Abstract
Cacospongionolide B is a novel marine metabolite isolated from the sponge Fasciospongia cavernosa. In in vitro studies, this compound inhibited phospholipase A2 (PLA2), showing selectivity for secretory PLA2 (sPLA2) versus cytosolic PLA2 (cPLA2), and its potency on the human synovial enzyme (group II) was similar to that of manoalide.
This activity was confirmed in vivo in the 8 h zymosan-injected rat air pouch, on the secretory enzyme accumulating in the pouch exudate. Cacospongionolide B, that is bioavailable when is given orally, reduced the elevated levels of sPLA2 present in paw homogenates of rats with adjuvant arthritis.
This marine metabolite showed topical anti-inflammatory activity on the mouse ear oedema induced by 12-O-tetradecanoylphorbol acetate (TPA) and decreased carrageenin paw oedema in mice after oral administration of 5, 10 or 20 mg kg−1.
In the mouse air pouch injected with zymosan, cacospongionolide B administered into the pouch, induced a dose-dependent reduction in the levels of eicosanoids and tumour necrosis factor α (TNFα) in the exudates 4 h after the stimulus. It also had a weak effect on cell migration.
The inflammatory response of adjuvant arthritis was reduced by cacospongionolide B, which did not significantly affect eicosanoid levels in serum, paw or stomach homogenates and did not induce toxic effects.
Cacospongionolide B is a new inhibitor of sPLA2 in vitro and in vivo, with anti-inflammatory properties in acute and chronic inflammation. This marine metabolite was active after oral administration and able to modify TNFα levels, and may offer an interesting approach in the search for new anti-inflammatory agents.
Keywords: Inflammation, phospholipase A2, rat and mouse air pouch, adjuvant arthritis, manoalide, cacospongionolide B
Introduction
The activation of different phospholipases is a critical step in the biosynthesis of lipid mediators. Phospholipase A2 (PLA2) is a class of enzymes that hydrolyze the acyl group from the sn-2 position of glycerophospholipids, yielding free fatty acids and lysophospholipids. These products or their metabolites are bioactive lipids modulating different cellular processes. Mammalian cells contain diverse PLA2 which may play a distinct role in cell activation and signal transduction. Moreover, in pathologic states, increased PLA2 activity causes alteration of membrane structure and function as well as an excessive production of lipid mediators and toxic species that contributes to tissue injury. Secretory PLA2 (sPLA2, groups I, II, III and V), cytosolic PLA2 (cPLA2, group IV) and calcium-independent PLA2 have been studied (for review see Serhan et al. (1996); Dennis (1997)). Calcium-independent PLA2 is present in the myocardium and other tissues. This enzyme may regulate the incorporation of arachidonic acid into membrane phospholipids in P388D1 macrophages (Balsinde et al., 1995) and could participate in arachidonic acid release and cell spreading in murine peritoneal macrophages (Teslenko et al., 1997).
It has been reported that cPLA2 play an important role in arachidonic acid release in a number of cell systems, e.g. human platelets stimulated with thrombin (Bartoli et al., 1994) or calcium ionophore (Riendeau et al., 1994), permeabilized human neutrophils (Bauldry & Wooten, 1996) or mouse peritoneal macrophages challenged with zymosan or 12-O-tetradecanoylphorbol acetate (TPA) (Qiu & Leslie, 1994). Inflammatory cytokines have been shown to induce cPLA2, resulting in high levels of eicosanoids in airway epithelial cells (Wu et al., 1997), rheumatoid synovial fibroblasts (Hulkower et al., 1994) or mouse osteoblasts (Chen et al., 1997).
Group II sPLA2 can act as a signalling agent that mediates cell growth induced by interleukin-1β (IL-1β) (Wada et al., 1997). In addition, it has a role in cell activation and contributes to the inflammatory response. sPLA2 activation may participate in signal transduction events such as CD11b/CD18 (MAC-1) expression on the surface of activated human neutrophils, and adhesion or degranulation (Takasaki et al., 1996; Jacobson & Schrier, 1993). This enzyme activity secreted at inflammatory sites becomes associated with cell surfaces and hydrolyzes phospholipids, thus releasing arachidonic acid, which enters the cell and participate in the increased generation of inflammatory lipid mediators (Pfeilschifter et al., 1993; Miyake et al., 1994). In fact, administration of different types of sPLA2 can induce or amplify inflammatory responses in animals (Vishwanath et al., 1988; Tanaka et al., 1995; Cirino et al., 1994). Interestingly, inflammatory cytokines increase group II PLA2 synthesis and secretion by rheumatoid synovial fibroblasts and other cell types (Pfeilschifter et al., 1993; Bomalaski & Clark, 1993). Thus, IL-1β induces an increase in group II sPLA2 gene expression, but does not increase cPLA2 gene expression or activity, and it provokes a parallel increase in prostaglandin E2 (PGE2) production by rabbit articular chondrocytes (Jacques et al., 1997). Group II sPLA2 has been reported to release arachidonic acid in some systems and may provide the substrate for both cyclo-oxygenase (COX) and 5-lipoxygenase (5-LO) product formation in mouse bone marrow-derived mast cells (Fonteh et al., 1994). In contrast, PLA2 secreted by guinea-pig peritoneal macrophages does not participate in the synthesis of PGE2 accumulating in the media (Marshall et al., 1994).
On the other hand, exocytosis of sPLA2 could modulate the activity of cPLA2 by initiating the formation of leukotriene B4 (LTB4), which after release stimulates its own receptor, thus leading to activation of cPLA2 in neutrophils (Wijkander et al., 1995). Exogenously added group I PLA2 is also believed to be involved in arachidonic acid release (Hara et al., 1991), in some cases accompanied by induction of group II PLA2, and recently a group V sPLA2 has been reported to participate in immediate prostanoid generation in the mouse macrophage cell line P388D1 (Balboa et al., 1996).
Arachidonic acid mobilization can be dependent on both types of PLA2 in some systems, as in the case of delayed PGD2 generation by COX-2 in rat peritoneal macrophages stimulated by lipopolysaccharide (LPS) (Naraba et al., 1998), as well as in receptor-stimulated P388D1 macrophages (Balsinde & Dennis, 1996), and human umbilical vein endothelial cells (Murakami et al., 1993). In human monocytes stimulated by ionophore or zymosan, cPLA2 would participate preferentially in the release of arachidonic acid for prostaglandin (PG) synthesis, whereas sPLA2 probably releases the substrate for LT synthesis (Marshall et al., 1997).
