide range of plants, causing enormous crop losses, and necessitates pesticide application to control its thrips vector (14). Bunyamwera virus (BUNV) is the prototype of both the family and the Orthobunyavirus genus and serves as a model for the many pathogens within this family.
Bunyaviruses share certain morphological and biochemical characteristics, including possession of a tripartite single-stranded RNA genome, but differ in the patterns of genome RNA and viral protein sizes, genome segment coding strategies, and the coding of nonstructural proteins (34). For BUNV, all three genome RNA segments are of negative-sense polarity. The large segment (L) codes for an RNA-dependent RNA polymerase (L protein), the medium segment (M) codes for a precursor polyprotein (NH2-Gn-NSm-Gc-COOH), which is cotranslationally cleaved to yield the two virion glycoproteins (Gn and Gc) and a nonstructural protein called NSm, and the smallest segment (S) codes for the nucleoprotein N and a second nonstructural protein NSs in overlapping reading frames (1, 36, 47). Viruses replicate in the cytoplasm of infected cells and mature by budding primarily at membranes of the Golgi apparatus (24, 25, 33, 47).
Proteins called NSm are also encoded by the M RNA segments of some phleboviruses and the tospoviruses (47) but do not show amino acid similarity to orthobunyavirus NSm proteins. Like orthobunyaviruses, phleboviruses encode their NSm proteins as part of a precursor with the glycoproteins (9, 18, 56), whereas NSm of tospoviruses is translated from a subgenomic mRNA in an ambisense manner (21, 27). Little is known about the function of NSm encoded by orthobunyaviruses or phleboviruses. The BUNV NSm protein was found to localize to the Golgi in virus-infected cells and, when expressed alone, from a transfected cDNA clone (26, 35). NSm did not affect the cotranslational cleavage of the BUNV glycoprotein precursor but seemed to be required for the efficient maturation of the two glycoproteins (50). The fact that the Golgi complex houses the bunyavirus factory where virus particles mature and bud (25, 37, 45) suggests that NSm may be involved in the process of virus assembly and morphogenesis, though this suggestion is tempered by the recent observation that a Maguari orthobunyavirus mutant lacking the C-terminal two-thirds of NSm is viable (41). The NSm protein of tospoviruses forms tubular structures in plant and insect cells (52) and is the viral “movement protein,” mediating cell-to-cell movement of the nonenveloped ribonucleocapsid structures across the plasmodesmata in infected plant cells (22, 51).
To investigate the role of BUNV NSm in viral replication, we first determined the intracellular location, membrane integrality, and topology of the protein. We also developed a novel virus assembly assay based on the BUNV minigenome (59) to evaluate the function of NSm in assembly and morphogenesis of infectious virus-like particles (VLPs). Using our efficient reverse genetics system, we rescued three recombinant BUNVs that contain deletions in the NSm coding region and a virus in which the green fluorescent protein (GFP) coding sequence was inserted into NSm. Our data show that the N-terminal region of NSm is essential for morphogenesis in infected mammalian cells.
MATERIALS AND METHODS
Cells and viruses.
Vero E6 (ATCC C1008), BHK-21, and BSR-T7/5 (4) were maintained as described previously (30, 48). Working stocks of wild-type BUNV were grown in BHK-21 cells, and titers were determined by plaque assay as previously described (2, 57).
Virus growth curves.
BHK-21 cells in 35-mm-diameter petri dishes were infected with 0.01 PFU per cell of the different viruses for 1 h, and the cells were washed twice with PBS to remove unattached viruses. Supernatants were harvested at various times after infection, and virus titers were determined by plaque assays on Vero E6 cells.
Antibodies.
A monoclonal antibody (MAb 742) against the BUNV Gc protein, anti-BUN, a rabbit antiserum against purified BUNV virions, and anti-NSm, a rabbit antiserum against a peptide TDQKYTLDEIADVLQA (residues 338 to 353 of the M segment precursor) derived from NSm, have been described previously (26, 35, 57). A rabbit polyclonal antibody against GM130, a cis-Golgi matrix protein (34), was provided by M. Lowe (School of Biological Sciences, University of Manchester, United Kingdom), and a monoclonal antibody against the same Golgi protein was purchased from BD Bioscience. Goat anti-rabbit antibody conjugated with fluorescein isothiocyanate was purchased from Sigma, and goat anti-mouse antibody conjugated with Cy5 was purchased from Amersham Pharmacia Biotech (Buckingham, United Kingdom).
Plasmids.
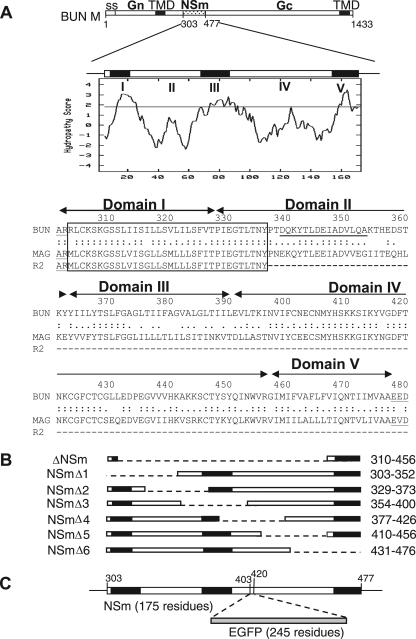
Plasmids pT7riboBUNL(+), pT7riboBUNM(+), and pT7riboBUNS(+) that contain full-length cDNAs of the BUNV genome segments under control of a T7 promoter and hepatitis delta virus ribozyme have been described previously (2). Eight BUNV M segment cDNA mutants, which contain internal deletions in the NSm coding region, were constructed from pT7riboBUNM(+) using a PCR mutagenesis approach (49) with Turbo Pfu DNA polymerase (Stratagene) (Fig. 1A). The mutated M cDNAs were also cloned into pTM1 (32) for use in the BUNV minigenome assembly assay. pT7riboBUNM-NSm-EGFP was made by inserting the enhanced GFP (EGFP) gene into the NSm coding region at PCR-created SacI restriction enzyme sites, replacing amino acid residues 403 to 420 (Fig. 1B). For the yeast two-hybrid protein-protein interaction assay, the predicted cytoplasmic domain (residues 391 to 457) of NSm, the cytoplasmic tail (residues 223 to 302) of Gn, or the complete NSs coding sequence was cloned into the binding domain (BD)-containing plasmid pGBKT7 or the activation domain (AD)-containing plasmid pGADT7 (28) (both from Clontech, Palo Alto, CA) to produce pBD-NSm, pAD-NSm, pBD-Gn, and pBD-NSs. All constructs were confirmed by DNA sequence analysis. The primers used and details of PCR are available upon request.
FIG. 1.
The BUNV M RNA segment and the encoded precursor polyprotein. (A) The gene layout of the M segment is shown at the top, with positions of amino acid residues marking protein boundaries indicated. ss, signal peptide; TMD, transmembrane domain. Below, the Kyte-Doolittle hydropathy plot and predicted domain structure of NSm are shown. Domains I to V were suggested by the program TMHMM (21). The amino acid alignment of the NSm proteins of BUNV, Maguari virus, and its mutant R2 are shown, with the conserved N-terminal region boxed. The peptide sequence used to raise the anti-NSm antibody is underlined. (B) Schematic of NSm deletion mutants. The regions deleted are indicated by the dashed line, and the residues deleted are indicated at the right. (C) Insertion of EGFP open reading frame into NSm. The EGFP coding sequence was cloned into the M segment cDNA at artificial SacI restriction enzyme sites created at codons 403 and 420 in NSm.
