Abstract
Sperm competition is a pervasive selective force in evolution, shaping reproductive anatomy, physiology and behaviour. Here, we present comparative evidence that varying sperm competition levels account for variation in the male reproductive anatomy of rodents, the largest and most diverse mammalian order. We focus on the sperm-producing testes and the accessory reproductive glands, which produce the seminal fluid fraction of the ejaculate. We demonstrate a positive association between relative testis size and the prevalence of within-litter multiple paternity, consistent with previous analyses in which relative testis size has been found to correlate with sperm competition levels inferred from social organization and mating systems. We further demonstrate an association between sperm competition level and the relative size of at least two accessory reproductive glands: the seminal vesicles and anterior prostate. The size of the major product of these glands—the copulatory plug—is also found to vary with sperm competition level. Our findings thus suggest that selection for larger plugs under sperm competition may explain variation in accessory gland size, and highlight the need to consider both sperm and non-sperm components of the male ejaculate in the context of post-copulatory sexual selection.
Keywords: accessory reproductive glands, copulatory plug, mammals, relative testis size, sexual selection, sperm competition
1. Introduction
As in other animal groups, female mammals frequently mate with more than one male in a single reproductive bout (Møller & Birkhead 1989; see also Birkhead & Møller 1998). Sexual selection on males (Darwin 1871) may thus extend beyond copulation, with competition occurring between the ejaculates of rival males for the fertilization of a given set of ova (i.e. sperm competition; Parker 1970, 1998). Although mammalian spermatozoa are generally short-lived and females do not store sperm for long periods (Austin 1975), ejaculates often overlap in the female reproductive tract and the importance of adaptations to sperm competition (and its avoidance) in this group is now well established (Gomendio et al. 1998; Stockley 2004).
Sperm competition typically selects for the production of numerous tiny sperm, accounting for the wide disparity between the sexes in gamete numbers (Parker 1982), and possibly even the evolution of anisogamy itself (Parker et al. 1972). Increasing the number of sperm inseminated may be particularly advantageous in many mammals, where, to a varying extent, fertilization success may commonly be determined according to a ‘raffle principle’ (Parker 1998); both absolute numbers of sperm (Weitze et al. 1993; Saacke et al. 1994) and, in a competitive context, relative numbers of sperm (Beatty 1960) have been shown to be important determinants of fertilization success. Nevertheless, sperm are costly to produce (Dewsbury 1982) and male gametic investment in each ejaculate should reflect species-specific levels of promiscuity (Parker 1998) and anticipated local degree of sperm competition (Wedell et al. 2002).
As the number of sperm per ejaculate increases, so too does the amount of testicular tissue required for sperm production. Thus, relative testis size (after controlling for body size) is expected to increase with predicted level of sperm competition (Parker et al. 1997). A strong association between mating system and relative testis size in the predicted direction has been demonstrated in numerous taxa (review in Parker et al. 1997), including mammals (e.g. Harcourt et al. 1981; Kenagy & Trombulak 1986; Ginsberg & Rubenstein 1990; Hosken 1997). Furthermore, mammalian species with relatively larger testes do indeed produce ejaculates containing more sperm (Møller 1988; Pierce et al. 1990) and, within species, larger testes enhance male reproductive success (Preston et al. 2003; Schulte-Hostedde & Millar 2004). Increasingly, molecular methods enable researchers to identify multiply sired litters in natural populations and this provides an alternative means to assess associations with sperm competition (Gomendio et al. 1998).
In addition to sperm, the mammalian ejaculate also contains a widely varying volume of seminal fluid, a diverse and complex combination of secretions from the accessory reproductive glands (Mann 1964; Brooks 1990). These additional components of the male reproductive tract—such as seminal vesicles, the multi-lobed prostate gland and bulbo-urethral and ampullary glands—are morphologically diverse (Engle 1926; Eckstein & Zuckerman 1956; Hamilton 1990) and their products may influence the outcome of sperm competition (as has been shown, for example, in insects; see Chapman 2001; Simmons 2001). Relevant functions commonly ascribed to mammalian seminal fluid components include maintenance of sperm viability, enhancement of sperm motility, hormonal stimulation of the female reproductive tract and suppression of female chemical and immunological challenges to sperm (see reviews in Rodger 1975; Mann & Lutwak-Mann 1981; Birkhead et al. 1993; Eberhard 1996) and, in at least one mammal (Sus scrofa), elements of the seminal fluid curtail oestrus (Waberski et al. 1995). In the only previous comparative study of variation in mammalian accessory gland size, Dixson (1998) found evidence for an enlargement in the seminal vesicles among primates with higher predicted levels of sperm competition, consistent with earlier observations by Short (1979).
