Abstract
Increasing ultraviolet-B radiation (UV-B) has recently captured the attention of ecologists as a key environmental stressor. Certain species may be particularly vulnerable as a result of either high natural exposure to UV-B or limited physiological capacity to withstand it. UV-B sensitivity has been examined at the cellular and individual level for a wide variety of taxa, but estimates of exposure to UV-B in natural systems are lacking and predictions of large-scale impacts are therefore limited. Here, we combine data on the physiological sensitivity to UV-B and patterns of field exposure across sites for embryos of several well-studied US Pacific Northwest amphibian species. We find substantial differences among species' physiological abilities to withstand UV-B and in the level of UV-B exposure of embryos in the field. More specifically, we find that species with the highest physiological sensitivity to UV-B are those with the lowest field exposures as a function of the location of embryos and the UV-B attenuation properties of water at each site. These results also suggest that conclusions made about species' vulnerability to UV-B in the absence of information on field exposures may often be misleading.
Keywords: amphibian declines, ultraviolet-B radiation, photoenzymatic repair, physiology, dissolved organic matter, oviposition behaviour
1. Introduction
As anthropogenic stressors to natural ecosystems escalate, predicting their impacts on diverse species and communities has emerged as a key challenge to ecologists and conservation biologists. Studies of environmental stressors have traditionally included human-produced contaminants such as pesticides, metals and industrial chemicals, but have come to include anthropogenic changes to natural features of environments like temperature, salinity and ultraviolet radiation. Assessments of the risk posed by particular stressors have generally been conducted at lower levels of biological organization and at small spatial and temporal scales. For example, assays of species' physiological tolerance are often based on standard laboratory or controlled field methodologies, such as estimates of acute lethal concentration or dose (LC50, LD50; Stark et al. 2004). However, there is broad recognition of the importance of processes and interactions operating at scales not easily captured by most manipulative experiments (Carpenter 1996), and this magnifies the difficulty of scaling-up results from traditional exposure experiments (Stark et al. 2004). Reconciling the level at which most studies of environmental risk are conducted, and the ultimate scales at which predictions and generalizations are made, remains a severe limitation to applied ecological risk analysis. Here, we capitalize on amphibian sensitivity to ultraviolet-B radiation (UV-B) in the context of declining amphibian populations as a case study to evaluate the consequences of estimating risk at two levels of biological organization.
Over the past decade, there has been intense interest in the role of UV-B in the decline of amphibians (Blaustein & Wake 1990; Blaustein et al. 1994). Anthropogenic increases in the flux of UV-B to the Earth's surface owing to stratospheric ozone depletion (Kerr & McElroy 1993; Madronich 1994) has been proposed as an important factor for the population dynamics of some amphibians, including several Pacific Northwest species (Blaustein et al. 1994, 2003; but see Blaustein et al. 1999; Olson 2001; Licht 2003). Tests of amphibian species sensitivity to UV-B have ranged from high mortality under ambient conditions to near insensitivity at artificially enhanced UV-B levels (Blaustein et al. 1994; Grant & Licht 1995; Hays et al. 1996; Ovaska et al. 1997). Although considerable progress has been made evaluating the relative physiological sensitivity of species to UV-B, much less effort has been expended in characterizing the natural context in which each species experiences UV-B (but see Belden et al. 2000; Diamond et al. 2002; Palen et al. 2002), though field-based measurements of actual UV-B exposures can be difficult to obtain.
UV-B includes the most biologically harmful wavelengths of light reaching the Earth's surface (290–320 nm), and has been a ubiquitous environmental stressor for most organisms over evolutionary time-scales. As such, organisms have developed a wide variety of mechanisms to cope with harmful levels of UV-B including behavioural avoidance, the synthesis or retention of photoprotective compounds and molecular processes to repair UV-B damage. Although behavioural avoidance can reduce UV-B exposure, the tolerance or sensitivity of an individual is determined by the effectiveness of photoprotective structures and the rate at which UV-B damage is repaired at a given level of exposure. Many organisms, including amphibians, produce compounds that absorb UV-B wavelengths of light (i.e., melanin, carotenoids, mycosporine-like amino acids), thus reducing the potential for DNA damage within cells (Kollias et al. 1991). However, once DNA is damaged by UV-B radiation, which occurs primarily through the formation of cyclobutane pyrimidine dimers (CPD), organisms rely on physiological mechanisms to repair or remove DNA damage (Mitchell & Karentz 1993), and these processes can be the major determinant of differences in species tolerance to UV-B.
Two general classes of repair mechanisms exist: light-dependent (UV-A and visible radiation 300–500 nm) enzymatic repair catalysed by photolyase (also called ‘photoenzymatic repair’; PER) and nucleotide excision repair, which can occur in the presence or absence of light (dark repair). The dependence of photolyase on UV-A and visible wavelengths of light to catalyse the repair of CPDs allows for experimental isolation of the contribution of PER compared with that of photoprotection and dark repair in the overall tolerance of particular species to UV-B (Worrest & Kimeldorf 1976; Williamson et al. 2001).