Generation of NSm mutant viruses by reverse genetics.
Rescue experiments were performed as described elsewhere (30). Briefly, BSR-T7/5 cells were transfected with a mixture of three plasmids, i.e., pT7riboBUNL(+), pT7riboBUNS(+), and either pT7riboBUNM (+) or one of the M cDNA mutant plasmids containing deletions in the NSm coding region. After 6 h, the cells were supplemented with 4 ml of growth medium, and incubation continued for 5 to 11 days. The transfectant viruses were isolated by plaque formation on Vero E6 cells as described previously (57).
Metabolic radiolabeling of viral proteins.
Vero E6 cells in 35-mm-diameter petri dishes were infected with wild-type (wt) or mutant BUNVs at a multiplicity of infection (MOI) of 1.0 PFU per cell and labeled with 50 μCi [35S]methionine (Amersham Pharmacia Biotech) for 1 to 2 h at various time points after infection. Cell lysates were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), as described previously (57).
Subcellular fractionation and sodium carbonate extraction of membranes.
Preparation of the total microsomal fraction of wt BUNV-infected Vero E6 cells was performed as described by Ehrenreich et al. (6) with minor modifications. Briefly, cells grown in 35-mm-diameter petri dishes were infected with an MOI of 5.0 PFU per cell and were radiolabeled for 2 h with 100 μCi [35S]methionine at 24 h postinfection. The cells were scraped into phosphate-buffered saline (PBS) and washed twice with cold PBS and once with 0.25 mM sucrose-10 mM HEPES buffer by centrifugation at 2,500 rpm for 3 min at 4°C. The cell pellet was resuspended in 400 μl of 0.25 mM sucrose-10 mM HEPES buffer and disrupted by three freeze-thaw cycles alternatively at 37°C and on dry ice, followed by three 10-s pulses of sonication in a water bath at 4°C. After centrifugation at 10,000 × g for 10 min at 4°C, the supernatant (total fraction) was further centrifuged at 65,000 rpm for 15 min (Beckman TL-100 rotor) to produce the soluble and pellet (microsomal) fractions. The microsomal fraction was resuspended in 400 μl 0.1 M Na2CO3 solution (pH, 11.0) and incubated for 30 min on ice. The sample was laid on top of 200 μl 0.25 M sucrose-10 mM HEPES buffer and centrifuged at 65,000 rpm at 4°C. The pellet was resuspended in 200 μl of 0.25 M sucrose-10 mM HEPES buffer (membrane fraction). The samples were then subjected to SDS-PAGE analysis and Western blotting.
Indirect immunofluorescence staining.
Immunofluorescence assays were performed as previously described (49). Briefly, the infected or transfected cells were fixed with 4% paraformaldehyde and then permeabilized with 0.1% Triton X-100 in PBS before staining with specific primary antibodies and secondary antibody conjugates. Some samples were semipermeabilized by the freeze-thawing method as described by Mardones and Gonzalez (31) with minor modifications. Cells on coverslips were placed on a precooled metal block (−70°C) for 10 s. Upon freezing, cells were put on a prewarmed metal block (40°C) for 10 s and were then fixed in PBS-4% paraformaldehyde. The localization of fluorescently labeled proteins was examined using either a Zeiss LSM confocal microscope or Delta Vision restoration microscope as indicated in the figure legends.
BUNV assembly assay.
BSR-T7/5 cells were transfected with three expression constructs, pTM1-BUNN (0.1 μg), pTM1-BUNL (0.25 μg), and either pTM1-BUNM (0.2 μg) or one of the NSm mutants cloned in pTM1 (also 0.2 μg), together with 0.3 μg of the BUNV-derived minigenome, pT7riboBUNMRen(−) (minigenome containing Renilla luciferase reporter gene) (59). pTM1-FFluc (0.1 μg), which contains the firefly luciferase gene (58), was cotransfected as an internal transfection control. Five hours later, the transfection mixture was removed and replaced with 2 ml of growth medium. At 24 h posttransfection, the supernatant was clarified by centrifugation for 3 min at 1,700 rpm, and 1.5 ml was used to infect BSR-T7/5 cells that had been transfected with pTM1-BUNN (0.1 μg) and pTM1-BUNL (0.2 μg) for 5 h. Renilla luciferase activity was measured after 24 h incubation using the dual-luciferase assay kit (Promega) as described previously (20). The level of Renilla luciferase activity of the infected cells was used as a measure of infectious VLP production. The specificity of passage of Renilla luciferase was demonstrated by the absence of firefly luciferase activity in the same cell extract.
Yeast two-hybrid assay.
Protein-protein interaction assays were performed using a commercial yeast two-hybrid system, Matchmaker 3 (Clontech, Palo Alto, CA), as described previously (28). Briefly, Saccharomyces cerevisiae strain AH109 (17) cells were cotransformed with GAL4 AD- and BD-containing plasmids expressing the genes of interest using the lithium acetate method (13). Cotransfection with the appropriate other empty vector (pGBKT7 or pGADT7) was used as a negative control. Further controls were provided in the Matchmaker 3 system and included pGADT7-T and pGBKT7-53 (positive control) and pGADT7-T and pGADT7-Lam (negative control). Yeast colonies were selected on SD-L-W-H-A selective medium containing various amounts of 3-amino-1,2,4 triazol (26).
RESULTS
Intracellular localization and membrane integrality of NSm.
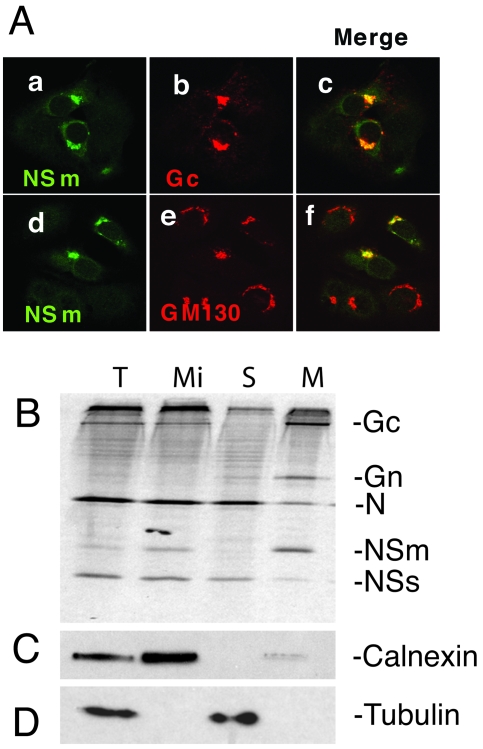
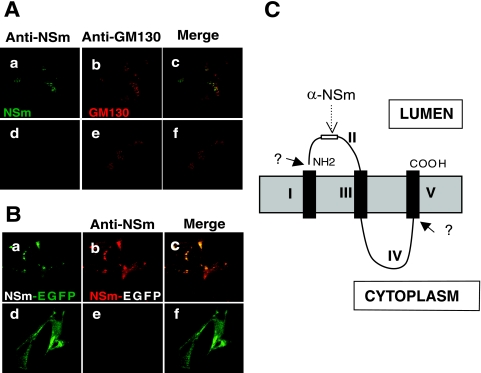
BUNV NSm comprises residues 303 to 477 of the 1,433-amino-acid precursor, sandwiched between the two viral glycoproteins, Gn and Gc (6) (Fig. 1A). It was reported previously that NSm localized in the Golgi region of infected cells based on its colocalization with wheat germ agglutinin (35). We reassessed this result by using more sensitive reagents, namely an antibody to the generic Golgi marker GM130, a cis-Golgi matrix protein (34), and a monoclonal antibody to Gc, to stain BUNV-infected cells. As shown in Fig. 2A, NSm colocalized with both Gc protein (Fig. 2A, panels a to c) and GM130 (panels d to f), confirming that the nonstructural protein is able to locate to, and accumulate in, the Golgi complex like the mature viral glycoproteins. However, these data do not distinguish whether NSm is an integral or peripheral membrane protein.