In many mammalian orders (e.g. some rodents, primates, marsupials, bats and insectivores), accessory gland secretions also form the copulatory plug, a coagulated mass of protein deposited in the female reproductive tract at mating (see Engle 1926; Voss 1979). This fact may account for the unusually well-developed nature of the accessory glands in these groups (Voss 1979; Bedford 2004) and, in primates, the degree of seminal coagulation has been linked to predicted levels of sperm competition (Dixson & Anderson 2002). Similar structures are also found in other taxa, where they are frequently interpreted as defensive adaptations to sperm competition, preventing insemination by subsequent males (e.g. Parker 1970; Devine 1975; Abele & Gilchrist 1977; Austad 1984; Barker 1994). Among rodents, experiments support a ‘chastity enforcement’ role for plugs in guinea pigs (Martan & Shepherd 1976), but not in other species that have been investigated (Dewsbury & Baumgardner 1981; Dewsbury 1988). Moreover, males of many species are able to remove previously deposited plugs (Milligan 1979; Wallach & Hart 1983; O'Hanlon & Sachs 1986). In rats, the presence (Blandau 1945), normal size (Carballada & Esponda 1993) and position (Matthews & Adler 1978; Toner et al. 1987) of the copulatory plug seems instead to be critical in stimulating sperm transport. Reviewing plug function(s) in rodents, Voss (1979) considered a chastity enforcement function to be most probable, but a continuing dearth of experimental evidence makes the adaptive significance of copulatory plugs uncertain, and the possibility of multiple functions or different functions in different species remains open.
Here, we first investigate the relationship between two predicted correlates of sperm competition level. Since multiple paternity within litters provides strong evidence that sperm competition has occurred (though the reverse cannot be claimed), across-species variation in the prevalence of multiple paternity should reflect variation in sperm competition levels. Using data on relative testis size to infer sperm competition levels, we test this hypothesis in rodents. We then test for correlations between sperm competition level and accessory components of the male reproductive tract in rodents. Specifically, if sperm competition underlies variation in accessory glands and their products, we predict a positive correlation (after controlling for body mass) between residual testis mass and the residual mass of the accessory glands. Finally, we analyse variation in the size of a major accessory gland product, the copulatory plug, to test for evidence of a similar association with sperm competition level.
2. Material and methods
(a) Comparative analyses
Because of the potential confounding effects of shared ancestry in comparative studies, data collected from individual species cannot necessarily be considered as independent observations (Felsenstein 1985; Harvey & Pagel 1991). To control for such phylogenetic non-independence in the data we adopted a generalized least-squares (GLS) approach (Martins & Hansen 1997; Pagel 1997, 1999; Freckleton et al. 2002), as implemented in the computer program Continuous (Pagel 1997, 1999). The program uses maximum likelihood (ML) models to investigate correlations between continuously varying characters while controlling for phylogenetic associations by reference to an internal matrix of expected covariances among species owing to their degree of shared ancestry (Pagel 1999). The ML estimate of a scaling parameter, λ, indicates the degree to which a specified phylogeny accounts for patterns of covariance observed in the data. If species values are independent, then the ML estimate of λ will not differ significantly from zero, and where λ>0, some form of phylogenetic correction is required. An ML estimate of λ=1 is consistent with a Brownian motion model of trait evolution and the results of any analysis will be equivalent to other methods of controlling for phylogeny that assume such a model (e.g. independent contrasts; Felsenstein 1985). The fit of models incorporating fixed or ML estimates of λ and allowing/disallowing traits to covary were compared using likelihood ratio tests in the Continuous program. The effects of varying two further scaling parameters, κ and δ, were not investigated, with their default value of one being retained. Phylogenies used to control for non-independence of species data were constructed using the supertree of Liu et al. (2001), and within-family relationships were estimated from other sources in the literature (see Electronic Appendix). Owing to incomplete branch length information, branch lengths were set equal to one in all analyses.
(b) Multiple paternity
Data on multiple paternity (percentage of litters showing more than one male to have sired offspring) were collated from Birkhead & Appleton (1998) and from other studies in the literature. Relative testis sizes were calculated according to the rodent regression equation of Kenagy & Trombulak (1986; i.e. as observed mass of both testes/expected mass, where expected mass=0.031×body mass0.77) using data from Kenagy & Trombulak (1986) or from other sources (see Electronic Appendix). We tested for a correlation between relative testis size and percentage of multiple-paternity litters, controlling for phylogeny using GLS as described above. Since variation in litter size among the species in the dataset might affect the detection of multiple paternity, we also tested for a correlation between relative testis size and residuals from a regression of percentage of multiple-paternity litters on litter size. Litter size values were extracted, where possible, from the same source as for multiple paternity or else from Hayssen et al. (1993; see Electronic Appendix).
(c) Accessory gland size
Sources of data on accessory gland masses were identified from Zoological Record and reviews of mammalian reproductive biology (Asdell 1964; Hayssen et al. 1993). Only data for mean paired accessory gland masses of sexually mature males were used. Similarly, only data for animals deemed from the description given to be in reproductive condition were used. In most cases, fresh (rather than fixed) masses were recorded, and, since there was no significant difference between residual masses of fresh and fixed glands, we included both types of data in our analyses. Most gland sources (39 out of 43 studies) quoted sample sizes; of these, the average was 35 individuals (see Electronic Appendix). Data on body and total (i.e. paired) testis masses were taken either from the same source as the accessory gland data or, where necessary, from Silva & Downing (1995) or Kenagy & Trombulak (1986), respectively. In total, data on body mass, testis mass and at least one accessory gland mass included in the study (seminal vesicles, anterior prostate and ventral prostate) were available for 42 rodent species (see Electronic Appendix). All data were log-transformed prior to analysis and, for each of the three accessory glands in turn, correlation coefficients were derived for male body mass, testis mass and gland mass. The strength of the correlation between relative testis mass and relative accessory gland mass was then assessed by calculating the partial correlation coefficient between testis mass and gland mass (holding body mass constant) using standard formulae and n−3 degrees of freedom (Sokal & Rohlf 1981).