Organisms have also evolved a number of behavioural responses to UV-B, ranging from positive effects including visual detection of prey and mates (Fleishman et al. 1993; Viitala et al. 1995) to spatial and temporal avoidance of the harmful effects of UV-B (Rhode et al. 2001; Van de Mortel & Buttemer 1998). Although visual acuity for UV wavelengths of light (290–400 nm) is highly conserved and present in some form across most animal taxa (Tovee 1995), behavioural responses to UV-B by amphibians have been documented in only a few cases (Nagl & Hofer 1997; Van de Mortel & Buttemer 1998). Despite the paucity of published evidence for amphibians, UV-B-sensitive species may exhibit any number of behavioural strategies to reduce UV-B-induced mortality. For embryos of physiologically sensitive species in particular, individual females may lay their eggs in ponds or locations within ponds that minimize exposure to high levels of UV-B, as has been suggested for amphibians (Blaustein et al. 1994, 1997; Lizana & Pedraza 1998) and quantitatively demonstrated for several fish species (Williamson et al. 1997; Gutierrez-Rodriguez & Williamson 1999; Huff et al. 2004). The role of UV-B in oviposition site choice by amphibians remains to be examined in detail but is probably moderated by the relative importance of other factors known to influence reproductive effort including predator presence, larval density, pond permanence and natal site fidelity (Resetarits & Wilbur 1989; Kats & Sih 1992; Petranka et al. 1994; Holomuzki 1995; Spieler & Linsenmair 1997; Murphy 2003). For lake and pond environments, it is well established that aromatic forms of dissolved organic matter (DOM), derived primarily from terrestrial plant decomposition, largely influence the penetration of UV-B in the water (Scully & Lean 1994; Morris et al. 1995), and this is known to be relevant for the distribution of pond-breeding amphibians and the exposure of embryos (Adams et al. 2001, in press; Palen et al. 2002; Brooks et al. in press).
We compared the response in embryos of two frog species, Rana cascadae (cascades frog) and Pseudacris regilla (Pacific treefrog), and two salamander species, Ambystoma macrodactylum (long-toed salamander) and Ambystoma gracile (northwestern salamander), with UV-B using novel laboratory-based exposure experiments, and combined these results with surveys of the UV-B exposure of embryos in the field to evaluate the context in which physiological sensitivity is embedded in natural ecosystems. To compare the relative importance of light-dependent photorepair of UV-B damage for these four species, we used a solar phototron instrument (Williamson et al. 2001) to control the exposure of embryos to different levels of UV-B with and without the longer-wavelength radiation (UV-A and visible) necessary to complete PER. We focus our assessment exclusively on the impacts of UV-B on embryonic amphibians, similar to the majority of other published studies. Embryos are experimentally tractable, require the fewest assumptions about the consequences of confinement in experiments and may be the most vulnerable to UV-B, based on exposure and the potential for magnified impacts early in development (reviewed by Licht 2003; but see Pahkala et al. 2000; Tietge et al. 2001). To incorporate the potential for behavioural adaptations to interact with physiological sensitivity to UV-B, we conducted surveys of amphibian embryos at breeding sites spanning high to low DOM concentrations, representing a broad range of UV-B environments. Our results show that among the amphibians we tested, the most physiologically sensitive species modify oviposition behaviours, resulting in reduced embryonic exposures to UV-B. This result provides an example of the trade-offs that may exist between species' physiological tolerance and behavioural responses to particular ecological stressors. We contend that although tests of physiological sensitivity are an essential component of risk assessment, incorporating factors operating over larger spatial and temporal scales in natural habitats has the potential to influence the determination of risk.
2. Methods
(a) Laboratory exposure experiments
To test for differences in the UV tolerance and the role of PER of four Pacific Northwest amphibian species, we conducted a series of experiments with a UV lamp phototron that simultaneously controls the dose of damaging short-wave UV radiation (UV-B) and exposure to longer-wave radiation necessary for PER. We compared the survival of embryos to hatching in either the presence or absence of this photoreactivating radiation (PRR=UV-A and visible) across a range of UV-B doses (Electronic Appendix 1) to determine the effect of PER on species' UV-B tolerance and its relative importance between species. Components of photoprotection and photorepair have been demonstrated to be up- or downregulated within individuals of some species as a function of light exposure, including carotenoid pigmentation in freshwater copepods (Hansson 2004), melanin production in hammerhead sharks (Lowe & Goodman-Lowe 1996) and both melanin and photorepair enzymes in some amphibians (Hofer & Mokri 2000; Smith et al. 2000; Belden & Blaustein 2002). The phototron experiments that we conducted allow for photoprotective compounds or photorepair activity to be induced by exposure to UV-B over the course of the experiment, if such abilities exist for the species tested (Smith et al. 2000), and would contribute to any differences we observe among species in their tolerance for UV-B.
Embryos of P. regilla and A. gracile were collected in March 2003 at one large wetland in the North Cascade mountains of Washington (UTM 10T 583144E, 5262556N, 950 m elevation). Embryos of R. cascadae and A. macrodactylum were collected at six ponds in the Sol Duc drainage of Olympic National Park, Washington (UTM 10T 441441E, 5307428N, 1249–1374 m elevation) in July 2003. The large difference in the timing of oviposition between the two collection sites is a result of factors including winter snow accumulation, elevation and aspect. In both locations, sites were monitored over 3–6 days during peak oviposition activity, and embryos were collected less than 48 h after deposition and held in approximately 10 °C incubators for 1–5 days until tested in the phototron array. In this way, embryos collected for all four species had variable light-exposure histories, from those collected within a few hours of sunrise after oviposition the previous night, to embryos that may have been exposed to nearly two full days of solar radiation (less than 48 h).
The UV phototron comprises three UV-B lamps (Spectronics XX15B) suspended approximately 20 cm above a rotating round platform containing 40 quartz dishes with lids (30 ml), half of which are exposed to beneficial PRR from below the platform. Each dish has a 2.5 cm tall black PVC collar to reduce stray light between dishes, and different UV-B doses are achieved with different gauge stainless steel mesh screens that uniformly reduce irradiance. The PRR was generated by two 40 W, cool white fluorescent bulbs and two 40 W, Q-Panel 340 bulbs aligned approximately 30 cm below the rotating platform, enclosed to prevent accidental stray light, and ventilated to prevent elevated temperatures. Each UV-B lamp was covered with new cellulose acetate film every 24 h to eliminate the shortest wavelength radiation (less than 295 nm) that does not normally reach the Earth's surface. The entire phototron array is housed within an incubator, and all experiments were conducted at 15 °C, which is within the range of natural oviposition temperatures for these species.