FIG. 2.
Intracellular localization and determination of the membrane integrality of NSm. (A) Colocalization of NSm with Gc (panels a to c) and Golgi matrix protein GM130 (panels d to f). wt BUNV-infected BSR-T7/5 cells were stained with a mixture of anti-NSm antibody and either anti-Gc MAb 742 or anti-GM130 MAb. NSm stains green (panels a and d), and Gc and GM130 stain red. Merged confocal microscopic images are also shown, with colocalization shown in yellow (panels c and f). (B) NSm is an integral membrane protein. Vero E6 cells were infected with wt BUNV and radiolabeled with [35S]methionine, and membrane fractions were prepared as described in Materials and Methods. Total (T) and microsomal (Mi) fractions were collected, and membranes were extracted with sodium carbonate to yield supernatant (S) and membrane (M) samples. The fractions were analyzed by SDS-PAGE. The positions of viral proteins are indicated at the right. (C). Western blot analysis of the gel using anti-calnexin antibodies as a marker for membranes. (D) Western blot analysis of the gel using anti-tubulin antibodies as a marker for the cytosolic fraction.
Five domains in NSm are predicted by the program TMHMM (23) (http://www.cbs.dtu.dk/services/TMHMM-2.0/): three hydrophobic domains (residues 306 to 327 [I], 362 to 390 [III], and 457 to 477 [V]) and two nonhydrophobic regions (residues 328 to 361 [II] and 391 to 456 [IV]) (Fig. 1A). The presence of hydrophobic domains in NSm and its association with the Golgi complex suggest that NSm is an integral membrane protein. NSm, together with the two viral glycoproteins Gn and Gc, N protein, and NSs protein were found in the microsomal fraction of infected cells (Fig. 2B). To investigate the membrane association of NSm, membrane fractions of wt BUNV-infected cells were extracted using sodium carbonate, which selectively strips extrinsic proteins off membranes without affecting the disposition of integral components (10). Western blotting using antibodies specific for the cellular integral membrane protein (calnexin) and cytosolic protein (tubulin) confirmed the fractionation procedure (Fig. 2C and D). SDS-PAGE analysis demonstrated that NSm was found exclusively in the membrane fraction, together with two known viral membrane proteins, Gn and Gc (Fig. 2B). The nucleocapsid protein (N) was shown predominantly in the supernatant fraction, though some N protein was also present in the membrane fraction. We suggest this likely represents N protein within virus particles that were entrapped in the membrane fractions.
Membrane topology of NSm.
The topology of NSm in intracellular membranes was examined by assessing the accessibility of an anti-NSm antibody, which was raised against NSm resides 338 to 353 (35), to its cognate epitope in semipermeabilized virus-infected cells. Cells on glass coverslips were frozen at −70°C for 10 s and then thawed at 40°C for 10 s. The freeze-thaw treatment allows the antibody to penetrate the plasma membrane but not the intracellular membranes, which remain impermeable (31). As a control, the semipermeabilized cells were further treated with 0.2% Triton X-100 to permeabilize them fully. As shown in Fig. 3A, both NSm and the cis-Golgi matrix protein GM130 were detected in fully permeabilized cells (panels a to c). However, in semipermeabilized cells, although GM130 was detected, no staining of NSm was evident (Fig. 3A, panels d to f), indicating that domain II, the first stretch of predominantly nonhydrophobic amino acids (residues 328 to 361) of NSm, was not accessible to the anti-NSm antibodies. We also analyzed cells infected with a newly constructed recombinant virus rBUNM-NSm-EGFP (see later for details) that expresses an NSm-EGFP hybrid protein. Anti-NSm antibodies were not able to access the same region of the recombinant NSm-EGFP fusion protein in semipermeabilized cells (Fig. 3B, d to f) but reacted with the protein in fully permeabilized cells (Fig. 3B, a to c). Thus, we conclude that NSm domain II is located inside an intracellular compartment, such as the endoplasmic reticulum (ER) or Golgi complex. A possible topological model of NSm is shown in Fig. 3C which is consistent with that suggested by Pettersson and Melin (40).
FIG. 3.
Determination of the topology of BUNV NSm. Vero E6 cells were infected with wt BUNV (A) or recombinant virus rBUNM-NSm-EGFP (B) and semipermeabilized by the freeze-thaw technique. Cells shown in the upper row of each set were further permeabilized with 0.2% Triton X-100-PBS (panels a to c in each case). Before examination by confocal microscopy, the wt BUNV-infected cells were costained with rabbit anti-NSm serum and anti-GM130 MAb and the rBUNM-NSm-EGFP-infected cells were stained only with anti-NSm serum. In panel A, NSm stains green and GM130 stains red, and in panel B, the EGFP autofluorescence of NSm-EGFP protein shows as green and the NSm antibody stains red. Merged confocal images are also shown. NSm antibodies can only react with NSm or NSm-EGFP in fully permeabilized cells. (C) The predicted topology of NSm. Hydrophobic domains are shown as black columns across the intracellular membrane. The positions of the predicted domains I to V and of the anti-NSm epitope are marked.
Role of NSm in virus particle assembly.
To investigate a possible role of NSm in virus morphogenesis, we developed a BUNV assembly assay by monitoring the formation of infectious VLPs based on the BUNV minigenome system (59). Briefly, BSR-T7/5 cells were transfected with the minigenome plasmid pT7riboBUNMRen(−), two expression constructs pTM1-BUNL and pTM1-BUNN that provide BUNV L and N proteins to transcribe and replicate the minigenome, and either pTM1-BUNM (expressing wt glycoproteins and NSm) or one of the seven pTM1-BUNM-derived constructs containing deletions in the NSm coding region (Fig. 1B). A plasmid encoding the firefly luciferase gene was cotransfected as an internal control to monitor transfection efficiency. Supernatants from the transfected cells were used to infect the fresh BSR-T7/5 cells that were previously transfected with the pTM1-BUNL and pTM1-BUNN plasmids. If infectious VLPs were formed in the initially transfected cells, their presence would be detected by the passage of the minigenome and, hence, subsequent Renilla luciferase activity to new cells.
The assay was used to analyze the requirement for NSm on VLP formation. As shown in Fig. 4A, infectious VLPs were produced in cells that were cotransfected with pTM1-BUNM (wt control, column 2), which expresses intact NSm, and with pTM1-BUNM-NSmΔ4 and pTM1-BUNM-NSmΔ5 (columns 7 and 8), which contain deletions in domains III and IV. No VLPs were detected in the supernatant from cells cotransfected with pTM1-BUNM-NSmΔ1, pTM1-BUNM-NSmΔ2, pTM1-BUNM-NSmΔ3, pTM1-BUNM-NSmΔ6, or pTM1-BUNMΔNSm, nor from the control cells transfected with empty pTM1 or an inactive L protein mutant or from nontransfected cells. The authenticity of the VLPs was validated by their effective neutralization by anti-BUN antiserum (column 3). In addition, there was no passage of firefly luciferase from the initially transfected cells to the new cells, indicating specific packaging of the BUNV minigenome (data not shown). These results showed that VLP formation with construct pTM1-BUNM-NSmΔ4 was as efficient as that with the wt M segment cDNA construct, whereas VLP formation in cells transfected with pTM1-BUNM-NSmΔ5 was compromised (about 35% of luciferase activity was passaged compared to wt).