(d) Copulatory plug size
To investigate variation in copulatory plug size among rodents, data on plug size were taken from Hartung & Dewsbury (1978) and Baumgardner et al. (1982). The latter of these studies found a correlation between plug length and vaginal length, and vaginal length was therefore also included in the analysis. Data on relative testis sizes were calculated as before using data from Kenagy & Trombulak (1986) or, for Peromyscus, calculated using the testis dimensions reported in Linzey & Layne (1969) and assuming each testis approximates to a prolate spheroid with a density of one (Mori & Christensen 1980; see Electronic Appendix). Correlation analyses were carried out controlling for phylogeny as before.
3. Results
(a) Multiple paternity
After controlling for phylogeny, we found a significant correlation between relative testis size and percentage multiple paternity (n=14, r=0.71, p=0.006, λ=0.589; see figure 1). The model incorporating the ML estimate of λ was not significantly preferred over models constraining λ to either zero (p=0.26) or one (p=0.12, although note the low sample size and limited power in this analysis; see Freckleton et al. 2002). Nevertheless, the weak phylogenetic signal is perhaps unsurprising given the structure of the data, much of which comes from studies of sister taxa (ordinarily expected to show heightened similarity) that have been specifically chosen for analysis because of their differing mating systems and, hence, the anticipated differences in the two variables under investigation. After controlling for phylogeny, we also found a correlation between litter size and percentage multiple paternity (n=14, r=0.68, p=0.003, λ=0.778). Using residuals from the GLS regression of percentage of multiple-paternity litters on litter size to control for this effect, the relationship between relative testis size and (residual) multiple paternity remained significant and positive (n=14, r=0.54, p=0.03, λ=0.457).
Figure 1.
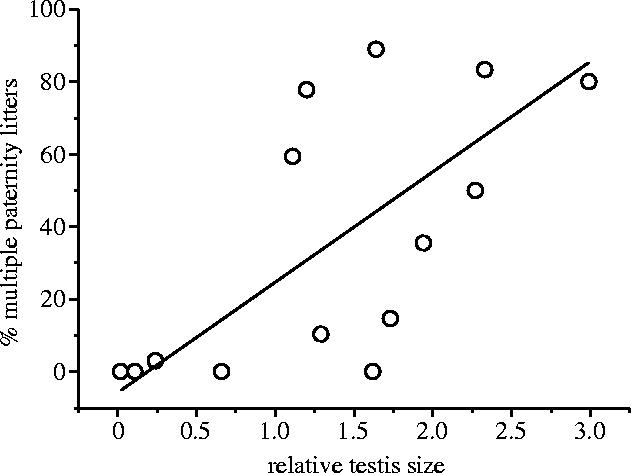
The relationship, after controlling for phylogeny, between relative testis size and percentage of litters with multiple paternity (y=30.482x−4.831; n=14, λ=0.589, r=0.71, p=0.006).
(b) Accessory gland size
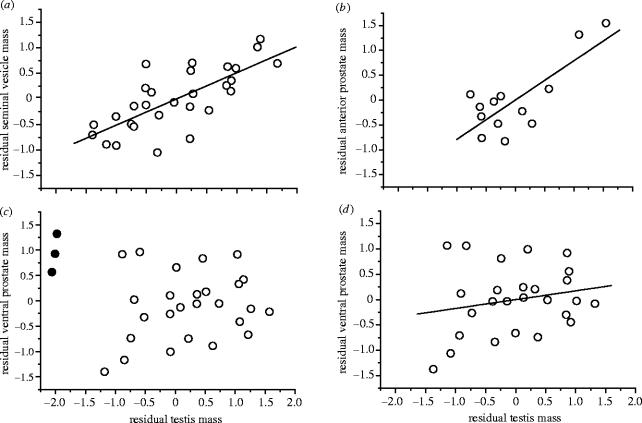
In all cases, models incorporating ML estimates of λ were significantly preferred over models where λ was constrained to one, indicating that the data did not fit a Brownian model of evolution. Nevertheless, some form of phylogenetic correction was usually justified. For two out of three glands, the model incorporating a ML estimate of λ was significantly preferred over a model where λ was constrained to zero (the exception being the anterior prostate, however, note the low sample size for this gland). After controlling for phylogeny and body mass, there was a significant correlation between seminal vesicle mass and testis mass (n=29, partial r=0.65, t=4.36, p=0.0002, λ=0.952; figure 2a) and between anterior prostate mass and testis mass (n=13, partial r=0.72, t=3.31, p=0.008, λ=0; figure 2b). This pattern was not repeated for the third gland included in this study. After controlling for phylogeny and body mass the correlation between ventral prostate mass and testis mass was non-significant (n=29, partial r=0.34, t=1.86, p=0.07, λ=0.997; figure 2c). However, this result is dependent upon the inclusion in the dataset of the somewhat unusual Notomys genus (see §4). Exclusion of three Notomys species revealed a significant positive correlation among the remaining species (n=26, partial r=0.53, t=3, p=0.006, λ=0.976; figure 2d).