Marine organisms are a rich source of molecules exhibiting PLA2 inhibitory properties in vitro, mainly on secretory enzymes (for review, see Potts et al., 1992). Some of these compounds have been found to reduce experimental inflammatory responses, preferentially after topical application. We have examined the PLA2 inhibitory activity of cacospongionolide B (Figure 1), a new marine metabolite isolated from the Mediterranean sponge Fasciospongia cavernosa. The results of our studies demonstrate that cacospongionolide B is a potent inhibitor of sPLA2. We have also assessed its effects on models of acute and chronic inflammation.
Figure 1.
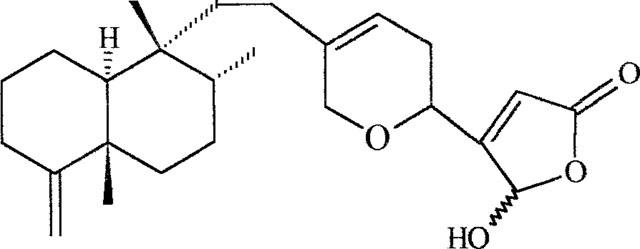
Chemical structure of cacospongionolide B.
Methods
sPLA2 assay
sPLA2 was assayed by using a modification of the method of Franson et al. (1974). E. coli strain CECT 101 were seeded in medium containing 1% tryptone, 0.5% NaCl and 0.6% sodium dihydrogen orthophosphate, pH 5.0, and grown for 6–8 h at 37°C in the presence of 5 μCi ml−1 [3H]-oleic acid (sp. act. 10 Ci mmol−1). After centrifugation at 2500×g for 10 min, the cells were washed in buffer (0.7 M Tris-HCl, 10 mM CaCl2, 0.1% bovine serum albumin, BSA, pH 8.0), resuspended in saline and autoclaved for 30–45 min. At least 95% of the radioactivity was incorporated into phospholipids. Naja naja venom, porcine pancreatic, bee venom and human recombinant synovial enzymes were diluted in 10 μl of 100 mM Tris-HCl, 1 mM CaCl2 buffer, pH 7.5. Supernatants (10 μl) of exudates from zymosan-injected rat air pouch (Payá et al., 1996) were also used as a source of sPLA2. Enzymes were preincubated at 37°C for 5 min with 2.5 μl of test compound solution or its vehicle in a final volume of 250 μl. Incubation proceeded for 15 min in the presence of 10 μl of autoclaved oleate-labelled membranes and was terminated by addition of 100 μl ice-cold solution of 0.25% BSA in saline to a final concentration of 0.07% w/v. After centrifugation at 2500×g for 10 min at 4°C, the radioactivity in the supernatants was determined by liquid scintillation counting.
cPLA2 assay
cPLA2 was prepared from human monocytic U937 cells (Cell Collection, Department of Animal Cell Culture, C.S.I.C., Madrid, Spain) grown in the above medium which were disrupted by sonication in 10 mM HEPES buffer pH 7.4, containing 0.32 M sucrose, 100 μM EDTA, 1 mM dithiothreitol, 2 mM phenylmethylsulphonylfluoride and 100 μM leupeptin. The homogenated cells were centrifuged at 2000×g for 10 min at 4°C and the resulting supernatant was further centrifuged at 100,000×g for 100 min at 4°C to obtain the cytosolic fraction. cPLA2 activity was measured as the release of radiolabelled arachidonic acid according to the method of Clark et al. (1990). 1-Palmitoyl-2-[14C]-arachidonyl-sn-glycero-3-phosphocholine (57.0 mCi mmol−1, 2×106 c.p.m.) was dried under nitrogen, then suspended in 1 ml of 100 mM glycine buffer pH 9.0 containing 200 μM Triton X-100, 10 mM CaCl2, 0.25 mg ml−1 BSA and 40% v/v glycerol. The suspension was then sonicated to form mixed micelles of phospholipid and Triton X-100. The reaction was started by adding the enzyme solution (approximately 24 μg protein of cytosolic fraction from human monocytes) to a final volume of 100 μl of the assay mixture which contained 1 mM CaCl2, 2 mM 2-mercaptoethanol, 150 mM NaCl, 40% glycerol, 1 mg ml−1 BSA and 50 mM HEPES pH 9.0. The substrate consisted of 5 μl of micelles (104 c.p.m.) containing dioleoyl glycerol at a molar ratio 2 : 1 (Kramer et al., 1987). Test compounds were dissolved in methanol and added to the reaction mixture just before the addition of the enzyme solution. The final concentration of methanol in the reaction mixture was less than 1%, which showed no effect on the enzyme activity. The reaction was stopped after a 60 min incubation period at 37°C by mixing with 0.5 ml of isopropyl alcohol/heptane/0.5 M H2SO4 (10 : 5 : 1). Heptane (0.7 ml) and water (0.2 ml) were then added, and the solution was vigorously mixed for 15 s. The heptane phase was mixed with 100 mg silica gel 60 (Merck, 70-230 mesh) and centrifuged, and the radioactivity in each supernatant was measured (Zhang et al., 1991).
Preparation of human leukocytes
The citrated blood of healthy volunteers was centrifuged at 200×g for 15 min at room temperature. The platelet-rich plasma was removed, and the leukocytes contained in the residual blood were isolated by sedimentation with 2% (w/v) dextran in 0.9% NaCl at room temperature. The supernatant was centrifuged at 1200×g for 10 min at 4°C. Contaminating erythrocytes were lysed by hypotonic treatment. The pellet was resuspended in phosphate buffered saline (PBS), and Ficoll-hypaque was layered under the cell mixture. The cell gradient mixture was centrifuged at 400×g for 40 min at 20°C. Neutrophils were separated and resuspended in PBS containing 1.26 mM Ca2+ and 0.9 mM Mg2+ (Bustos et al., 1995). Viability was greater than 95% by the Trypan blue exclusion test. The monocyte and lymphocyte layer was removed and pelleted by centrifugation. The cell pellet was resuspended in RPMI-1640 media, pH 7.4, with 10% foetal bovine serum, 2 mM L-glutamine, 50 u ml−1 penicillin and 50 μg ml−1 streptomycin and was incubated at a cell density of 107 ml−1 in 60/15 mm tissue culture dishes. The cells were allowed to adhere for 2 h at 37°C in a 5% CO2 atmosphere incubator. The nonadherent cells were removed by vacuum suction of media followed by two washes with 1 ml of RPMI-1640. The adherent cells resulted in a greater than 90% pure monocyte population as assessed by differential staining.