The lights in the phototron were on a 12L : 12D cycle, and experiments were run for 1–3 days depending on species' sensitivity to UV-B (Electronic Appendix 1). To allow for gas exchange, the lids of the quartz dishes were removed at night for experiments lasting longer than 24 h. The combination of irradiance-reducing mesh and experimental duration resulted in four different UV-B doses for each species (+PRR and −PRR), plus a control that was kept in the dark for the extent of development (Electronic Appendix 1), resulting in nine different experimental treatments. For species laying egg masses rather than single eggs (R. cascadae, P. regilla, A. gracile), the embryos of 3–15 masses were separated and mixed to reduce the potential for maternal effects, and to ensure that any differences in light-exposure history prior to collection were represented equally in all treatments. External embryonic jelly remained intact for all species except A. gracile, whose egg mass architecture is such that approximately 20–200 embryos are clustered at the core of a thick (5–15 cm) extracellular jelly matrix. This jelly is initially very clear and thin, and probably has limited optical absorbance properties during the embryonic stages coinciding with our laboratory experiments (R. Moeller, personal communication; but see Licht 2003). For each experiment, 7–10 embryos were randomly assigned per dish, and each treatment was replicated five times. After experimental exposure, embryos remained in dark 15 °C incubators until hatching. Further details about the phototron array are described in Williamson et al. (2001).
To evaluate differences in species' physiological tolerance to UV-B exposure, we estimated the lethal dose of UV-B that coincided with 50% survival to hatching (LD50) in the presence and absence of PRR from our phototron experiments. We modelled survival across UV-B doses (kJ m−2) for each species as a sigmoid function according to
| 2.1 |
where a is the steepness, and b the LD50. Parameters were fit by the Gauss–Newton method for nonlinear least-squares estimation (Systat 9.0). In addition to nonlinear model fits, we arcsine square-root transformed embryonic survival in each treatment for normality, and performed a two-way ANOVA with UV-B dose and PRR level as factors for each species.
(b) Field oviposition surveys
To characterize the relative exposure of embryos to UV-B at natural oviposition sites, we surveyed 43 amphibian breeding sites in the Sol Duc drainage of Olympic National Park, WA (UTM 10T 441443 5307426) in 2002 and 2003. These sites represent a subset of ponds surveyed over the past 4 years as part of a larger project following the spatial and temporal distribution of DOM, and include all breeding sites within several sub-basins of the Sol Duc drainage. Most amphibian breeding within the Sol Duc drainage is restricted to smaller, fishless, permanent ponds (surface area range 24–823 m2, average 172 m2±222 s.d.). Sites surveyed span an elevational gradient from subalpine to alpine (elevation range 1077–1472 m, average 1239 m±133 s.d.), and include the sites where R. cascadae and A. macrodactylum eggs were collected for the laboratory-based physiology experiments during the 2003 breeding season (July). The limited elevation of our study sites prevented us from collecting sufficient data on the oviposition habits of P. regilla, a species found primarily below 1100 m in this region, which restricted our conclusions to three out of the four species tested in the phototron array (R. cascadae, A. gracile and A. macrodactylum). Each site was visited approximately every 1–2 days during initial thaw to determine the extent of the breeding period. Once the deposition of new eggs had ceased at an individual site, between two and four experienced observers thoroughly searched all habitats for amphibian eggs, measured the depth below the surface of the water to the top of each egg or egg mass (cm) and characterized its exposure to light as either exposed or shaded. We considered eggs or egg masses to be shaded if they were covered by an immediate and complete barrier to light including rocks, woody debris, vegetation or the bank of the pond. We did not take into account shading by the landscape that could also reduce the total exposure of eggs to UV-B, such as location in the pond relative to the dominant aspect of the sun or the proximity to large objects (trees or rocks) outside of the pond edge. Survey data are presented as averages by site for the depth of eggs and the proportion of eggs scored as shaded from light. For sites surveyed in both years (n=13), we present the average across years.
(c) Optical transparency of water at breeding sites
As part of a larger project documenting the annual and seasonal variation in the UV-B transparency of water at amphibian breeding sites, we collected water samples at each site to estimate the diffuse attenuation coefficient (Kd) for UV-B wavelengths of light and to compare relative transparency (Morris et al. 1995; Palen et al. 2002). Samples were collected 1–3 times a month across the breeding season (June–August), filtered in the field through a 0.2 μm syringe filter (Whatman Membra-fil, 47 mm diameter), and refrigerated (4–10 °C) in the dark until laboratory analysis. We estimated Kd at each site based on a previously established relationship, with the absorption of 440 nm light passed through a 10 cm quartz cuvette in a Shimadzu UV-2100 double beam spectrophotometer (see Palen et al. 2002 for details). To characterize the optical clarity of water at breeding sites over the embryonic incubation period (2–6 weeks), we calculated the average Kd from 2 to 3 water samples taken most closely to the beginning of breeding at each site each year. To compare the UV-B exposure of amphibian eggs at breeding sites with different transparencies to UV-B, we calculated the proportion of surface UV-B that would penetrate to an arbitrary depth of 10 cm based on our estimates of Kd. We regressed these values with the average depth of eggs and proportion of eggs, scored as fully shaded from light for each species at each site. Proportions (egg exposure and surface UV-B penetration) were arcsine square-root transformed for normality and are presented as untransformed means.
3. Results
(a) Laboratory exposure experiments
All four amphibian species suffered higher mortality as a function of both increasing UV-B dose (two-way ANOVA for each species, UV-B: p<0.001) and when denied the long-wave radiation necessary for photoenzymatic repair (two-way ANOVA for each species, PER: p<0.001). In addition, there were significant interaction effects with UV-B exposure, having a greater effect in the absence of photoenzymatic repair (figure 1; two-way ANOVA for each species, UV-B×PER: p<0.001). Based on our nonlinear model fits (Electronic Appendix 2), we estimated the lethal dose of UV-B (kJ m−2) resulting in 50% embryonic survival in the presence and absence of long-wave radiation, and found that photoenzymatic repair on average increased species' tolerance for UV-B between 2.5 and 49 times that in the absence of long-wave radiation (figure 2; R. cascadae=2.8, P. regilla=2.6, A. macrodactylum=4.2, A. gracile=48.7). More importantly, these differences in photoenzymatic repair capability are largely responsible for the substantial differences in the overall tolerance to UV-B across species, where the rank order of UV-B sensitivity from highest to lowest was A. gracile, A. macrodactylum, P. regilla and R. cascadae (figure 2).