Figure 2.
Residual plots (after controlling for body mass) of log-transformed accessory gland masses against log-transformed testis mass. Residuals have been calculated manually from the phylogenetically corrected GLS regression equation (see text for explanation): (a) seminal vesicles, n=29, λ=0.952, partial r=0.65, t=4.36, p=0.0002; (b) anterior prostate, n=13, λ=0, partial r=0.72, t=3.31, p=0.008; (c) ventral prostate, n=29, λ=0.997, partial r=0.34, t=1.86, p=0.07 (filled circles represent Notomys species known to have unusual reproductive anatomy, see §4); (d) ventral prostate, residuals recalculated after fitting the GLS regression line excluding the three unusual Notomys species, n=26, λ=0.976, partial r=0.53, t=3, p=0.006. Regression lines for the residual plots (a), (b) and (d) have been fitted by ordinary least squares.
(c) Copulatory plug size
Without controlling for vaginal length, there was a significant phylogenetically controlled correlation between relative testis size and plug length (n=12, r=0.56, p=0.04, λ=0.179). Controlling for variation in female vaginal length, there was also a significant correlation between relative testis size and plug length (n=12, partial r=0.65, t=2.54, p=0.03, λ=0). Similarly, holding relative testis size constant revealed a significant correlation between vaginal length and plug length (n=12, partial r=0.84, t=4.63, p=0.001, λ=0), as reported by Baumgardner et al. (1982).
4. Discussion
(a) Multiple paternity and testis size
The well-established relationship between sperm competition level and relative testis size is frequently used in comparative studies, permitting analysis of a wider range of species than would otherwise be possible. To our knowledge, this is the first study to demonstrate a relationship between relative testis size and the prevalence of within-litter multiple paternity in mammals. Although multiple mating is, of course, a prerequisite for multiple paternity, the latter is not an inevitable consequence of the former since numerous factors could prevent mixed paternity. For example, male defensive adaptations may be effective in preventing rival males from successfully inseminating mated females, even when extra matings are attempted (Parker 1970), or events in the female tract might bias paternity towards favoured/compatible males (Eberhard 1996). In either case, the likelihood of multiple paternity may be diminished. Similarly, comparing multiple paternity estimates across species is complicated by differences in such factors as the number of individuals studied and a sampling bias (controlled for here) towards detecting multiple paternity in species with large litter sizes. Despite these considerations, we nevertheless found that species where males have larger testes for their body size also produce more multiply sired litters. We attribute this result to the operation of a raffle principle over fertilization (see §1), and consequent arms race among males in sperm numbers (Parker 1982, 1998) selecting for increased testis size in more promiscuous species (Parker et al. 1997).
(b) Sperm competition and accessory glands
The morphological and biochemical diversity of mammalian accessory reproductive glands and their products has long been recognized (e.g. Eckstein & Zuckerman 1956; Mann 1964), but the underlying selective pressures are poorly understood and the role of many accessory gland products remains obscure (Rodger 1975; Bedford 2004). Evidence that sexual selection and, more specifically, sperm competition, might be behind the elaboration of accessory glands in polyandrous species has been lacking. Recently, Dixson (1998) provided comparative evidence in primates that one pair of glands, the seminal vesicles, are relatively larger in species that are likely to experience sperm competition. The present study expands this conclusion to a new mammalian order, and to additional accessory glands. In rodents, we find that at least two of the three glands studied vary positively with an indirect measure of predicted sperm competition level.
After controlling for body mass, interspecific variation in the mass of seminal vesicles and the anterior lobe of the prostate closely correlates with variation in testis mass. Although a third gland, the ventral prostate, appears not to show this relationship, the unusual Notomys genus may explain this result. Whereas Notomys cervinus has a full complement of accessory glands, the other three Notomys species all have small testes and much reduced seminal vesicles and anterior prostates, but an enlarged ventral prostate (Breed 1982). This arrangement seems to preclude copulatory plug formation, although Notomys alexis shows some degree of seminal coagulation (Breed 1990). Therefore, the unusually large contribution from the ventral prostate appears to fulfil some function ordinarily performed by seminal vesicle or anterior prostate secretions (but not plug formation). A minimum volume of fluid is perhaps always required for successful sperm transfer. However, the overall contribution to the ejaculate from this reduced accessory gland complement probably does not exceed that of other groups with a more typical anatomical arrangement. Excluding the three unusual Notomys species from the analysis results in a significant correlation after controlling for phylogeny and body mass. Hence, variation in the size of the ventral prostate may also be associated with variation in the level of sperm competition (figure 2d).