Cytotoxicity assays
The cytoplasmic marker enzyme lactate dehydrogenase (LDH) (Bergmeyer & Bernt, 1974) and the mitochondrial dependent reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to formazan (Gross & Levi, 1992) were used to assess the possible cytotoxic effect of cacospongionolide B on human neutrophils.
Phospholipase C (PLC)
Human neutrophils (1×107 cells ml−1) were suspended in HBSS with Ca2+ and Mg2+ and preincubated with test drugs for 5 min at 37°C, before the reaction was initiated by adding 1 μM N-formyl-methionyl-leucyl-phenylalanine (fMLP) for 5 min at 37°C. Inositol triphosphate (IP3) was quantified by the method of Palmer (1989).
Synthesis of LTB4 by high speed supernatants from human neutrophils
High speed (100,000×g) supernatants from sonicated human neutrophils were obtained as previously described (Tateson et al., 1988). Aliquots (50 μg of protein/tube) in PBS containing 2 mM CaCl2 were incubated with 5 μM arachidonic acid at 37°C for 5 min, in the presence of test compounds or vehicle. The samples were then heated at 90°C for 5 min and centrifuged at 10,000×g at 4°C for 30 min. The LTB4 levels in supernatants were measured by radioimmunoassay (Moroney et al., 1988).
Cyclo-oxygenase-1
Human platelets were sonicated at 4°C in an ultrasonicator at maximum potency. Microsomes were prepared by centrifugation at 2000×g for 5 min at 4°C followed by centrifugation of the supernatant at 100,000×g for 100 min at 4°C. Microsomes (20 μg of protein/tube) were incubated for 30 min at 37°C in 50 mM Tris HCl, pH 7.4 with 5 μM arachidonic acid and test compound or vehicle in the presence of 2 μM hematin and 1 mM L-tryptophan. The reaction was terminated boiling the samples for 5 min and PGE2 levels were determined by radioimmunoassay (Moroney et al., 1988).
Cyclo-oxygenase-2
Human monocytes cells were resuspended in RPMI-1640 culture medium containing aspirin (300 μM) and incubated at 37°C for 2 h. The cells were washed twice, resuspended in RPMI-1640 with 10% foetal bovine serum and incubated with E. coli lipopolysaccharide (10 μg ml−1) at 37°C for 24 h (Grossman et al., 1995). After centrifugation the cells were sonicated at 4°C in an ultrasonicator at maximum potency, and microsomes were prepared as above. Microsomes (40 μg of protein/tube) were used as a source of cyclo-oxygenase-2 and reactions were carried out in the same conditions as above. PGE2 synthesis was determined by radioimmunoassay (Moroney et al., 1988).
Inducible nitric oxide synthase (iNOS) assay
NOS activity was induced by i.p. injection of LPS (2 mg kg−1) to rats. After 24 h the animals were killed and livers were excised and homogenated in 10 mM HEPES, pH 7.4, containing sacarose (0.32 M), EDTA (100 μM), dithiothreitol (1 mM), phenylmethylsulfonyl fluoride (1 mg ml−1) and leupeptin (10 μg ml−1) (Knowles et al., 1990). The homogenate was centrifuged at 1200×g for 10 min at 4°C, followed by centrifugation of the supernatant at 100,000×g for 100 min at 4°C. NOS activity was determined in supernatants by monitoring the conversion of L-[3H]-arginine to L-[3H]-citrulline, (Mitchell et al., 1991). Samples (40 μg protein) were incubated at room temperature for 60 min with 100 μl of the above buffer in the presence of NADPH (1 mM) and a mixture of unlabelled and L-[3H]-arginine (10 μM, 1 μCi ml−1). Incubations were terminated by the addition of 20 mM HEPES (1 ml, pH 5.5) containing 1 mM EGTA and 1 mM EDTA. L-[3H]-citrulline was separated from arginine by adding 1.5 ml of a 1 : 1 suspension of Dowex (50 W) in water. Radioactivity was measured in supernatants by liquid scintillation counting.
Rat air pouch
Male Wistar rats (120–150 g) were used. Air pouches were formed as previously described (Edwards et al., 1981). The animals were anaesthetized with ethyl ether and given a 20 ml injection of sterile air in the subcutaneous tissue of the back, and 3 days later 10 ml of sterile air was injected into the same cavity. After 3 days 1 ml of sterile saline (saline group), 1 ml of 1% (w/v) zymosan in saline+10 μl ethanol (zymosan control group) or 1 ml of 1% w/v zymosan in saline+test drug (dissolved in 10 μl of ethanol: treated groups), was administered into the air pouch. After 8 h, rats were sacrificed and the exudate was collected in 1 ml of saline. Leukocytes in exudate fluids were counted by Coulter counter. After centrifugation of the exudate at 1200×g at 4°C for 10 min, the supernatants were used to measure PLA2 activity as above. Protein was quantified by the Bradford technique (Bradford, 1976) using BSA as standard.
Mouse ear oedema
The protocols were approved by the institutional Animal Care and Use Commitee. All studies were performed in accordance with European Union regulations for the handling and use of laboratory animals. TPA (5 μg) dissolved in 20 μl of acetone was applied in 10 μl volumes to both inner and outer surfaces of the right ear of Swiss mice (20–25 g). Test compounds were applied topically in acetone before TPA administration. The left ear (control) received only acetone. The animals were killed by cervical dislocation after 4 h, and equal sections of both ears were punched out and weighed. The increase in the weight of the right ear punch over that of the left indicated the oedema (Carlson et al., 1985). The ear sections were homogenized in 750 μl saline, and after centrifugation at 10,000×g for 15 min at 4°C, the myeloperoxidase activity was measured in aliquots of supernatants. The reaction mixture contained 50 μl supernatant, 150 μl phosphate buffered saline, 20 μl 0.22 M NaH2PO4 pH 5.4, 20 μl 0.026 (v/v) % H2O2 and 20 μl 18 mM tetramethylbenzidine in 8% (v/v) aqueous dimethylformamide. After 10 min reaction at 37°C, 30 μl 1.46 M sodium acetate, pH 3.0 was added and absorbance at 620 nm was read using a microtiter plate reader (De Young et al., 1989).