Figure 1.
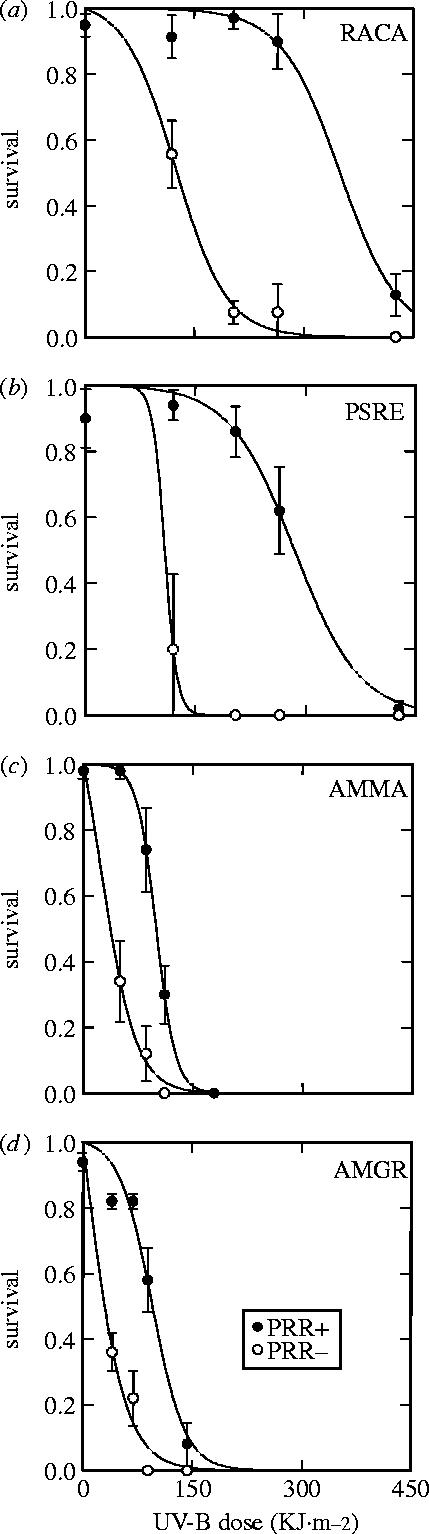
Average proportion of embryos surviving to hatching when exposed to five experimental levels of UV-B (kJ m−2) in the presence (filled circles) and absence (open circles) of photorepair radiation (PRR) for (a) R. cascadae (RACA), (b) P. regilla (PSRE), (c) A. macrodactylum (AMMA) and (d) A. gracile (AMGR). Solid line represents the best-fit nonlinear model for each series of experiments. Data are plotted as means±1 s.e.
Figure 2.
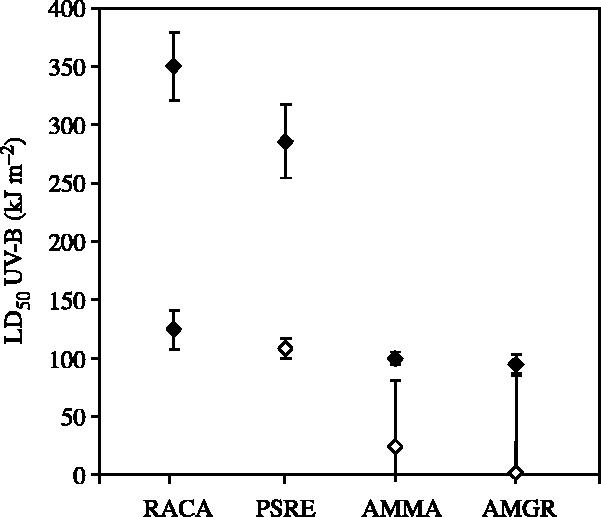
Estimated dose of UV-B (kJ m−2) corresponding to 50% embryonic survival (LD50) for RACA, PSRE, AMMA and AMGR in the presence (filled diamonds) and absence (open diamonds) of PRR. LD50 for each species is estimated from nonlinear model fits and plotted with 95% confidence intervals.
(b) Field surveys
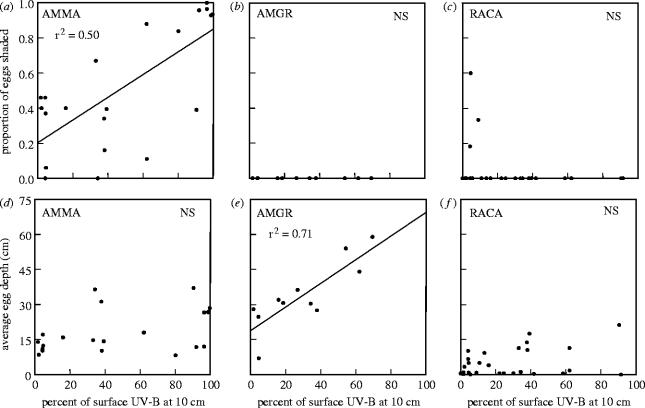
Breeding-site surveys from 2002 and 2003 coupled with our extensive sampling of the optical clarity of water revealed two strong relationships between amphibian oviposition behaviour (average egg depth and proportion of eggs shaded) and the UV-B transparency of individual breeding sites (figure 3). As the transparency of water to UV-B increased, we found that a greater proportion of A. macrodactylum eggs were laid in habitats fully shaded from light . Additionally, we found that the average depth of A. gracile eggs increased with increasing water transparency to . We found no significant pattern with either the proportion of eggs shaded or the average egg depth for R. cascadae as a function of UV-B penetration (Electronic Appendix 3, figure 3).