(c) Function of accessory gland products
Perhaps the most intuitive explanation for relatively larger accessory glands with increased relative testis size is that an increased volume of seminal fluid is required for the transfer and maintenance of greater numbers of sperm, reinforcing the non-trivial costs to males of increases in ejaculate size (Dewsbury 1982; Wedell et al. 2002). If each unit of sperm simply requires a fixed volume of seminal fluid (itself presumably an optimal mixture from the various accessory glands), then we might expect that the mass of all glands should vary with testis size to the same degree. On the other hand, it is not immediately obvious why an increased number of sperm should necessarily require an increase in the size of the accessory glands' major product, the copulatory plug. Indeed, according to the results presented here, it is the two glands that produce components of the copulatory plug (formed when proteins from the seminal vesicles cross-link and coagulate upon mixing with a transglutaminase enzyme from the anterior prostate at ejaculation; see Williams-Ashman 1984) that vary most closely with sperm competition level (figure 2). This suggests that sperm competition may have selected for larger accessory glands to produce a larger copulatory plug. Because plug components are not the only products of these glands, we addressed this question by analysing variation in plug size directly. We found evidence that the size of the copulatory plug does indeed vary with relative testis size and thus sperm competition level, even after a correlation between plug size and vaginal length (Baumgardner et al. 1982) is taken into account. Hence, selection for increased copulatory plug size under conditions of sperm competition may be partially responsible for the increased size of seminal vesicles and the anterior prostate in promiscuous species.
Plugs have frequently been interpreted as defensive adaptations to sperm competition in a variety of animal groups. An all-pervading ‘chastity enforcement’ function in rodents seems unlikely (see §1), although even an incompletely effective plug may still be favoured if it delays insemination by a rival male. (Of course, if plugs were always completely effective, then the sperm competition risk would be zero and there would be no advantage to males ejaculating large numbers of sperm. However, as we have illustrated, multiple paternity is common and plugs are clearly not 100% effective.) Presumably, a larger plug may be harder to remove, and this often requires multiple intromissions (Wallach & Hart 1983) and fragmentation of the plug material (Hartung & Dewsbury 1978). In insects, for example, plug efficacy does indeed increase with plug size (see Simmons 2001). The main alternative hypothesis for plug function, stimulating the transport of greater numbers of sperm towards the site of fertilization, can again be easily interpreted in terms of male–male competition, and experiments on rats with partially removed seminal vesicles suggest a direct correlation between the size of the gland left intact (and presumably the size of the plug) and the proportion of sperm that are successfully transferred from the vagina to the uterus (Carballada & Esponda 1992). Both functional hypotheses might plausibly lead to selection on males for an increased plug size under sperm competition. Under either scenario, there may be sexual conflict over plug deposition (Parker 1970; Voss 1979) and, consistent with this, plug removal by female rodents has been documented (Koprowski 1992).
(d) Sexual selection and reproductive anatomy
Ever since Short's (1979) observations in primates, post-copulatory sexual selection frequently has been invoked to account for variation in the reproductive anatomy of male mammals, although surprisingly little evidence has been available for rodents, the largest and most diverse mammalian order. Here, we have been able to demonstrate that variation in relative testis size across rodents correlates with the percentage of multiply sired litters. This finding is consistent with current theory and recent intraspecific studies of rodents that have demonstrated the ability of males to ejaculate more sperm under local sperm competition risk (Pound & Gage 2004; delBarco-Trillo & Ferkin 2004), and the importance of relative testis to male reproductive success in natural populations (Schulte-Hostedde & Millar 2004). Our findings also indicate that an increase in testis size, though undoubtedly important, may not be the only anatomical adaptation to sperm competition in this group. We show that varying sperm competition levels also account for variation in the size of the accessory glands (probably partly as a result of selection on copulatory plug size), highlighting the likely importance of additional, non-sperm components of the male ejaculate in influencing the outcome of post-copulatory sexual selection among males. The extent to which accessory gland products influence the outcome of sperm competition in this and other mammalian taxa remains to be elucidated.
Acknowledgments
We are extremely grateful to A. Schulte-Hostedde for supplying and discussing unpublished data, P. L. Schwagmeyer for suggesting data sources, M. Anderson and M. Thom for helpful discussions, and two anonymous referees for many useful comments on an earlier version of the manuscript. S.A.R. is supported by a studentship from the University of Liverpool. P.S. is also grateful for generous support provided by a visiting scholarship to St John's College, University of Oxford.
Footnotes
As this paper exceeds the maximum length normally permitted, the authors have agreed to contribute to production costs.