FIG. 4.
Effect of internal deletions in NSm on virus assembly, protein processing, and intracellular transport. (A) Production of virus-like particles. BSR-T7/5 cells were transfected with minigenome-component plasmids and either wt BUNV M segment cDNA or mutated NSm-containing plasmids as indicated. Controls included pTM1 instead of pTM1-BUNM, substitution of wt BUNV L segment cDNA with an inactive L cDNA mutant, and mock-infected cells. Supernatants from these cells were taken 24 h posttransfection and used to infect fresh BSR-T7/5 cells that had been transfected with BUNV L and N protein-expressing plasmids 5 h previously. In one case, the supernatant was reacted with anti-BUN antibody prior to infection. Renilla luciferase activity in all extracts of these cells was measured after 24 h and is shown in arbitrary light units. 1, pTM1 vector control; 2, pTM1-BUNM (wt control); 3, anti-BUNV serum-neutralized supernatant from pTM1-BUN-M-transfected cells; 4, pTM1-BUNM-NSmΔ1; 5, pTM1-BUNM-NSmΔ2; 6, pTM1-BUNM-NSmΔ3; 7, pTM1-BUNM-NSmΔ4; 8, pTM1-BUNM-NSmΔ5; 9, pTM1-BUNM-NSmΔ6; 10, pTM1-BUNMΔNSm; 11, substitution with inactive L mutant; 12, mock-transfected cells. (B) Processing of BUNV glycoproteins from Vero E6 cells transfected with wt or NSm deletion mutant cDNA clones. Vero E6 cells were infected with recombinant vaccinia virus vTF7-3, followed by transfection with pTM1-BUNM (wt) or NSm mutant cDNA constructs. Cells were labeled with [35S]methionine for 15 h, extracts were prepared, and viral proteins were immunoprecipitated with anti-BUN serum. The labeled glycoproteins were subjected to endo H (H) or mock digestion (C) and analyzed by SDS-10% PAGE under reducing conditions. The relevant portions of the gels are shown. (C) Intracellular localization of Gc expressed from wt and mutated NSm-containing M segment cDNAs. BSR-T7/5 cells were transfected cDNA constructs as indicated and were stained with a mixture of anti-Gc MAb 742 and anti-GM130 antibodies and 4′,6′-diamidino-2-phenylindole (DAPI). Cells were examined using the DeltaVision microscopy system (AppliedPrecision), and triple-stained images are shown. Gc proteins stain red, the Golgi stains green, and cell nuclei, stained with DAPI, are shown in blue.
To ensure that the effect of deletions in the NSm coding region on VLP assembly was not simply due to defective processing of the precursor polyprotein, we monitored the synthesis of the glycoproteins by radiolabeling and the intracellular transport of Gc by immunofluorescent staining. SDS-PAGE analysis of radiolabeled and endo-β-N-acetylglucosaminidase H (endo H)-treated BUNV glycoproteins in cells transfected with wt or mutant constructs (Fig. 4B) showed that glycoproteins expressed from constructs pTM1-BUNM-NSmΔ1 to pTM1-BUNM-NSmΔ5 (lanes 3 to 13) were correctly cleaved. The Gc proteins expressed from pTM1-BUNM-NSmΔ1 and pTM1-BUNM-NSmΔ2 were predominantly endo H sensitive (lanes 4 and 6), while both endo H-sensitive and -resistant forms of Gc (evident as two closely migrating bands) could be seen in extracts from cells transfected with mutants pTM1-BUNM-NSmΔ3 (lane 8), pTM1-BUNM-NSmΔ4 (lane 11), pTM1-BUNM-NSmΔ5 (lane13), and pTM1-BUNMΔNSm (lane 17) as well as with wt BUNV M segment cDNA (lane 2). No glycoproteins of the correct size were detected in extracts of cells transfected with mutant pTM1-BUNM-NSmΔ6 that lacks domain V (lanes 14 and 15).
The intracellular localization of the expressed glycoproteins was monitored by immunofluorescent staining of transfected cells, using MAb 742 to detect Gc. Golgi targeting was influenced by deletion of the complete NSm coding region (pTM1-BUNMΔNSm) (Fig. 4C, panel h) and deletion of domain I (pTM1-BUNM-NSmΔ1) (panel b) where most Gc staining appears to be cytoplasmic. The pattern of staining was distinct from that observed in cells in which the intact NSm was expressed (panel a). However, Golgi localization of Gc appeared similar to the wt observed in cells transfected with pTM1-BUNM-NSmΔ2, -NSmΔ3, -NSmΔ4, and -NSmΔ5 (Fig. 4C, panels c to f). Deletion in NSm domain V completely abrogated intracellular transport of the glycoproteins to the Golgi, as Gc showed a cytoplasmic staining pattern (Fig. 4C, panel g). Together with the radiolabeling result (Fig. 4B, lanes 14 and 15), this indicates that the precursor polyprotein from transfected pTM1-BUNM-NSmΔ6 was not correctly processed.
Generation of recombinant BUNVs containing mutations in NSm.
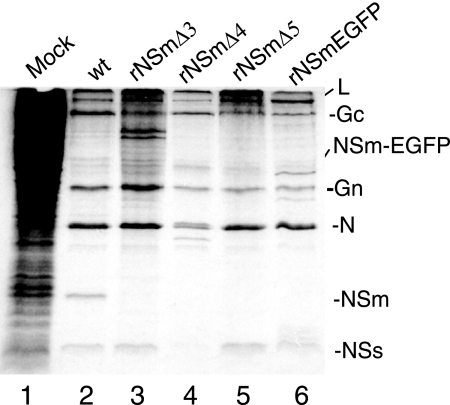
The role of NSm in viral replication was further evaluated using our improved reverse genetics system (2, 30) to recover NSm-deficient recombinant viruses. pT7riboBUNM(+) derivatives containing deletions in NSm were transfected into BSRT-7/5 cells, together with plasmids pT7riboBUNL(+) and pT7riboBUNS(+) that contain wt L and S segment cDNAs. Consistent with results form the VLP assay, no viable virus was recovered from repeated virus rescue attempts with the constructs that either lacked the complete NSm (in the case of pT7riboBUNMΔNSm) or contained internal deletions in domains I, II, and V (in the cases of pT7riboBUNM-NSmΔ1, -NSmΔ2, and -NSmΔ6). We were successful in rescuing three recombinant viruses from the NSm deletion constructs pT7riboBUNM-NSmΔ3, -NSmΔ4, and -NSmΔ5 that contain deletions covering domains II to IV (Fig. 1B) (the rescued recombinant viruses were designated rBUNM-NSmΔ3, rBUNM-NSmΔ4, rBUNM-NSmΔ5). The recovery of rBUNM-NSmΔ3 was somewhat unexpected, as no VLP production was detected using the construct pTM1-BUNM-NSmΔ3. No band equivalent to NSm was detected in the protein profiles of cells infected with the mutant viruses with viruses rBUNM-NSmΔ3, rBUNM-NSmΔ4, and rBUNM-NSmΔ5 (Fig. 5, lanes 3, 4, and 5). Two prominent bands below Gc were seen in cells infected with rBUNM-NSmΔ3. Their identity has not been pursued, but they may represent aberrant processing products of the M segment precursor. DNA sequence analysis of reverse transcription-PCR products of recombinant virus RNA confirmed that the viral genomes carried the expected deletions (data not shown).