Figure 3.
Relationship between two features of embryonic oviposition, (a–c) proportion of eggs shaded and (d–f) mean depth of eggs, at breeding sites of (a,d) AMMA, (b,e) AMGR and (c,f) RACA across a range of water transparency to UV-B (percentage surface UV-B at 10 cm water depth). A greater proportion of AMMA embryos are shaded from ambient light with increasing water transparency to , and the average depth (cm) of AMGR eggs in the water column increases with increasing water transparency to . No other relationships were found to be statistically significant with α=0.05. Transmission of surface UV-B through the water column is determined by the diffuse attenuation coefficient (Kd) for UV-B wavelengths (290–320 nm), estimated by the absorption of 440 nm light in a spectrophotometer.
4. Discussion
(a) Laboratory exposure experiments
Our laboratory experiments manipulating UV-B dose and access to photorepair radiation (PRR) demonstrated that physiological sensitivity to UV-B was highly variable among species, as indicated by the substantial differences in LD50 estimates in +PRR treatments (figure 2). Further, in the absence of PRR when light-dependent repair of UV-B damage is disabled, there remain moderate differences in LD50 estimates among species, reflecting differences in the concentration of photoprotective compounds of the embryo or jelly (Grant & Licht 1995; Belden & Blaustein 2002; Licht 2003) or in the role of nucleotide excision repair (dark repair; figure 2). Surveys across broad animal taxa suggest that PER is the key physiological mechanism by which most organisms cope with UV-B-induced DNA damage, and that organisms with high capabilities for PER often have highly reduced capabilities to undergo nucleotide excision repair (dark repair; reviewed by Mitchell & Karentz 1993). Our results support this pattern, where the contribution of PER to overall UV-B tolerance (+PRR LD50) was greater than that of photoprotection and dark repair combined (−PRR LD50) for all four species of amphibians.
We cautiously interpret the relative sensitivity of A. gracile embryos given the necessity of removing the extracellular jelly matrix for our solar phototron experiments. Optical properties of amphibian jelly have been examined for several species including A. gracile, the results of which suggest that the attenuation of UV-B wavelengths by jelly is highly variable among species and developmental stages, ranging from strong to very limited attenuation in the UV-B range (Grant & Licht 1995; Ovaska et al. 1997; Smith et al. 2002; Licht 2003; Rasanen et al. 2003). Owing to this uncertainty surrounding the importance of extracellular jelly for A. gracile exposure to UV-B, we suggest that the LD50 reported here probably represent minimum estimates, although the relative difference between +PRR LD50 and −PRR LD50 should be unaffected. If A. gracile jelly during the early embryonic stages coinciding with our experiment (stages 8–10) does not affect UV-B transmission, then our LD50s should be representative, and in contrast, if it strongly attenuates UV-B then the true LD50s will be more similar to those estimated for the frog species.
Although a number of studies have documented the correlation between the activity of the enzyme photolyase in vitro and the survival of embryos in laboratory or field UV-B exposure experiments (Blaustein et al. 1994, 1999; Grant & Licht 1995; Van de Mortel et al. 1998; Smith et al. 2002), our study is among the first to follow the fate of individual embryos to hatching while testing the importance of PER in each species' overall sensitivity to UV-B (but see Worrest & Kimeldorf 1976). Because enzymatic photorepair processes are inherently temperature dependent, it is probable that the exact LD50 we estimate for each species is also dependent on the temperature at which the experiments were performed (15 °C), as has been demonstrated for a suite of zooplankton species (Williamson et al. 2002). Therefore, the sensitivity of particular species to UV-B will probably depend on site-specific incubation temperatures in addition to underlying physiological capabilities, optical properties of water and oviposition behaviours.
(b) Field surveys
UV-B is attenuated exponentially as a function of depth, where the rate of attenuation (Kd) depends on the concentration of DOM (Scully & Lean 1994; Morris et al. 1995). As a result, the UV-B exposure and dose received through time at any point in the water column is a function of the ambient flux of UV-B to the pond surface Kd and water depth. The simplicity of these calculations highlights the relative ease of calculating first approximations of embryonic UV-B exposures by combining depth and microhabitat distribution data with estimates of the Kd for each breeding site. Our field surveys indicate that females of A. macrodactylum and A. gracile adjust the microhabitats in which they lay eggs, and that this pattern is directly correlated with the UV-B transparency of water at individual breeding sites. Williamson et al. (1997) found a similar pattern in the depth distribution of yellow perch eggs in two lakes with contrasting UV-B transparency, and experimental incubations of eggs within these lakes confirm that eggs suffer higher mortality when moved to shallower depths (Huff et al. 2004). Field experiments with amphibians have also verified that embryonic mortality due to UV-B declines with increasing water depth (Kiesecker et al. 2001). Therefore, we expect that A. gracile eggs laid deeper in the water column experience a reduced UV-B environment both in terms of the peak flux and the dose of UV-B through time, and suffer less UV-B-induced mortality as a result.
Factors influencing the oviposition behaviour of female amphibians have been studied for over 30 years. Though the primary focus has been on the choice of individual breeding sites rather than the microhabitat distribution of oviposition within sites, many of the same factors could influence choices made by individual amphibians. For example, amphibian breeding-site selection is known to be influenced by the combination of predators including fishes and amphibians (Resetarits & Wilbur 1989; Kats & Sih 1992; Murphy 2003), embryonic and larval densities of other amphibians (Resetarits & Wilbur 1989; Murphy 2003), temperature (Seale 1982) and pond drying (Spieler & Linsenmair 1997). Because our results are correlative, it is possible that the patterns of oviposition we detected for A. gracile and A. macrodactylum embryos in optically clear sites may be the result of females responding to one or more of these physical or biological features, although they would be expected to covary with optical clarity. We have followed each of these sites for 3 or more years, having collected a variety of data on physical, chemical and biological features. Primary predators in these relatively simple communities are cannibalistic and predatory amphibians (A. gracile, A. macrodactylum, Taricha granulosa and adult R. cascadae) and omnivorous larval insects (Libellulidae, Limnephilidae). Analyses of community composition and environmental data have not revealed any strong species associations or avoidance patterns, or changes in the suite of predators, temperature or pond permanence with optical clarity (W. J. Palen & D. E. Schindler, unpublished work).