Mouse paw oedema
Swelling was induced following a modification of the technique of Sugishita et al. (1981). Female Swiss mice (20–25 g) were fasted for 12 h with free access to water. Drugs or vehicle (ethanol, tween 80, distilled water: 5/5/90, v/v/v) were administered p.o. (0.5 ml) 1 h before the injection of carrageenin (0.05 ml; 3% w/v in saline) into the subplantar area of the right hind paws of groups of six animals. The volumes of injected and contralateral paws were measured at 1, 3 and 5 h after induction of oedema by using a plethysmometer (Ugo Basile, Comerio, Italy). The volume of oedema was expressed for each animal as the difference between the carrageenin-injected and contralateral paws.
Mouse air pouch
Female Swiss mice (25–30 g) were anaesthetized with ethyl ether, and 10 ml of sterile air was injected into the subcutaneous tissue of the back. Three days later, pouches were reinflated with 5 ml of sterile air. In 6 days-old air pouches, mice were administered saline (saline group), zymosan+vehicle (control group) or zymosan+test drug, as indicated in the rat air pouch method. Four hours after administration, the animals were killed by cervical dislocation, and the exudate in the pouch was collected with 1 ml of saline (Edwards et al., 1981). Leukocytes present in exudates were measured using a Coulter counter. After centrifugation of exudates at 1200×g at 4°C for 10 min, the supernatants were used to measure LTB4 and PGE2 levels as indicated above or TNFα by ELISA.
Adyuvant arthritis
Adyuvant arthritis was elicited in female Lewis rats (126–150 g) by injecting 0.1 ml of Mycobacterium butyricum (10 mg ml−1) in mineral oil into the base of the tail (Taurog et al., 1988). Paw volumes were measured at the beginning of the experiment by using a plethysmometer. Animals were housed in propylene cages with food and water ad libitum. The light cycle was automatically controlled (on 0700 h; off 1900 h) and the room temperature thermostatically regulated to 21±1°C. The magnitude of the inflammatory response was evaluated by measuring the volume of both paws at day 13. The oedema was calculated as the mean increase in paw volume. Animals with oedema paw volumes 0.60 ml larger than normal paws were then randomized into treatment groups. Cacospongionolide B (20 mg kg−1), indomethacin (5 mg kg−1) or vehicle (ethanol, tween 80, distilled water: 5/5/90, v/v/v) were administered p.o. (1.0 ml) once-daily on days 13–17. Serum was collected on the last day of the experiment (day 18) for the determination of PGE2, thromboxane B2 (TXB2) and LTB4. After death, paws from arthritic, treated groups and non-arthritic normal animals were amputated above the ankle and homogenized in 2.5 ml saline. After centrifugation at 10,000×g for 15 min at 4°C, supernatants were used for the determination of PGE2 and sPLA2. Prior to evaluating the sPLA2 content, a purification by acidic treatment was performed (Bolognese et al., 1995). Stomachs were homogenized in 2 ml of methanol and aliquots of supernatants were used to determine the content of PGE2 as above.
Materials
Cacospongionolide B was isolated from the sponge Faciospongia cavernosa following known procedures (De Rosa et al., 1995). Antibody against LTB4 was kindly provided by Zeneca Pharmaceuticals, Macclesfield, Cheshire, U.K. Human synovial recombinant PLA2 was a gift from Dr R.M. Kramer (Lilly Research Laboratories, Indianapolis, U.S.A). [9,10-3H]-oleic acid and L-3-phosphatidylcholine 1-palmitoyl-2-arachidonyl [arachidonyl-1-14C] were purchased from Du Pont, (Itisa, Madrid, Spain). [5,6,8,11,12,14,15(n)-3H]-PGE2, [5,6,8,9,11,12,14,15(n)-3H]-LTB4, [5,6,8,9,11,12,14,15(n)3H]-TXB2, L-[3H]-argnine, IP3 measurement kit and the TNFα ELISA kit were from Amersham Iberica, (Madrid, Spain). Palmityl trifluoromethyl ketone (PTK) was purchased from Cayman Chem. (MI, U.S.A.). M. butyricum was obtained from Difco (MI, U.S.A). The rest of reagents were from Sigma Chem.(MO, U.S.A). E. coli strain CECT 101 was a gift from Prof Uruburu, Department of Microbiology, University of Valencia, Spain.
Statistical analysis
The results are presented as means±s.e.mean; n represents the number of experiments. Inhibitory concentration 50% (IC50) values were calculated from at least four significant concentrations (n=6). The level of statistical significance was determined by analysis of variance (ANOVA) followed by Dunnett's t-test for multiple comparisons.
Results
Effect on PLA2 and other enzyme activities in vitro
The effect of cacospongionolide B on sPLA2 was determined using different assay systems in vitro. As shown in Table 1, this marine compound preferentially inhibited the synovial and pancreatic secretory enzymes, and its potency on the human synovial enzyme was comparable to that of the reference inhibitor, manoalide. Other sPLA2, including the enzyme present in the inflammatory exudates of zymosan-injected rat air pouch and bee venom PLA2, were inhibited by cacospongionolide B to a lesser extent, whereas it exerted no effect on the Naja naja venom enzyme. We also determined the concentration-dependent inhibition of human recombinant synovial PLA2 of cacospongionolide B and manoalide at 10 μM, without and with 100 μg ml−1 BSA in reaction buffer, resulting a partial reduction in their potency (cacospongionolide B, 86.7±2.5% and 60.1±1.9% of inhibition without and with BSA, respectively, n=6, P<0.01 and manoalide, 93.2±0.2% and 53.1±5.2% of inhibition without and with BSA, respectively, n=6, P<0.01). This type of non-specific action has been reported previously in manoalide (Jacobson et al., 1990). In contrast, cacospongionolide B had no inhibitory effects on cPLA2, which was partially inhibited by manoalide at 10 μM (Table 2). We also tested the possible influence of cacospongionolide B on other enzymes involved in the release or metabolism of arachidonic acid, such as PLC, COX-1, COX-2 and 5-LO, as well as on iNOS. Only 5-LO was slightly inhibited by cacospongionolide B in a manner similar to manoalide. In contrast, this reference compound also inhibited PLC. Cacospongionolide B did not exert significant cytotoxic effects on human neutrophils at concentrations up to 50 μM, as assessed by the release of LDH and the MTT method (data not shown). Otherwise manoalide showed a slight cytotoxicity effect at 50 μM, as assessed by the MTT method (18.9±3.0% of cytotoxicity, n=6, P<0.01).