FIG. 5.
Protein profiles of cells infected with wild-type and recombinant BUNV. Vero E6 cells were infected with wt BUNV (lane 2), rBUNM-NSmΔ3 (lane 3), rBUNM-NSmΔ4 (lane 4), rBUNM-NSmΔ5 (lane 5), and rBUNM-NSm-EGFP (lane 6) at 5 PFU/cell. At 24 hpi, cells were labeled with 100 μCi [35S]methionine for 2 h, and then equal amounts of cell lysate were analyzed by SDS-12.5% PAGE under reducing conditions. Positions of viral proteins are indicated.
The results from the above experiments, as well as those from studying Maguari virus mutants (41), suggest that the internal region of NSm, particularly residues in domains III and IV, is not required for virus growth cell culture. Thus, it was reasonable to predict that the internal region of NSm might tolerate insertion of a foreign gene. Therefore, to generate a recombinant virus capable of expressing GFP, we fused the enhanced GFP open reading frame to NSm in domain IV between residues 403 and 420 (Fig. 1B). We succeeded in rescuing a recombinant virus, designated rBUNM-NSm-EGFP, and used it for examining the membrane topology of NSm protein as described earlier. The expected size of the NSm-EGFP fusion protein is approximately 45,000 kDa, and a band of this size was shown above the Gn band by SDS-PAGE analysis of infected cell extracts (Fig. 5, lane 6). The expression of the NSm-EGFP was also evidenced by the EGFP fluorescence of rBUNM-NSm-EGFP-infected cells (Fig. 3B).
Effect of internal deletions in NSm on virus viability and growth in cell culture.
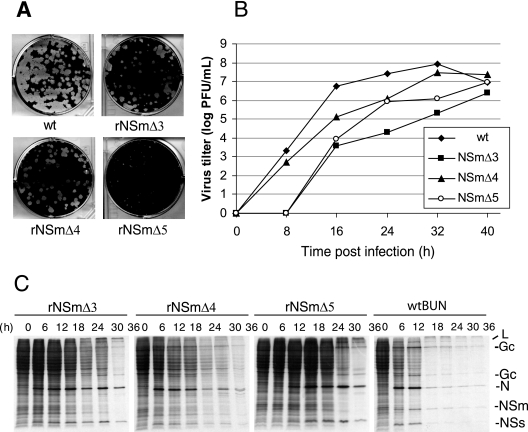
We compared the plaque size, growth kinetics, and ability to shutoff host cell protein synthesis of the three recombinant viruses carrying deletions in NSm (Fig. 6). The plaque morphology of the mutant viruses rBUNM-NSmΔ3 and rBUNM-NSmΔ4 were comparable to those of wt BUNV, while plaques produced by rBUNM-NSmΔ5 were much smaller (Fig. 6A). Analysis of the growth curves of the mutant viruses revealed that replication of all mutant viruses was compromised, to different extents, in comparison with that of wt BUNV (Fig. 6B). rBUNM-NSmΔ3 grew slowest, generating titers nearly 1,000-fold less than that of wt BUNV at 24 h postinfection, and maximal virus yield was almost 100-fold lower. rBUNM-NSmΔ5 and rBUNM-NSmΔ4 also grew more slowly than wt BUNV but reached maximal yields only 10-fold lower than wt BUNV. Analysis of the time course of protein synthesis (Fig. 6C), performed under similar multiplicities of infection, showed that shutoff of host cell protein was delayed in cells infected with the mutant viruses. Shutoff was already evident at 6 h postinfection (hpi) in wt BUNV-infected cells and was complete by 18 hpi; in the mutant virus-infected cells, shutoff was delayed by 6 h (rBUNM-NSmΔ4) to 24 h (rBUNM-NSmΔ5). Viral N protein synthesis was only significantly delayed in cells infected with rBUNM-NSmΔ5, though continued for longer than in cells infected with wt BUNV.
FIG. 6.
Plaque phenotype, growth kinetics, and protein synthesis shutoff of wt and mutant BUNV. (A) Comparison of plaque morphology on Vero E6 cells. Cell monolayers were fixed with 4% formaldehyde and stained with Giemsa solution 4 days after infection. (B) Viral growth curves. Vero E6 cells were infected with either wt or recombinant viruses at an MOI of 0.01 PFU/cell. Virus was harvested at 8-h intervals and titrated by plaque assay. The results shown are the averages from two independent titrations. (C) Time course of protein synthesis. Vero E6 cells infected at an MOI of 1.0 PFU/cell were labeled with 100 μCi [35S]methionine for 20 min at the time points indicated, and cell lysates were analyzed by SDS-15% PAGE. The positions of the viral proteins are indicated at the right.
Interaction of NSm with itself and other BUNV proteins.
Virus morphogenesis involves participation and interaction of multiple viral proteins and host proteins (55). We investigated the interaction of NSm with other viral components using coimmunoprecipitation and the yeast two-hybrid system. Coimmunoprecipitation analysis of BUNV-infected cell lysates with anti-BUN, anti-Gc, anti-NSm, and anti-N antibodies demonstrated that NSm did interact with other BUNV structural proteins and NSs (Fig. 7A). Anti-BUN, a rabbit antiserum raised against purified virus particles, coprecipitated both nonstructural proteins NSm and NSs in addition to the four structural proteins (Fig. 7A, lane 1). Anti-Gc MAb 742 coprecipitated not only Gc protein and Gn but also NSm and trace amounts of N protein (lane 3). Anti-NSm predominantly precipitated the NSm band, but minor amounts of the two glycoproteins and N were also visible (lane 5). The anti-N serum precipitated significant amounts of N and L proteins and trace amounts of all the other viral proteins. Comparison of the intensities of the bands suggests that specific interactions occur between N and L (lane 7) and Gc and Gn (lane 3). However, the increased intensity of the NSm band in lane 3 compared to lane 1 might be indicative of its interaction with one, or both, of the viral glycoproteins.
FIG. 7.
Interaction of Bunyamwera virus proteins. (A) Coimmunoprecipitation. BUNV-infected Vero E6 cells (at 5 PFU/cell) were labeled with 80 μCi [35S]methionine for 4 h at 30 h postinfection, and equal volumes of cell lysate were immunoprecipitated with anti (α)-BUN serum (lanes 1 and 2), anti-Gc MAb 742 (lanes 3 and 4), anti-NSm serum (lanes 5 and 6), or anti-N serum (lanes 7 and 8). I, BUNV-infected cells; C, mock-infected control cells. The positions of the viral proteins are indicated. (B) Interaction studied by yeast two-hybrid analysis. S. cerevisiae AH109 cells were cotransformed with plasmids as listed below and plated on selective medium as described in Materials and Methods. Plasmid combinations: a, pBK-NSm + pAD-NSm; b, pBK + pAD-NSm; c, pBK-NSm + pAD; d, pBK-NSs + pAD-NSm; e, pBK-NSs + pAD; f, negative control; g, pBK-T + pAD-p53; h, pBK-T + pAD-Lam; i, pBK-Gn + pAD; j, pBK-Gn + pAD-NSm; k, pBK + pAD-NSm; l, negative control. Growth on the plates indicates interaction of NSm with itself (sector a), with NSs (sector d), and the cytoplasmic tail of Gn (sector j).