Although our study does not include enough species to make a rigorous comparison across broad taxa, the differences that we identified between species in physiological sensitivity to UV-B exposure and the complementary differences in oviposition behaviour in the field suggest that trade-offs exist in the way that species confront environmental stressors. We propose that the level of behavioural adaptation by a species to avoid a particular environmental stressor is probably related to the degree of physiological capacity to withstand it. Nonetheless, it remains to be determined how widespread UV-B-moderating behaviours may be among taxa known to be physiologically sensitive to UV-B (Rhode et al. 2001). This highlights the long-standing debate surrounding the role that environmental stress plays in the evolution of adaptive physiologies (Mayr 1963). Recent work has proposed that behavioural responses to natural environmental stressors may reduce selection pressure for adaptive physiology by dampening the effect of environmental variation (Huey et al. 2003). Amphibian sensitivity to UV-B may also fit this paradigm, as it seems plausible that species that modify the exposure of their embryos may temper the negative effects of UV-B on embryonic survival, thereby reducing the potential for selection on photoprotection or photorepair physiology. Although this mechanism has been generally proposed for amphibians (Blaustein et al. 1994, 1997; Smith et al. 2002; Licht 2003), our study represents the most quantitative comparison to date of the physiological sensitivity of individuals and broad patterns of oviposition that influence UV-B exposure.
Our controlled laboratory experiments and field surveys present a more complete picture of the susceptibility of these four amphibian species to current and increasing levels of UV-B. We find that although embryonic survival can be influenced strongly by exposure to UV-B for each species, photoenzymatic repair of UV-B damage is a critical component to interpreting variation in the overall tolerance of individual species. When embryos were prevented from undergoing PER, each species suffered substantially higher mortality for a given level of UV-B exposure (figure 1). These laboratory experiments also suggest that the embryos of both salamander species tested—A. macrodactylum and A. gracile—are more susceptible to UV-B-induced mortality than either of the frog species, P. regilla and R. cascadae, as has been suggested previously (Blaustein et al. 1994, 1997). However, the degree of UV-B sensitivity determined at the level of individual physiology contrasts with the patterns of embryonic UV-B exposure that we documented across individual breeding sites in the field, where both salamander species exhibit strong oviposition behaviours limiting embryonic UV-B exposure (figure 3). Were we to estimate the risk posed by increasing UV-B to each of these species based exclusively on the results of physiological sensitivity, we would probably overstate the vulnerability of at least one (A. macrodactylum) if not both (A. gracile) of the salamander species relative to the frog species, P. regilla and R. cascadae.
Amphibian species of the US Pacific Northwest, including those we tested, have been at the forefront of research on the effects of UV-B on amphibians, and our study reveals that the evaluation of the risk posed by current and increasing levels of UV-B is still maturing. Although there is an increasing appreciation for the consequences of investigating ecological processes at particular scales, our study suggests that physiological assessments of sensitivity to UV-B alone may overstate the vulnerability of species like A. macrodactylum and A. gracile that exhibit behaviours reducing their exposure to harmful levels of UV-B. We find that our predictions as to which species are most vulnerable to UV-B change as a function of our level of investigation, but when combined, we improve our assessment of when and where UV-B exposure influence embryonic survival.
Acknowledgments
We thank the US National Park Service for access to field sites; Jennifer Jones, Adam Goodwin, Justin Fox, Eric Wagner and the Sol Duc backcountry rangers for field assistance; Klaus Richter for information about North Cascades breeding sites; and Steve Diamond, Lawrence Licht and Robert Moeller for discussions about light attenuation in jelly. Comments from Bob Paine, Mike Adams, Bruce Bury, Steve Corn, Jennie Hoffman and three anonymous reviewers greatly improved this manuscript. This work was supported by the Canon National Park Science Scholars Program, US Geological Survey, US National Park Service and the Department of Biology, University of Washington.
Footnotes
As this paper exceeds the maximum length normally permitted, the authors have agreed to contribute to production costs.