Table 1.
Effect of cacospongionolide B and manoalide on different secretory PLA2 activities

Table 2.
Effect of cacospongionolide B and manolide on hr-sPLA2, cPLA2, PLC, 5-LO, COX-2, COX-1 and iNOS activities in vitro
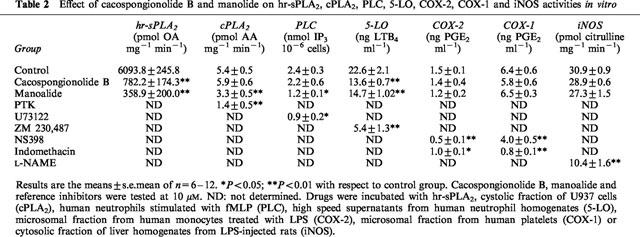
To further characterize the inhibitory activity of cacospongionolide B on sPLA2 we also studied the type of inhibition, which was apparently irreversible as assessed by the kinetic analysis of enzyme activity as a function of the human synovial PLA2 concentration in the absence or presence of cacospongionolide B (Figure 2) because there was no significant difference in the slopes of the straight lines (Segel, 1975). This type of inhibition has been reported for other marine compounds such us manoalide (Jacobson et al., 1990).
Figure 2.
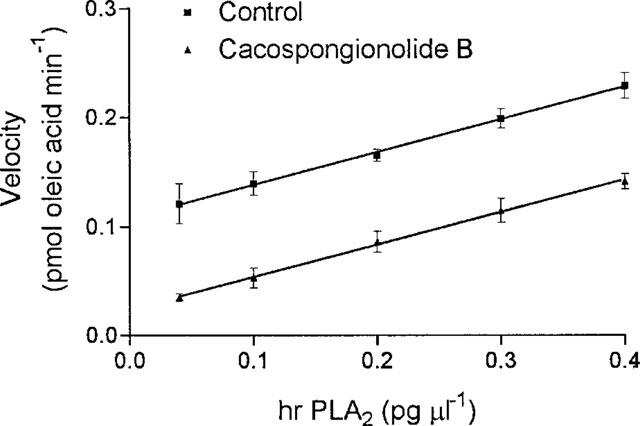
Activity of human synovial sPLA2 as a function of enzyme concentration in the absence or presence of cacospongionolide B. Data are the means±s.e.mean of n=6. Different enzyme concentrations were preincubated with vehicle (control) or cacospongionolide B (1 μM) for 5 min at 37°C, and after addition of substrate, incubation proceeded for 15 min.
Effect on sPLA2 in vivo
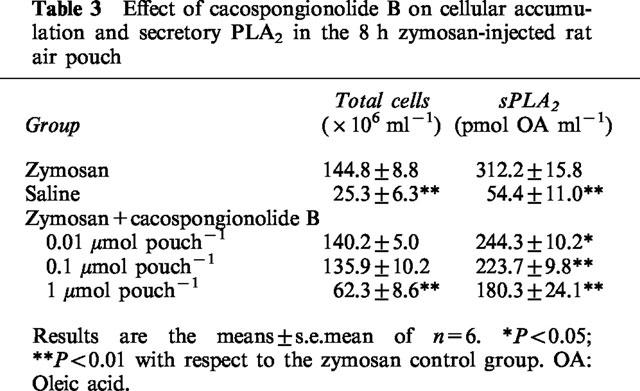
Having established the inhibitory properties of cacospongionolide B on sPLA2 in vitro, we tested the effects of this marine compound on an animal model in which high levels of secretory type II PLA2 is generated (Payá et al., 1996). As shown in Table 3, cacospongionolide B administered into the air pouch dose-dependently inhibited the PLA2 activity present in the 8 h zymosan-injected rat air pouch, whereas the accumulation of leukocytes in the pouch was reduced by CB treatment only at the highest dose tested (1 μmol pouch−1).
Table 3.
Effect of cacospongionolide B on cellular accumulation and secretory PLA2 in the 8 h zymosan-injected rat air pouch
Effect on mouse ear oedema
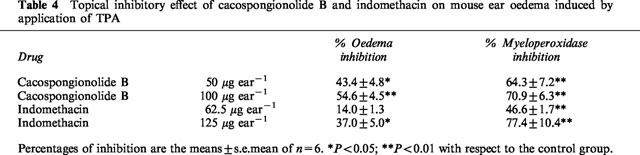
Evaluation of the topical anti-inflammatory activity of cacospongionolide B was performed in the TPA-induced mouse ear oedema. Control animals showed an oedema of 17.8±1.0 mg and myeloperoxidase levels of 0.852±0.104 O.D.620 units 4 h after TPA administration (Table 4). Topical treatment with cacospongionolide B resulted in a dose-dependent inhibition of TPA-induced ear oedema, in addition to a decrease in myeloperoxidase levels measured in ear homogenates. Indomethacin was more effective on myeloperoxidase than on oedema, which was also attenuated, but to a lesser extent than in animals treated with cacospongionolide B.
Table 4.
Topical inhibitory effect of cacospongionolide B and indomethacin on mouse ear oedema induced by application of TPA
Effect on mouse paw oedema
Oral pretreatment (1 h before carrageenin) with 5, 10 or 20 mg kg−1 of cacospongionolide B significantly reduced oedema (Figure 3). This inhibitory effect was observed at the three time points considered, 1, 3 and 5 h after carrageenin, for the doses of 10 and 20 mg kg−1, whereas the dose of 5 mg kg−1 caused significant inhibition at 1 and 3 h. Interestingly, cacospongionolide B was more effective than indomethacin in this model, mainly at 3 h carrageenin administration.