Supplementary Material
References
- Abele L.G, Gilchrist S. Homosexual rape and sexual selection in acanthocephalan worms. Science. 1977;197:81–83. doi: 10.1126/science.867055. [DOI] [PubMed] [Google Scholar]
- Asdell S.A. 2nd edn. Constable; London: 1964. Patterns of mammalian reproduction. [Google Scholar]
- Austad S.N. Evolution of sperm priority patterns in spiders. In: Smith R.L, editor. Sperm competition and the evolution of animal mating systems. Academic Press; London: 1984. pp. 223–249. [Google Scholar]
- Austin C.R. Sperm fertility, viability and persistence in the female tract. J. Reprod. Fertil. Suppl. 1975;22:75–89. [PubMed] [Google Scholar]
- Barker D.M. Copulatory plugs and paternity assurance in the nematode Caenorhabditis elegans. Anim. Behav. 1994;48:147–156. [Google Scholar]
- Baumgardner D.J, Hartung T.G, Sawrey D.K, Webster D.G, Dewsbury D.A. Muroid copulatory plugs and female reproductive tracts: a comparative investigation. J. Mammal. 1982;63:110–117. [Google Scholar]
- Beatty R.A. Fertility of mixed semen from different rabbits. J. Reprod. Fertil. 1960;1:52–60. doi: 10.1530/jrf.0.0010052. [DOI] [PubMed] [Google Scholar]
- Bedford J.M. Enigmas of mammalian gamete form and function. Biol. Rev. 2004;79:429–460. doi: 10.1017/s146479310300633x. [DOI] [PubMed] [Google Scholar]
- Birkhead T.R, Appleton A.C. Multiple paternity in mammals. In: Birkhead T.R, Møller A.P, editors. Sperm competition and sexual selection. Academic Press; London: 1998. pp. 752–755. [Google Scholar]
- Birkhead T.R, Møller A.P. Academic Press; London: 1998. Sperm competition and sexual selection. [Google Scholar]
- Birkhead T.R, Møller A.P, Sutherland W.J. Why do females make it so difficult for males to fertilize their eggs? J. Theor. Biol. 1993;161:51–60. [Google Scholar]
- Blandau R.J. On the factors involved in sperm transport through the cervix uteri of the albino rat. Am. J. Anat. 1945;77:253–272. [Google Scholar]
- Breed W.G. Morphological variation in the testes and accessory sex organs of Australian rodents in the genera Pseudomys and Notomys. J. Reprod. Fertil. 1982;66:607–613. doi: 10.1530/jrf.0.0660607. [DOI] [PubMed] [Google Scholar]
- Breed W.G. Copulatory behaviour and coagulum formation in the female reproductive tract of the Australian hopping mouse, Notomys alexis. J. Reprod. Fertil. 1990;88:17–24. doi: 10.1530/jrf.0.0880017. [DOI] [PubMed] [Google Scholar]
- Brooks D.E. Biochemistry of the male accessory glands. In: Lamming G.E, editor. Marshall's physiology of reproduction. 4th edn. Churchill Livingstone; Edinburgh: 1990. pp. 569–690. [Google Scholar]
- Carballada R, Esponda P. Role of fluid from seminal vesicles and coagulating glands in sperm transport into the uterus and fertility in rats. J. Reprod. Fertil. 1992;95:639–648. doi: 10.1530/jrf.0.0950639. [DOI] [PubMed] [Google Scholar]
- Carballada R, Esponda P. Structure of the vaginal plugs generated by normal rats and by rats with partially removed seminal vesicles. J. Exp. Zool. 1993;265:61–68. doi: 10.1002/jez.1402650109. [DOI] [PubMed] [Google Scholar]
- Chapman T. Seminal fluid-mediated fitness traits in Drosophila. Heredity. 2001;87:511–521. doi: 10.1046/j.1365-2540.2001.00961.x. [DOI] [PubMed] [Google Scholar]
- Darwin C. John Murray; London: 1871. The descent of man, and selection in relation to sex. [Google Scholar]
- delBarco-Trillo J, Ferkin M.H. Male mammals respond to a risk of sperm competition conveyed by odours of conspecific males. Nature. 2004;431:446–449. doi: 10.1038/nature02845. [DOI] [PubMed] [Google Scholar]
- Devine M.C. Copulatory plugs in snakes: enforced chastity. Science. 1975;187:844–845. doi: 10.1126/science.1114329. [DOI] [PubMed] [Google Scholar]
- Dewsbury D.A. Ejaculate cost and male choice. Am. Nat. 1982;119:601–610. [Google Scholar]
- Dewsbury D.A. A test of the role of copulatory plugs in sperm competition in deer mice (Peromyscus maniculatus) J. Mammal. 1988;69:854–857. [Google Scholar]
- Dewsbury D.A, Baumgardner D.J. Studies of sperm competition in two species of muroid rodents. Behav. Ecol. Sociobiol. 1981;9:121–133. [Google Scholar]
- Dixson A.F. Sexual selection and evolution of the seminal vesicles in primates. Folia Primatol. 1998;69:300–306. doi: 10.1159/000021643. [DOI] [PubMed] [Google Scholar]
- Dixson A.F, Anderson M.J. Sexual selection, seminal coagulation and copulatory plug formation in primates. Folia Primatol. 2002;73:63–69. doi: 10.1159/000064784. [DOI] [PubMed] [Google Scholar]