Supplementary Material
References
- Adams M.J, Schindler D.E, Bury R.B. Association of amphibians with attenuation of ultraviolet-B radiation in montane ponds. Oecologia. 2001;128:519–525. doi: 10.1007/s004420100688. [DOI] [PubMed] [Google Scholar]
- Adams, M. J., Hossack, B. R., Knapp, R. A., Corn, P. S., Diamond, S. A., Trenham, P. C. & Fagre, D. C. In press. Distribution patterns of lentic-breeding amphibians in relation to ultraviolet radiation exposure in Western North America. Ecosystems
- Belden L.K, Blaustein A.R. UV-B induced skin darkening in larval salamanders does not prevent sublethal effects of exposure on growth. Copeia. 2002;3:748–754. [Google Scholar]
- Belden L.K, Wildy E.L, Blaustein A.R. Growth, survival and behaviour of larval long-toed salamanders (Ambystoma macrodactylum) exposed to ambient levels of UV-B radiation. J. Zool. 2000;251:473–479. [Google Scholar]
- Blaustein A.R, Wake D.B. Declining amphibian populations: a global phenomenon? Trends Ecol. Evol. 1990;5:203–204. [Google Scholar]
- Blaustein A.R, Hoffman P.D, Hokit D.G, Kiesecker J.M, Walls S.C, Hays J.B. UV repair and resistance to solar UV-B in amphibian eggs: a link to population declines? Proc. Natl Acad. Sci. USA. 1994;91:1791–1795. doi: 10.1073/pnas.91.5.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein A.R, Kiesecker J.M, Chivers D.P, Anthony R.G. Ambient UV-B radiation causes deformities in amphibian embryos. Proc. Natl Acad. Sci. USA. 1997;94:13 735–13 737. doi: 10.1073/pnas.94.25.13735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein A.R, et al. DNA repair and resistance to UV-B radiation in western spotted frogs. Ecol. Appl. 1999;9:1100–1105. [Google Scholar]
- Blaustein A.R, Romansic J.M, Kiesecker J.M, Hatch A.C. Ultraviolet radiation, toxic chemicals and amphibian population declines. Divers. Distrib. 2003;9:123–140. [Google Scholar]
- Brooks, P. D., O'Reilly, C. M., Diamond, S. A., Campbell, D. H., Knapp, R., Bradford, D., Corn, P. S., Hossack, B. & Tonnesson, K. In press. Spatial and temporal variability in the amount and source of dissolved organic carbon: implications for UV exposure in amphibian habitats. Ecosystems
- Carpenter S.R. Microcosm experiments have limited relevance for community and ecosystem ecology. Ecology. 1996;77:677–680. [Google Scholar]
- Diamond S.A, Peterson G.S, Tietge J.E, Ankley G.T. Assessment of the risk of solar ultraviolet radiation to amphibians. III. Prediction of impacts in selected northern mid-western wetlands. Environ. Sci. Tech. 2002;36:2866–2874. doi: 10.1021/es011197d. [DOI] [PubMed] [Google Scholar]
- Fleishman L.J, Loew E.R, Leal M. Ultraviolet vision in lizards. Nature. 1993;365:397. [Google Scholar]
- Grant K.E, Licht L.E. Effects of ultraviolet radiation on life-history stages of anurans from Ontario, Canada. Can. J. Zool. 1995;73:2292–2301. [Google Scholar]
- Gutierrez-Rodriguez C, Williamson C.E. Influence of solar ultraviolet radiation on early life-history stages of the bluegill sunfish, Lepomis macrochirus. Environ. Biol. Fish. 1999;55:307–319. [Google Scholar]
- Hansson L.-A. Plasticity in pigmentation induced by conflicting threats from predation and UV radiation. Ecology. 2004;85:1005–1016. [Google Scholar]
- Hays J.B, Blaustein A.R, Kiesecker J.M, Hoffman P.D, Pandelova I, Coyle D, Richardson T. Developmental responses of amphibians to solar and artificial UVB sources: a comparative study. Photochem. Photobiol. 1996;64:449–456. doi: 10.1111/j.1751-1097.1996.tb03090.x. [DOI] [PubMed] [Google Scholar]
- Hofer R, Mokri C. Photoprotection in tadpoles of the common frog, Rana temporaria. Photochem. Photobiol. 2000;59:48–53. doi: 10.1016/s1011-1344(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Holomuzki J.R. Oviposition sites and fish-deterrent mechanisms of two stream anurans. Copeia. 1995;1995:607–613. [Google Scholar]
- Huey R.B, Hertz P.E, Sinervo B. Behavioral drive versus behavioral inertia in evolution: a null model approach. Am. Nat. 2003;161:357–366. doi: 10.1086/346135. [DOI] [PubMed] [Google Scholar]
- Huff D.D, Grad G, Williamson C.E. Environmental constraints on spawning depth of yellow perch: the roles of low temperature and high solar ultraviolet radiation. Trans. Am. Fish. Soc. 2004;133:718–726. [Google Scholar]
- Kats L.B, Sih A. Oviposition site selection and avoidance of fish by streamside salamanders (Ambystoma barbouri) Copeia. 1992;1992:468–473. [Google Scholar]
- Kerr J.B, McElroy C.J. Evidence for large upward trends of ultraviolet-B radiation linked to ozone depletion. Science. 1993;262:1032–1034. doi: 10.1126/science.262.5136.1032. [DOI] [PubMed] [Google Scholar]
- Kiesecker J.M, Blaustein A.R, Belden L.K. Complex causes of amphibian population declines. Nature. 2001;410:681–684. doi: 10.1038/35070552. [DOI] [PubMed] [Google Scholar]
- Kollias N, Sayre R.M, Zeise L, Chedekel M.R. Photoprotection by melanin. J. Photochem. Photobiol. B. 1991;9:135–160. doi: 10.1016/1011-1344(91)80147-a. [DOI] [PubMed] [Google Scholar]
- Licht L.E. Shedding light on ultraviolet radiation and amphibian embryos. BioScience. 2003;53:551–561. [Google Scholar]
- Lizana M, Pedraza E.M. The effects of UV-B radiation on toad mortality in mountainous areas of central Spain. Conserv. Biol. 1998;12:703–707. [Google Scholar]
- Lowe C, Goodman-Lowe G. Suntanning in hammerhead sharks. Nature. 1996;383:677. doi: 10.1038/383677a0. [DOI] [PubMed] [Google Scholar]
- Madronich S. Increases in biologically damaging UV-B radiation due to stratospheric ozone reductions: a brief review. Archiv für Hydrobiologie Beiheft Ergebnisse der Limnologie. 1994;43:17–30. [Google Scholar]
- Mayr E. Harvard University Press; Cambridge: 1963. Animal species and evolution. [Google Scholar]