Figure 3.
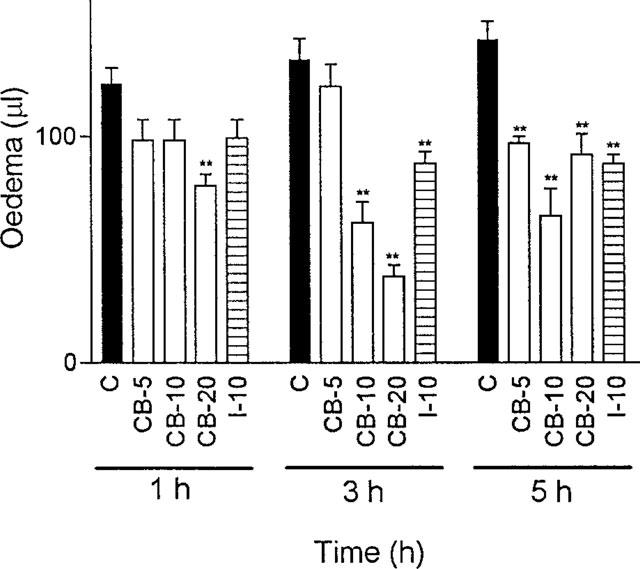
Effect of cacospongionolide B (CB) and indomethacin (I) on mouse paw oedema induced by carrageenin. Data represent means±s.e.mean of n=6. *P<0.05; **P<0.01, significantly different from control (C). Cacospongionolide B (5–20 mg kg−1) and indomethacin (10 mg kg−1) were administered p.o. 1 h before the injection of carrageenin.
Effect on the mouse air pouch
An important increase in leukocyte migration was observed in zymosan-injected animals in comparison with the saline-injected group 4 h after the induction of inflammation. Cacospongionolide B blocked cell accumulation in exudates at the dose of 1 μmol pouch−1, but it was ineffective at lower doses (Figure 4a). This inflammatory response also showed high levels of PGE2, LTB4 and TNFα in the air pouch exudates of control animals injected with zymosan (Figure 4b, c and d). Treatment with cacospongionolide B resulted in a significant decrease in PGE2 levels at a dose as low as 1 nmol pouch−1, whereas LTB4 levels were significantly reduced at higher doses (0.5 and 1 μmol pouch−1). In this model, TNFα levels were very sensitive to cacospongionolide B; 1 nmol pouch−1 of this compound achieved a significant reduction in TNFα and at 1 μmol pouch−1 the levels of this cytokine were abolished. As expected, the 5-LO inhibitor ZM 230,487 (0.1 μmol pouch−1) strongly reduced LTB4 levels (87.3±5.8% of inhibition, n=6, P<0.01) and cell migration (36.7±3.1% of inhibition, n=6, P<0.01), whereas the COX inhibitor indomethacin (0.1 μmol pouch−1) decreased PGE2 levels (89.2±4.8% of inhibition, n=6, P<0.01). Dexamethasone (2 mg kg−1 i.p.) inhibited cell migration (37.8±3.3% of inhibition, n=6, P<0.01) as well as PGE2 and LTB4 (74.8±3.6% and 82.8±4.0 of inhibition, respectively, n=6, P<0.01) and TNFα (75.7±7.1% of inhibition, n=6, P<0.01) levels in exudates. The effects of cacospongionolide B this experimental model were also confirmed after oral administration at a single dose of 20 mg kg−1, which reduced PGE2, LTB4 and TNFα levels (58.6±4.8, 64.7±8.2 and 59.5±7.1% of inhibition, respectively; n=6, P<0.01) to a similar extent, and had a lower effect on cell accumulation in exudates (38.5±4.1% of inhibition, n=6, P<0.01).
Figure 4.
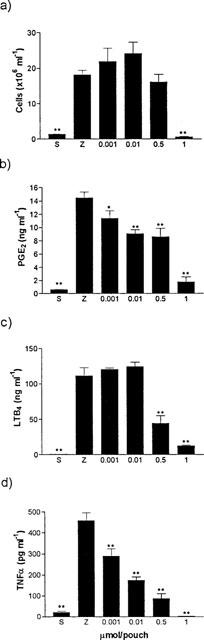
Effect of cacospongionolide B on the mouse air pouch injected with zymosan. Data represent means±s.e.mean of n=6. *P<0.05; **P<0.01 with respect to the zymosan control group. Cacospongionolide B was injected into the air pouch at the same time as zymosan. S=saline, Z=zymosan. (a) Number of cells present in exudates 4 h after zymosan. (b) PGE2 levels in exudates. (c) LTB4 levels in exudates. (d) TNFα levels in exudates.
Effect on adjuvant arthritis
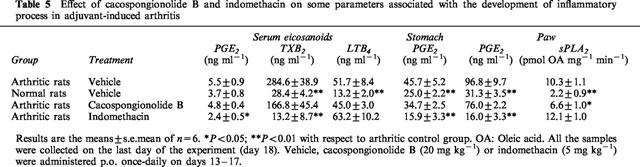
We tested the effects of cacospongionolide B on a model of chronic inflammation, the established adjuvant-induced arthritis, to further characterize its anti-inflammatory properties. Administration of 20 mg kg−1 daily on days 13–17 after adjuvant injection, to animals with developed arthritis, significantly reduced mean paw oedema on the two measures performed, on days 15 and 18 after adjuvant (Figure 5). As shown in Table 5, arthritic animals showed a significant increase in eicosanoid levels in different tissues measured at the end of the experiment (day 18), with respect to the non-arthritic control. Cacospongionolide B did not modify eicosanoid levels in serum, stomach and paw homogenates. Interestingly, an increase in sPLA2 activity was detected in paw homogenates of arthritic rats which was significantly inhibited in animals treated with this marine metabolite. Indomethacin was very effective in oedema reduction, and with it a striking inhibition of prostanoid levels was obtained in serum, stomach and paw homogenates. Nevertheless, the stomachs of the animals treated with this NSAID showed redness and perforations, and suppurative peritonitis was observed in two animals. All these toxic effects were absent in the rats treated with cacospongionolide B.