By using the Matchmaker 3 yeast two-hybrid system, we demonstrated that the predicated cytoplasmic domain (residues 391 to 456) of NSm was able to self-interact (Fig. 7B, sector a). Moreover, the same domain was also able to interact with the NSs protein (Fig. 7B, sector d) and with the cytoplasmic tail of Gn (Fig. 7B, sector j). However, the interaction between N and NSm, weakly seen in the coimmunoprecipitation assay, was not confirmed in the yeast two-hybrid system (V. H. J. Leonard and R. M. Elliott, unpublished data).
DISCUSSION
Virus assembly and budding at the Golgi is one of the characteristic features of the Bunyaviridae family (25, 45) and is likely mediated by the accumulation of the two virion glycoproteins, Gn and Gc, in this organelle (40). Recently, electron microscopic examination of BUNV-infected cells provided evidence of a “viral factory” in the perinuclear area (45), similar to the factories described for other enveloped viruses (16, 38, 43-45). In addition, the BUNV nonstructural protein NSm also localizes to the Golgi (26, 35), which suggested that NSm may be involved in virus assembly and morphogenesis. However, characterization of a mutant of the related Maguari virus indicated that the C-terminal two-thirds of NSm are not essential (41). To understand further the role of NSm, we determined its membrane integrality, intracellular localization, topology on intracellular membranes, and interaction with other viral proteins. Furthermore, we generated recombinant viruses carrying mutations in NSm using our improved reverse genetics techniques.
The hydropathy profile and computer-aided domain prediction analysis of NSm (Fig. 1A) revealed that the protein has features common to transmembrane proteins (8). We demonstrated that NSm was indeed an integral membrane protein by its resistance to sodium carbonate extraction (10) and its partitioning with the viral glycoproteins in membranes isolated from infected cells. The orientation of integral membrane proteins is defined by the distribution of charged residues flanking the hydrophobic core of the signal-anchor sequence (15). On this basis, it was predicted that NSm domain II should be located in the ER lumen, with the second hydrophobic domain (domain III) acting as a transmembrane anchor (Fig. 3C). This prediction correlated with the result from experiments to assess the accessibility of an epitope on NSm to its cognate antibody in semipermeabilized cells infected by wt BUNV or a recombinant BUNV expressing an NSm-EGFP fusion protein.
The production of VLPs from recombinant expressed proteins has proved a valuable technique to study virus assembly (39). The assembly of infectious VLPs probably requires all the components for virus replication, including the structural and nonstructural proteins as well as the virus genome or genome analogue. We developed an assay to produce infectious BUNV VLPs that were capable of transferring a minigenome RNA-expressing luciferase (59), by infection, into new cells. It was found that no infectious VLPs were produced when either the full-length NSm or regions in domains I, II, III, or V were removed from the M segment polyprotein precursor. This correlated with the inability to rescue infectious virus by reverse genetics from the cDNA constructs that contained the same deletions in domains I, II, and V. However, we were able to recover a viable virus from pT7riboBUN-NSmΔ3 that contains the deletion in domain III; this mutant virus exhibited the poorest growth kinetics of the mutant viruses rescued, perhaps indicative of inefficient virus assembly. Presumably, the VLP assay in its present form is not sufficiently sensitive for use with precursor proteins containing such disruptive mutations. Infectious VLPs and recombinant viruses were obtained from constructs containing deletions in domain IV. In addition, we were able to recover a viable virus expressing a hybrid NSm-EGFP protein, indicating that incorporation of a foreign sequence did not interfere with NSm function or, indeed, processing of the M segment precursor protein. This correlates with data from Bupp et al. (5), who demonstrated that insertion of a portion of the beta-galactosidase gene into the La Crosse virus NSm coding region did not affect processing or intracellular targeting of the glycoproteins when expressed via vaccinia virus vectors.
Correct processing of the glycoprotein precursor depends both on the sequence and protease accessibility of the Gn-NSm and NSm-Gc cleavage sites and on the presence of N-terminal signal sequences to translocate the proteins across the ER membrane. The suggested topology of the precursor (40) and of NSm (Fig. 3C) is similar to that of the Semliki Forest virus membrane protein precursor that comprises p62-6K-E1 proteins (46). In this case, the C-terminal hydrophobic domains of p62 and 6K act as an internal signal sequences for 6K and E1, respectively. Deletion of 6K does not destroy virus viability, and the hydrophobic domain in p62 can substitute as the E1 signal sequence (29). Analyses of the mutants described in this paper and of the Maguari virus R2 mutant (41) suggest a similar arrangement for the orthobunyavirus precursor. We rescued three mutant viruses (rBUNM-NSmΔ3, -NSmΔ4, and -NSmΔ5) that contain deletions in part of domains II, III, and IV, but we failed to recover viable virus from cDNA constructs that contain deletions in domains I and V or in which the entire NSm coding region was removed. Thus, we suggest that domain I acts as the internal signal sequence for NSm itself and domain V acts as the internal signal sequence for Gc, but this role is assumed by domain I in Maguari virus R2, which contains an extensive deletion in NSm (40). However, it is still not clear whether hydrophobic domains I and V remain attached to or are cleaved from the mature NSm protein. In snowshoe hare orthobunyavirus-infected cells, proteins of 11,000 molecular weight (11K) and 10K were mapped to the NSm region of the polyprotein, but the relationship between these proteins remains unknown (9). For Germiston orthobunyavirus, NSm appeared as a 16K doublet upon SDS-PAGE (12). These data may indicate that NSm does indeed undergo further processing.
The alignment in Fig. 1A shows that the sequence of the portion of NSm remaining in Maguari virus R2 is very similar to that in BUNV and extends into a conserved region in domain II. The maintenance of this conserved sequence in Maguari virus R2 and the failure to recover a recombinant BUNV with this region deleted (from pT7riboBUNM-NSmΔ1, pT7riboBUNM-NSmΔ2, and pT7riboBUNMΔNSm) are indicative that this region may be critical in virus assembly. Note that, in pT7riboBUNMΔNSm, domain V was retained, which permitted polyprotein cleavage and acquisition of endo H resistance (Fig. 4B) and partial Golgi localization (Fig. 4C). At present, it is unclear how Gc derived from pT7riboBUNM-NSmΔ3 can be correctly translocated into the cell membrane. The lack of transmembrane domain III suggests that the C terminus of the mutated NSm protein would be on the cytoplasmic side rather than in the lumen of the ER, and the program TMHMM gave ambiguous predictions on membrane topology (data not shown). However, since the precursor is cotranslationally cleaved, it is possible that the presence of domain V could allow Gc to translocate independently.
It is well known that viral assembly and morphogenesis involve interaction between viral proteins and interaction between the viral and host cell proteins (11, 39, 42, 55). NSm was shown to interact with itself and the cytoplasmic tail of Gn protein, interactions that would appear to be relevant (though not obligatory) to viral assembly. The interaction with the other nonstructural protein NSs is less obvious. NSs is a virulence factor and is involved in interferon antagonism and shutoff of host cell protein synthesis (3, 19, 53, 54, 58). NSm does not seem to be involved in interferon antagonism, as the NSm mutants had not lost their ability to inhibit the interferon response (unpublished data). Although host protein shutoff was significantly delayed by the mutant viruses, especially rBUNM-NSmΔ5, this is more likely because of slower growth of the viruses; however, other effects of NSm, such as on the ER stress response, cannot be discounted at this stage. Likewise, whether the deletions in NSm affect pathogenesis in animals requires further investigation.