- Mitchell D.L, Karentz D. The induction and repair of DNA photodamage in the environment. In: Young A.R, et al., editors. Environmental UV photobiology. Plenum Press; New York: 1993. pp. 345–377. [Google Scholar]
- Morris D.P, Zagarese H, Williamson C.E, Balseiro E.G, Hargreaves B.R, Modenutti B, Moeller R, Queimalinos C. The attenuation of solar UV radiation in lakes and the role of dissolved organic carbon. Limnol. Oceanogr. 1995;40:1381–1391. [Google Scholar]
- Murphy P.J. Context-dependent reproductive site choice in a Neotropical frog. Behav. Ecol. 2003;14:626–633. [Google Scholar]
- Nagl A.M, Hofer R. Effects of ultraviolet radiation on early larval stages of the Alpine newt, Triturus alpestris, under natural and laboratory conditions. Oecologia. 1997;110:514–519. doi: 10.1007/s004420050188. [DOI] [PubMed] [Google Scholar]
- Olson D.H. Ecology and management of montane amphibians of the US Pacific Northwest. Biota. 2001;2:51–74. [Google Scholar]
- Ovaska K, Davis T.M, Flamarique I.N. Hatching success and larval survival of the frogs Hyla regilla and Rana aurora under ambient and artificially enhanced solar ultraviolet radiation. Can. J. Zool. 1997;75:1081–1088. [Google Scholar]
- Pahkala M, Laurila A, Merila J. Ambient ultraviolet-B radiation reduces hatchling size in the common frog Rana temporaria. Ecography. 2000;23:531–538. [Google Scholar]
- Palen W.J, Schindler D.E, Adams M.J, Pearl C.A, Bury R.B, Diamond S.A. Optical characteristics of natural waters protect amphibians from UV-B in the U.S. Pacific Northwest. Ecology. 2002;83:2951–2957. [Google Scholar]
- Petranka J.W, Hopey M.E, Jennings B.T, Baird S.D, Boone S.J. Breeding habitat segregation of wood frogs and american toads—the role of interspecific tadpole predation and adult choice. Copeia. 1994;3:691–697. [Google Scholar]
- Rasanen K, Pahkala M, Laurila A, Merila J. Does jelly envelope protect the common frog Rana temporaria embryos from UV-B radiation? Herpetologica. 2003;59:293–300. [Google Scholar]
- Resetarits W.J, Wilbur H.M. Choice of oviposition site by Hyla chrysoscelis: role of predators and competitors. Ecology. 1989;70:220–228. [Google Scholar]
- Rhode S.C, Pawlowski M, Tollrian R. The impact of ultraviolet radiation on the vertical distribution of zooplankton of the genus Daphnia. Nature. 2001;412:69–72. doi: 10.1038/35083567. [DOI] [PubMed] [Google Scholar]
- Scully N.M, Lean D.R.S. The attenuation of ultraviolet radiation in temperate lakes. Archiv für Hydrobiologie Beiheft Ergebnisse der Limnologie. 1994;43:135–144. [Google Scholar]
- Seale D.B. Physical factors influencing oviposition by the wood frog, Rana sylvatica, in Pennsylvania. Copeia. 1982;1982:627–635. [Google Scholar]
- Smith M.A, Kapron C.M, Berrill M. Induction of photolyase activity in wood frog (Rana sylvatica) embryos. Photochem. Photobiol. 2000;72:575–578. doi: 10.1562/0031-8655(2000)072<0575:iopaiw>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Smith M.A, Berrill M, Kapron C.M. Photolyase activity of the embryo and the ultraviolet absorbance of embryo jelly for several Ontario amphibian species. Can. J. Zool. 2002;80:1109–1116. [Google Scholar]
- Spieler M, Linsenmair K.E. Choice of optimal oviposition sites by Holobatrachus occipitalis (Anura: Ranidae) in an unpredictable and patchy environment. Oecologia. 1997;109:184–199. doi: 10.1007/s004420050073. [DOI] [PubMed] [Google Scholar]
- Stark J.D, Banks J.E, Vargas R. How risky is risk assessment: the role that life history strategies play in susceptibility of species to stress. Proc. Natl Acad. Sci. USA. 2004;101:732–736. doi: 10.1073/pnas.0304903101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovee M.J. Ultra-violet photoreceptors in the animal kingdom—their distribution and function. Trends Ecol. Evol. 1995;10:455–460. doi: 10.1016/s0169-5347(00)89179-x. [DOI] [PubMed] [Google Scholar]
- Tietge J.E, Diamond S.A, Ankley G.T, DeFoe D.L, Holcombe G.W, Jensen K.M, Degitz S.J, Elonen G.E, Hammer E. Ambient solar UV radiation causes mortality in larvae of three species of Rana under controlled exposure conditions. Photochem. Photobiol. 2001;74:261–268. doi: 10.1562/0031-8655(2001)074<0261:asurcm>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Van de Mortel T.F, Buttemer W.A. Avoidance of ultraviolet-B radiation in frogs and tadpoles of the species Litoria aurea, L. dentata, and L. peronii. Proc. Linn. Soc. NSW. 1998;119:173–179. [Google Scholar]
- Van de Mortel T, Buttemer W, Hoffman P, Hays J, Blaustein A. A comparison of photolyase activity in three Australian tree frogs. Oecologia. 1998;115:366–369. doi: 10.1007/s004420050529. [DOI] [PubMed] [Google Scholar]
- Viitala J, Korpimaki E, Palokangas P, Koivula M. Attraction of kestrels to vole scent marks visible in ultraviolet light. Nature. 1995;373:425–427. [Google Scholar]
- Williamson C.E, Metzgar S.L, Lovera P.A, Moeller R.E. Solar ultraviolet radiation and the spawning habitat of yellow perch, Perca flavescens. Ecol. Appl. 1997;7:1017–1023. [Google Scholar]
- Williamson C.E, Neale P.J, Grad G, De Lange H, Hargreaves B. Beneficial and detrimental effects of UV on aquatic organisms: implications of spectral variation. Ecol. Appl. 2001;11:1843–1857. [Google Scholar]
- Williamson C.E, Grad G, De Lange H.J, Gilroy S. Temperature-dependent ultraviolet responses in zooplankton: implications of climate change. Limnol. Oceanogr. 2002;47:1844–1848. [Google Scholar]
- Worrest R.C, Kimeldorf D.J. Photoreactivation of potentially lethal, UV-induced damage to Boreal toad (Bufo boreas boreas) tadpoles. Life Sci. 1976;17:1545–1550. doi: 10.1016/0024-3205(75)90175-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.