- Eberhard W.G. Princeton University Press; Princeton: 1996. Female control: sexual selection by cryptic female choice. [Google Scholar]
- Eckstein P, Zuckerman S. Morphology of the reproductive tract. In: Parkes A.S, editor. Marshall's physiology of reproduction. 3rd edn. Longmans; London: 1956. pp. 43–155. [Google Scholar]
- Engle E.T. The copulation plug and the accessory genital glands of mammals. J. Mammal. 1926;7:119–126. [Google Scholar]
- Felsenstein J. Phylogenies and the comparative method. Am. Nat. 1985;125:1–15. doi: 10.1086/703055. [DOI] [PubMed] [Google Scholar]
- Freckleton R.P, Harvey P.H, Pagel M. Phylogenetic analysis and comparative data: a test and review of evidence. Am. Nat. 2002;160:712–726. doi: 10.1086/343873. [DOI] [PubMed] [Google Scholar]
- Ginsberg J.R, Rubenstein D.I. Sperm competition and variation in zebra mating behavior. Behav. Ecol. Sociobiol. 1990;26:427–434. [Google Scholar]
- Gomendio M, Harcourt A.H, Roldán E.R.S. Sperm competition in mammals. In: Birkhead T.R, Møller A.P, editors. Sperm competition and sexual selection. Academic Press; London: 1998. pp. 667–755. [Google Scholar]
- Hamilton D.W. Anatomy of mammalian male accessory reproductive organs. In: Lamming G.E, editor. Marshall's physiology of reproduction. 4th edn. Churchill Livingstone; Edinburgh: 1990. pp. 691–746. [Google Scholar]
- Harcourt A.H, Harvey P.H, Larson S.G, Short R.V. Testis weight, body weight and breeding system in primates. Nature. 1981;293:55–57. doi: 10.1038/293055a0. [DOI] [PubMed] [Google Scholar]
- Hartung T.G, Dewsbury D.A. A comparative analysis of copulatory plugs in muroid rodents and their relationship to copulatory behavior. J. Mammal. 1978;59:717–723. [Google Scholar]
- Harvey P.H, Pagel M.D. Oxford University Press; Oxford: 1991. The comparative method in evolutionary biology. [Google Scholar]
- Hayssen V, van Tienhoven A, van Tienhoven A. Comstock; London: 1993. Asdell's patterns of mammalian reproduction. [Google Scholar]
- Hosken D.J. Sperm competition in bats. Proc. R. Soc. B. 1997;264:385–392. doi: 10.1098/rspb.1997.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenagy G.J, Trombulak S.C. Size and function of mammalian testes in relation to body size. J. Mammal. 1986;67:1–22. [Google Scholar]
- Koprowski J.L. Removal of copulatory plugs by female tree squirrels. J. Mammal. 1992;73:572–576. [Google Scholar]
- Linzey A.V, Layne J.N. Comparative morphology of the male reproductive tract in the rodent genus Peromyscus (Muridae) Am. Mus. Novit. 1969;2355:1–47. [Google Scholar]
- Liu F.-G.R, Miyamoto M.M, Freire N.P, Ong P.Q, Tennant M.R, Young T.S, Gugel K.F. Molecular and morphological supertrees for Eutherian (placental) mammals. Science. 2001;291:1786–1789. doi: 10.1126/science.1056346. [DOI] [PubMed] [Google Scholar]
- Mann T. Methuen; London: 1964. The biochemistry of semen. [Google Scholar]
- Mann T, Lutwak-Mann C. Springer; Berlin: 1981. Male reproductive function and semen. [Google Scholar]
- Martan J, Shepherd B.A. The role of the copulatory plug in reproduction of the guinea pig. J. Exp. Zool. 1976;196:79–83. doi: 10.1002/jez.1401960108. [DOI] [PubMed] [Google Scholar]
- Martins E.P, Hansen T.F. Phylogenies and the comparative method: a general approach to incorporating phylogenetic information into the analysis of interspecific data. Am. Nat. 1997;149:646–667. [Google Scholar]
- Matthews M.K, Adler N.T. Systematic interrelationship of mating, vaginal plug position, and sperm transport in the rat. Physiol. Behav. 1978;20:303–309. doi: 10.1016/0031-9384(78)90224-x. [DOI] [PubMed] [Google Scholar]
- Milligan S.R. The copulatory pattern of the bank vole (Clethrionomys glareolus) and speculation on the role of penile spines. J. Zool. 1979;188:279–300. [Google Scholar]
- Møller A.P. Ejaculate quality, testes size and sperm competition in primates. J. Hum. Evol. 1988;17:479–488. [Google Scholar]
- Møller A.P, Birkhead T.R. Copulation behaviour in mammals: evidence that sperm competition is widespread. Biol. J. Linn. Soc. 1989;38:119–131. [Google Scholar]
- Mori H, Christensen A.K. Morphometric analysis of Leydig cells in the normal rat testis. J. Cell Biol. 1980;84:340–354. doi: 10.1083/jcb.84.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hanlon J.K, Sachs B.D. Fertility of mating in rats (Rattus norvegicus): contributions of androgen-dependent morphology and actions of the penis. J. Comp. Psychol. 1986;100:178–187. [PubMed] [Google Scholar]
- Pagel M. Inferring evolutionary processes from phylogenies. Zool. Scr. 1997;26:331–348. [Google Scholar]