In short, we characterized the BUNV NSm protein by determining the membrane integrality, topology, and role in virus replication. A novel virus assembly assay based on a reverse minigenome system was developed and applied to assess the role of NSm in virus assembly and packaging. Our results demonstrated that the N-terminal region of NSm plays an important role in assembly and morphogenesis of orthobunyaviruses.
Acknowledgments
We thank Klaus Conzelmann, Martin Lowe, and Bernard Moss for provision of reagents used in this study.
The work was supported by Wellcome Trust grants to R.M.E.
REFERENCES
- 1.Bishop, D. H. 1996. Biology and molecular biology of bunyaviruses, p. 19-61. In R. M. Elliott (ed.), The Bunyaviridae. Plenum Press, New York, N.Y.
- 2.Bridgen, A., and R. M. Elliott. 1996. Rescue of a segmented negative-strand RNA virus entirely from cloned complementary DNAs. Proc. Natl. Acad. Sci. USA 93:15400-15404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bridgen, A., F. Weber, J. K. Fazakerley, and R. M. Elliott. 2001. Bunyamwera bunyavirus nonstructural protein NSs is a nonessential gene product that contributes to viral pathogenesis. Proc. Natl. Acad. Sci. USA 98:664-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchholz, U. J., S. Finke, and K. K. Conzelmann. 1999. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J. Virol. 73:251-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bupp, K., K. Stillmock, and F. Gonzalez-Scarano. 1996. Analysis of the intracellular transport properties of recombinant La Crosse virus glycoproteins. Virology 220:485-490. [DOI] [PubMed] [Google Scholar]
- 6.Ehrenreich, J. H., J. J. Bergeron, P. Siekevitz, and G. E. Palade. 1973. Golgi fractions prepared from rat liver homogenates. I. Isolation procedure and morphological characterization. J. Cell Biol. 59:45-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elliott, R. M. 1997. Emerging viruses: the Bunyaviridae. Mol. Med. 3:572-577. [PMC free article] [PubMed] [Google Scholar]
- 8.Elliott, R. M. 1990. Molecular biology of the Bunyaviridae. J. Gen. Virol. 71:501-522. [DOI] [PubMed] [Google Scholar]
- 9.Fazakerley, J. K., F. Gonzalez-Scarano, J. Strickler, B. Dietzschold, F. Karush, and N. Nathanson. 1988. Organization of the middle RNA segment of snowshoe hare Bunyavirus. Virology 167:422-432. [PubMed] [Google Scholar]
- 10.Fujiki, Y., A. L. Hubbard, S. Fowler, and P. B. Lazarow. 1982. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J. Cell Biol. 93:97-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garoff, H., R. Hewson, and D. J. Opstelten. 1998. Virus maturation by budding. Microbiol. Mol. Biol. Rev. 62:1171-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerbaud, S., N. Pardigon, P. Vialat, and M. Bouloy. 1992. Organization of Germiston bunyavirus M open reading frame and physicochemical properties of the envelope glycoproteins. J Gen. Virol. 73:2245-2254. [DOI] [PubMed] [Google Scholar]
- 13.Gietz, D., A. St Jean, R. A. Woods, and R. H. Schiestl. 1992. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 20:1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldbach, R., and D. Peters. 1996. Molecular and biological aspects of tospoviruses, p. 129-157. In R. M. Elliott (ed.), The Bunyaviridae. Plenum Press, New York, N.Y.
- 15.High, S., and B. Dobberstein. 1992. Mechanisms that determine the transmembrane disposition of proteins. Curr. Opin. Cell Biol. 4:581-586. [DOI] [PubMed] [Google Scholar]
- 16.Hobman, T. C., L. Woodward, and M. G. Farquhar. 1993. The rubella virus E2 and E1 spike glycoproteins are targeted to the Golgi complex. J. Cell Biol. 121:269-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.James, P., J. Halladay, and E. A. Craig. 1996. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics 144:1425-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kakach, L. T., T. L. Wasmoen, and M. S. Collett. 1988. Rift Valley fever virus M segment: use of recombinant vaccinia viruses to study phlebovirus gene expression. J. Virol. 62:826-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kohl, A., R. F. Clayton, F. Weber, A. Bridgen, R. E. Randall, and R. M. Elliott. 2003. Bunyamwera virus nonstructural protein NSs counteracts interferon regulatory factor 3-mediated induction of early cell death. J. Virol. 77:7999-8008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohl, A., T. J. Hart, C. Noonan, E. Royall, L. O. Roberts, and R. M. Elliott. 2004. A bunyamwera virus minireplicon system in mosquito cells. J. Virol. 78:5679-5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kormelink, R., P. de Haan, C. Meurs, D. Peters, and R. Goldbach. 1992. The nucleotide sequence of the M RNA segment of tomato spotted wilt virus, a bunyavirus with two ambisense RNA segments. J. Gen. Virol. 73:2795-2804. [DOI] [PubMed] [Google Scholar]
- 22.Kormelink, R., M. Storms, J. Van Lent, D. Peters, and R. Goldbach. 1994. Expression and subcellular location of the NSm protein of tomato spotted wilt virus (TSWV), a putative viral movement protein. Virology 200:56-65. [DOI] [PubMed] [Google Scholar]
- 23.Krogh, A., B. Larsson, G. von Heijne, and E. L. Sonnhammer. 2001. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 305:567-580. [DOI] [PubMed] [Google Scholar]
- 24.Kuismanen, E., B. Bang, M. Hurme, and R. F. Pettersson. 1984. Uukuniemi virus maturation: immunofluorescence microscopy with monoclonal glycoprotein-specific antibodies. J. Virol. 51:137-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuismanen, E., K. Hedman, J. Saraste, and R. F. Pettersson. 1982. Uukuniemi virus maturation: accumulation of virus particles and viral antigens in the Golgi complex. Mol. Cell. Biol. 2:1444-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lappin, D. F., G. W. Nakitare, J. W. Palfreyman, and R. M. Elliott. 1994. Localization of Bunyamwera bunyavirus G1 glycoprotein to the Golgi requires association with G2 but not with NSm. J. Gen. Virol. 75:3441-3451. [DOI] [PubMed] [Google Scholar]
- 27.Law, M. D., J. Speck, and J. W. Moyer. 1992. The M RNA of impatiens necrotic spot Tospovirus (Bunyaviridae) has an ambisense genomic organization. Virology 188:732-741. [DOI] [PubMed] [Google Scholar]
- 28.Leonard, V. H., A. Kohl, J. C. Osborne, A. McLees, and R. M. Elliott. 2005. Homotypic interaction of Bunyamwera virus nucleocapsid protein. J. Virol. 79:13166-13172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liljestrom, P., and H. Garoff. 1991. Internally located cleavable signal sequences direct the formation of Semliki Forest virus membrane proteins from a polyprotein precursor. J. Virol. 65:147-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lowen, A. C., C. Noonan, A. McLees, and R. M. Elliott. 2004. Efficient bunyavirus rescue from cloned cDNA. Virology 330:493-500. [DOI] [PubMed] [Google Scholar]
- 31.Mardones, G., and A. Gonzalez. 2003. Selective plasma membrane permeabilization by freeze-thawing and immunofluorescence epitope access to determine the topology of intracellular membrane proteins. J. Immunol. Methods 275:169-177. [DOI] [PubMed] [Google Scholar]
- 32.Moss, B., O. Elroy-Stein, T. Mizukami, W. A. Alexander, and T. R. Fuerst. 1990. Product review. New mammalian expression vectors. Nature 348:91-92. [DOI] [PubMed] [Google Scholar]
- 33.Murphy, F. A., A. K. Harrison, and S. G. Whitfield. 1973. Bunyaviridae: morphologic and morphogenetic similarities of Bunyamwera serologic supergroup viruses and several other arthropod-borne viruses. Intervirology 1:297-316. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura, N., C. Rabouille, R. Watson, T. Nilsson, N. Hui, P. Slusarewicz, T. E. Kreis, and G. Warren. 1995. Characterization of a cis-Golgi matrix protein, GM130. J. Cell Biol. 131:1715-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakitare, G. W., and R. M. Elliott. 1993. Expression of the Bunyamwera virus M genome segment and intracellular localization of NSm. Virology 195:511-520. [DOI] [PubMed] [Google Scholar]
- 36.Nichol, S. T., B. Beaty, R. M. Elliott, R. Goldbach, A. Plyusnin, A. L. Schmaljohn, and R. B. Tesh. 2005. Bunyaviridae, p. 695-716. In C. M. Fauquet, M. A. Mayo, J. Maniloff, U. Desselberger, and L. A. Ball (ed.), Virus taxonomy. Elsevier, Amsterdam, The Netherlands.