Figure 5.
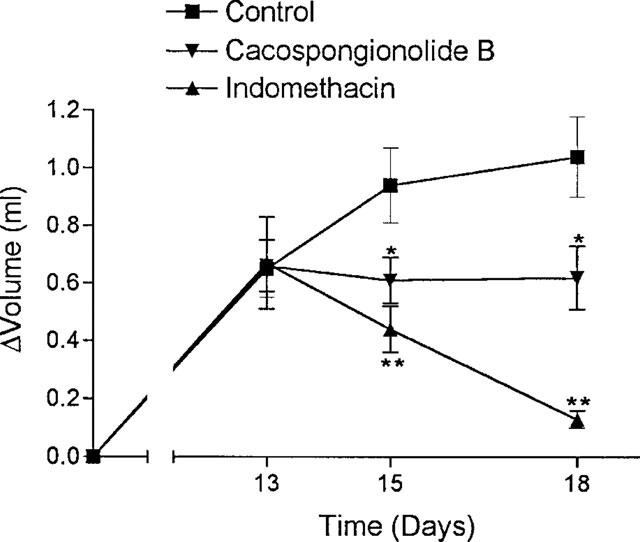
Effect of cacospongionolide B (20 mg kg−1) p.o. and indomethacin (5 mg kg−1) p.o. on the development of adjuvant-induced arthritis in female Lewis rats. Values are the means ±s.e.mean of n=6. *P<0.05; **P<0.01 with respect to the vehicle-treated arthritic rats.
Table 5.
Effect of cacospongionolide B and indomethacin on some parameters associated with the development of inflammatory process in adjuvant-induced arthritis
Discussion
Our results indicate that cacospongionolide B is a new inhibitor of sPLA2 with a strong effect on human synovial PLA2 (group II), and a somewhat milder effect on the pancreatic enzyme (group I) and bee venom PLA2 (group III). Acting in an irreversible way, this marine metabolite showed in vitro a selectivity of inhibition for sPLA2 versus cPLA2, and a potency on the human synovial enzyme similar to that of manoalide. Several reports on the structure-activity relationship of manoalide indicate that the most likely initial reaction between manoalide and PLA2 is the formation of a Schiff base between a lysine residue and the aldehyde generated upon opening of the manoalide γ-lactone ring (Jacobson et al., 1990; Glaser et al., 1989). Manoalide and cacospongionolide B are very close chemically, differing from each other by the hydroxylic substitution in the dihydropyran ring and in the hydrophobic region of the molecule that is cyclic in cacospongionolide B structure. Sharing both compounds a non-specific action on lysine-containing proteins, the in vitro loss of potency observed is lower for cacospongionolide B. Interestingly, we have demonstrated that cacospongionolide B also inhibits group II sPLA2 in vivo, in the 8 h zymosan-injected rat air pouch, a model sensitive to this type of inhibitor (Payá et al., 1996). This effect was not due to inhibition of neutrophil accumulation in the air pouch exudate, which is the main source of this secretory enzyme (Payá et al., 1996), for inhibition of this activity was also observed at doses that did not affect cell migration. The bioavailability by oral route of this compound was also confirmed in the adjuvant-induced arthritis model of chronic inflammation.
A potential protective role of group II sPLA2 inhibitors can be inferred from studies showing that this type of enzymes participates in the inflammatory process, which leads to the generation of mediators and production of tissue injury in different pathological states. High levels of this enzyme are known to be present in synovial fluids, articular cartilage and blood from patients with rheumatic diseases (Pruzanski et al., 1987; Bomalaski & Clark, 1993). In addition, recent studies suggest that group II sPLA2 may play a broad role in tissue injury. Elevated levels of this enzyme, for example, have been associated with a poor clinical outcome in the acute respiratory distress syndrome (Arbibe et al., 1997). This enzyme activity has also been detected in human atherosclerotic plaques and it could increase the atherogenicity of LDL by hydrolyzing the phospholipids present in this lipoprotein (Eckey et al., 1997). A high expression of group II sPLA2 has been demonstrated in colon biopsies from patients of ulcerative colitis (Haapamaki et al., 1997), and its levels are also increased in effusions from cancer patients, associated with a high mRNA expression in carcinoma cells (Abe et al., 1997).
On the other hand, sPLA2 could also play a role in cellular defence against infection, as this enzyme activity is bactericidal against E. coli (Weiss et al., 1994), L. monocytogenes (Weiss et al., 1994; Harwig et al., 1995) and S. aureus (Weinrauch et al., 1996). In addition, it has been suggested that cPLA2, sPLA2 and diacylglycerol/monoacylglycerol lipase participate in arachidonic acid release in rat NK cells, which plays a crucial role in the lytic activity of these cells (Cifone et al., 1997).
Cacospongionolide B can act as a topical anti-inflammatory agent and has also shown oral efficacy. Our data thus demonstrate that a selective inhibitor of sPLA2 is able to decrease the inflammatory reaction in models of acute and chronic inflammation. The production of eicosanoids derived from the COX and 5-LO pathways was reduced by cacospongionolide B in an acute inflammatory response, the mouse air pouch. This effect is probably the consequence of a reduction in substrate availability, since this compound is not an inhibitor of COX and is only a weak inhibitor of 5-LO. This weak effect would explain the slightly higher inhibition of LTB4. In contrast, the fact that in the model of chronic inflammation, cacospongionolide B did not significantly affect the levels of eicosanoids suggests the participation of other pathways in the regulation of arachidonic acid availability. It has been suggested that inhibition of cPLA2 interferes with cellular activation and therefore results in the control of adjuvant arthritis (Amandi-Burgermeister et al., 1997). This model of chronic inflammation is a complex response involving different mediators, and therefore there is a possibility of multiple interactions, e.g. inhibition of iNOS results in the control of the inflammatory features (Connor et al., 1995). Our results indicate that inhibition of sPLA2 can also interfere with this model of chronic inflammation without producing toxic effects.