- Pagel M. Inferring the historical patterns of biological evolution. Nature. 1999;401:877–884. doi: 10.1038/44766. [DOI] [PubMed] [Google Scholar]
- Parker G.A. Sperm competition and its evolutionary consequences in the insects. Biol. Rev. 1970;45:525–567. [Google Scholar]
- Parker G.A. Why are there so many tiny sperm? Sperm competition and the maintenance of two sexes. J. Theor. Biol. 1982;96:281–294. doi: 10.1016/0022-5193(82)90225-9. [DOI] [PubMed] [Google Scholar]
- Parker G.A. Sperm competition and the evolution of ejaculates: towards a theory base. In: Birkhead T.R, Møller A.P, editors. Sperm competition and sexual selection. Academic Press; London: 1998. pp. 3–54. [Google Scholar]
- Parker G.A, Baker R.R, Smith V.G.F. The origin and evolution of gamete dimorphism and the male–female phenomenon. J. Theor. Biol. 1972;36:529–553. doi: 10.1016/0022-5193(72)90007-0. [DOI] [PubMed] [Google Scholar]
- Parker G.A, Ball M.A, Stockley P, Gage M.J.G. Sperm competition games: a prospective analysis of risk assessment. Proc. R. Soc. B. 1997;264:1793–1802. doi: 10.1098/rspb.1997.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J.D, Jr, Ferguson B, Salo A.L, Sawrey D.K, Shapiro L.E, Taylor S.A, Dewsbury D.A. Patterns of sperm allocation across successive ejaculates in four species of voles (Microtus) J. Reprod. Fertil. 1990;88:141–149. doi: 10.1530/jrf.0.0880141. [DOI] [PubMed] [Google Scholar]
- Pound N, Gage M.J.G. Prudent sperm allocation in Norway rats, Rattus norvegicus: a mammalian model of adaptive ejaculate adjustment. Anim. Behav. 2004;68:819–823. [Google Scholar]
- Preston B.T, Stevenson I.R, Pemberton J.M, Coltman D.W, Wilson K. Overt and covert competition in a promiscuous mammal: the importance of weaponry and testes size to male reproductive success. Proc. R. Soc. B. 2003;270:633–640. doi: 10.1098/rspb.2002.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger J.C. Seminal plasma, an unnecessary evil? Theriogenology. 1975;3:237–247. doi: 10.1016/0093-691x(75)90141-7. [DOI] [PubMed] [Google Scholar]
- Saacke R.G, Nadir S, Nebel R.L. Relationship of semen quality to sperm transport, fertilization, and embryo quality in ruminants. Theriogenology. 1994;41:45–50. [Google Scholar]
- Schulte-Hostedde A.I, Millar J.S. Intraspecific variation of testis size and sperm length in the yellow-pine chipmunk (Tamias amoenus): implications for sperm competition and reproductive success. Behav. Ecol. Sociobiol. 2004;55:272–277. [Google Scholar]
- Short R.V. Sexual selection and its component parts, somatic and genital selection, as illustrated by man and the great apes. Adv. Stud. Behav. 1979;9:131–158. [Google Scholar]
- Silva M, Downing J.A. CRC Press; Boca Raton, FL: 1995. CRC Handbook of mammalian body masses. [Google Scholar]
- Simmons L.W. Princeton University Press; Princeton: 2001. Sperm competition and its evolutionary consequences in the insects. [Google Scholar]
- Sokal R.R, Rohlf F.J. 2nd edn. W.H. Freeman; New York: 1981. Biometry. [Google Scholar]
- Stockley P. Sperm competition in mammals. Hum. Fertil. 2004;7:91–97. doi: 10.1080/14647270410001699054. [DOI] [PubMed] [Google Scholar]
- Toner J.P, Attas A.I, Adler N.T. Transcervical sperm transport in the rat: the roles of pre-ejaculatory behavior and copulatory plug fit. Physiol. Behav. 1987;39:371–375. doi: 10.1016/0031-9384(87)90237-x. [DOI] [PubMed] [Google Scholar]
- Voss R.S. Male accessory glands and the evolution of copulatory plugs in rodents. Occas. Pap. Mus. Zool. Univ. Mich. 1979;689:1–27. [Google Scholar]
- Waberski D, et al. Advanced ovulation in gilts by the intrauterine application of a low molecular mass pronase-sensitive fraction of boar seminal plasma. J. Reprod. Fertil. 1995;105:247–252. doi: 10.1530/jrf.0.1050247. [DOI] [PubMed] [Google Scholar]
- Wallach S.J.R, Hart B.L. The role of the striated penile muscles of the male rat in seminal plug dislodgement and deposition. Physiol. Behav. 1983;31:815–821. doi: 10.1016/0031-9384(83)90278-0. [DOI] [PubMed] [Google Scholar]
- Wedell N, Gage M.J.G, Parker G.A. Sperm competition, male prudence and sperm-limited females. Trends Ecol. Evol. 2002;17:313–320. [Google Scholar]
- Weitze K.F, Waberski D, Töpfer-Petersen E. Number of spermatozoa in the inseminate, sperm transport and success of fertilization in pigs. Reprod. Domest. Anim. 1993;28:406–415. [Google Scholar]
- Williams-Ashman H.G. Transglutaminases and the clotting of mammalian seminal fluids. Mol. Cell. Biochem. 1984;58:51–61. doi: 10.1007/BF00240604. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.