- 37.Novoa, R. R., G. Calderita, R. Arranz, J. Fontana, H. Granzow, and C. Risco. 2005. Virus factories: associations of cell organelles for viral replication and morphogenesis. Biol. Cell 97:147-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Novoa, R. R., G. Calderita, P. Cabezas, R. M. Elliott, and C. Risco. 2005. Key Golgi factors for structural and functional maturation of bunyamwera virus. J. Virol. 79:10852-10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palucha, A., A. Loniewska, S. Satheshkumar, A. M. Boguszewska-Chachulska, M. Umashankar, M. Milner, A.-L. Haenni, and H. S. Savithri. 2005. Virus-like particles: models for assembly studies and foreign epitope carriers. Prog. Nucleic Acid Res. Mol. Biol. 80:135-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pettersson, R. F., and L. Melin. 1996. Snythesis, assembly, and intracellular transport of Bunyaviridae membrane proteins, p. 159-188. In R. M. Elliott (ed.), The Bunyaviridae. Plenum Press, New York, N.Y.
- 41.Pollitt, E., J. Zhao, P. Muscat, and R. M. Elliott. 2006. Characterizatioin of Maguari orthobunyavirus mutants suggests the nonstructural protein NSm is not essecial for growth in tissue culture. Virology 348:224-232. [DOI] [PubMed] [Google Scholar]
- 42.Pornillos, O., J. E. Garrus, and W. I. Sundquist. 2002. Mechanisms of enveloped RNA virus budding. Trends Cell Biol. 12:569-579. [DOI] [PubMed] [Google Scholar]
- 43.Risco, C., J. L. Carrascosa, and T. K. Frey. 2003. Structural maturation of rubella virus in the Golgi complex. Virology 312:261-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salanueva, I. J., J. L. Carrascosa, and C. Risco. 1999. Structural maturation of the transmissible gastroenteritis coronavirus. J. Virol. 73:7952-7964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salanueva, I. J., R. R. Novoa, P. Cabezas, C. Lopez-Iglesias, J. L. Carrascosa, R. M. Elliott, and C. Risco. 2003. Polymorphism and structural maturation of bunyamwera virus in Golgi and post-Golgi compartments. J. Virol. 77:1368-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schlesinger, S., and M. J. Schlesinger. 2001. Togaviridae: the viruses and their replication, p. 895-916. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott, Williams, & Wilkins, Philadelphia, Pa.
- 47.Schmaljohn, C., and J. W. Hooper. 2001. Bunyaviridae: the viruses and their replication, p. 1581-1602. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott, Williams, & Wilkins, Philadelphia, Pa.
- 48.Shi, X., and R. M. Elliott. 2004. Analysis of N-linked glycosylation of hantaan virus glycoproteins and the role of oligosaccharide side chains in protein folding and intracellular trafficking. J. Virol. 78:5414-5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi, X., and R. M. Elliott. 2002. Golgi localization of Hantaan virus glycoproteins requires coexpression of G1 and G2. Virology 300:31-38. [DOI] [PubMed] [Google Scholar]
- 50.Shi, X., D. F. Lappin, and R. M. Elliott. 2004. Mapping the Golgi targeting and retention signal of Bunyamwera virus glycoproteins. J. Virol. 78:10793-10802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soellick, T.-R., J. F. Uhrig, G. L. Bucher, J.-W. Kellmann, and P. H. Schreier. 2000. The movement protein NSm of tomato spotted wilt tospovirus (TSWV): RNA binding, interaction with the TSWV N protein, and identification of interacting plant proteins. Proc. Natl. Acad. Sci. USA 97:2373-2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Storms, M. M., R. Kormelink, D. Peters, J. W. Van Lent, and R. W. Goldbach. 1995. The nonstructural NSm protein of tomato spotted wilt virus induces tubular structures in plant and insect cells. Virology 214:485-493. [DOI] [PubMed] [Google Scholar]
- 53.Streitenfeld, H., A. Boyd, J. K. Fazakerley, A. Bridgen, R. M. Elliott, and F. Weber. 2003. Activation of PKR by Bunyamwera virus is independent of the viral interferon antagonist NSs. J. Virol. 77:5507-5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thomas, D., G. Blakqori, V. Wagner, M. Banholzer, N. Kessler, R. M. Elliott, O. Haller, and F. Weber. 2004. Inhibition of RNA polymerase II phosphorylation by a viral interferon antagonist. J. Biol. Chem. 279:31471-31477. [DOI] [PubMed] [Google Scholar]
- 55.Villanueva, R. A., Y. Rouillé, and J. Dubuisson. 2005. Interactions between virus proteins and host cell membranes during the viral life cycle. Int. Rev. Cytol. 245:171-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wasmoen, T. L., L. T. Kakach, and M. S. Collett. 1988. Rift Valley fever virus M segment: cellular localization of M segment-encoded proteins. Virology 166:275-280. [DOI] [PubMed] [Google Scholar]
- 57.Watret, G. E., C. R. Pringle, and R. M. Elliott. 1985. Synthesis of bunyavirus-specific proteins in a continuous cell line (XTC-2) derived from Xenopus laevis. J. Gen. Virol. 66:473-482. [DOI] [PubMed] [Google Scholar]
- 58.Weber, F., A. Bridgen, J. K. Fazakerley, H. Streitenfeld, N. Kessler, R. E. Randall, and R. M. Elliott. 2002. Bunyamwera bunyavirus nonstructural protein NSs counteracts the induction of alpha/beta interferon. J. Virol. 76:7949-7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weber, F., E. F. Dunn, A. Bridgen, and R. M. Elliott. 2001. The Bunyamwera virus nonstructural protein NSs inhibits viral RNA synthesis in a minireplicon system. Virology 281:67-74. [DOI] [PubMed] [Google Scholar]