Interestingly, cacospongionolide B inhibited TNFα levels in the mouse air pouch dose-dependently. This effect is absent in COX and 5-LO inhibitors and is similar to that of dexamethasone. The molecular mechanism of this inhibitory effect remains to be established. Inflammatory cytokines induce the enzymes responsible for arachidonic acid release and metabolism, thus leading to increased levels of eicosanoids, and they are involved in the chronification of the inflammatory response. Eicosanoids, in turn, may regulate in part the synthesis of inflammatory cytokines, although there are differences depending on the cell type and the experimental conditions. In certain cases, LTB4 can increase the generation of IL-1β (Rola-Pleszczynski & Lemaire, 1985), whereas PGE2 inhibits the production of TNFα and IL-1β in human monocytes (Caughey et al., 1997). In contrast, thromboxane A2 (TXA2) facilitates cytokine production in these cells, and the balance between different eicosanoids may determine the resulting effect on cytokines (Caughey et al., 1997).
Of the anti-inflammatory drugs in use, NSAID can not prevent the progression of chronic inflammation. This group is able to affect cytokine synthesis by human monocytes stimulated by TPA at high concentrations only (Jiang et al., 1998), and in addition, the inhibition of PG synthesis can potentiate the expression of TNFα induced by LPS (Bondeson & Sundler, 1996). Glucocorticoids are potent agents for the treatment of chronic inflammatory diseases, although severe side effects limit the long term administration required in chronic disorders. These drugs exert complex effects on inflammation, with inhibition of G protein-dependent activation of cPLA2 activity (Croxtall et al., 1995) and the expression of cPLA2, sPLA2 and COX-2, thus leading to inhibition of eicosanoid biosynthesis. These anti-inflammatory agents also inhibit the biosynthesis of other enzymes, cytokines and adhesion molecules, and act on gene regulation by different mechanisms (Goppelt-Struebe, 1997). The inhibition of cytokine generation by compounds active after oral administration, such us cacospongionolide B, can offer an interesting approach to the search for new anti-inflammatory agents, since this type of drugs can have disease modifying properties (Geiger et al., 1994).
Acknowledgments
This study was supported by grant SAF98-0119, C.I.C.Y T., Spanish Ministerio de Educación y Ciencia. We wish to thank Dr S.J. Foster, (Zeneca Pharmaceuticals, Macclesfield, Cheshire, U.K.) for the kind gift of antibody against LTB4 and ZM230,487, and Dr R.M. Kramer (Lilly Research Laboratories, Indianapolis, U.S.A.) for providing human synovial recombinant PLA2.
Abbreviations
- COX
cyclo-oxygenase
- cPLA2
cytosolic PLA2
- fMLP
N-formyl-methionyl-leucyl-phenylalanine
- iNOS
inducible nitric oxide synthase
- IP3
inositol triphosphate
- IL-1β
interleukin-1β
- LDH
lactate dehydrogenase
- LTB4
leukotriene B4
- LPS
lipopolysaccharide
- 5-LO
5-lipoxygenase
- MAC-1
CD11b/CD18
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NSAID
non-steroidal anti-inflammatory drug
- PTK
palmityl trifluoromethyl ketone
- PBS
phosphate buffered saline
- PLA2
phospholipase A2
- PLC
phospholipase C, PG, prostaglandin
- PGD2
prostaglandin D2
- PGE2
prostaglandin E2
- sPLA2
secretory phospholipase A2
- TPA
12-O-tetradecanoylphorbol acetate
- TXA2
thromboxane A2
- TXB2
thromboxane B2
- TNFα
tumour necrosis factor α
References
- ABE T., SAKAMOTO K., KAMOHARA H., HIRANO Y., KUWAHARA N., OGAWA M. Group II phospholipase A2 is increased in peritoneal and pleural effusions in patients with various types of cancer. Int. J. Cancer. 1997;74:245–250. doi: 10.1002/(sici)1097-0215(19970620)74:3<245::aid-ijc2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- AMANDI-BURGERMEISTER E., TIBES U., KAISER B.M., FRIEBE W.G., SCHEUER W.V. Suppression of cytokine synthesis, integrin expression and chronic inflammation by inhibitors of cytosolic phospholipase A2. Eur. J. Pharmacol. 1997;326:237–250. doi: 10.1016/s0014-2999(97)85419-2. [DOI] [PubMed] [Google Scholar]
- ARBIBE L., VIAL D., ROSINSKI-CHUPIN I., HAVET N., HUERRE M., VARGAFTIG B.B., TOUQUI L. Endotoxin induces expression of type II phospholipase A2 in macrophages during acute lung injury in guinea pigs. Involvement of TNF-α in lipopolysaccharide-induced type II phospholipase A2 synthesis. J. Immunol. 1997;159:391–400. [PubMed] [Google Scholar]
- BALBOA M.A., BALSINDE J., WINSTEAD M.V., TISCHFIELD J.A., DENNIS E.A. Novel group V phospholipase A2 involved in arachidonic acid mobilization in murine P388D1 macrophages. J. Biol. Chem. 1996;271:32381–32384. doi: 10.1074/jbc.271.50.32381. [DOI] [PubMed] [Google Scholar]
- BALSINDE J., BIANCO I.D., ACKERMANN E.J., CONDE-FRIEBOES K., DENNIS E.A. Inhibition of calcium-independent phospholipase A2 prevents arachidonic acid incorporation and phospholipid remodeling in P388D1 macrophages. Proc. Natl. Acad. Sci. U.S.A. 1995;92:8527–8531. doi: 10.1073/pnas.92.18.8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALSINDE J., DENNIS E.A. Distinct roles in signal transduction for each of the phospholipase A2 enzymes present in P388D1 macrophages. J. Biol. Chem. 1996;271:6758–6765. doi: 10.1074/jbc.271.12.6758. [DOI] [PubMed] [Google Scholar]
- BARTOLI F., LIN H.-K., GHOMASHCHI F., GELB M.H., JAIN M.K., APITZ-CASTRO R. Tight binding inhibitors of 85-kDa phospholipase A2 but not 14-kDa phospholipase A2 inhibit release of free arachidonate in thrombin-stimulated human platelets. J. Biol. Chem. 1994;269:15625–15630. [PubMed] [Google Scholar]
- BAULDRY S.A., WOOTEN R.E. Leukotriene B4 and platelet activating factor production in permeabilized human neutrophils: role of cytosolic PLA2 in LTB4 and PAF generation. Biochim. Biophys. Acta. 1996;1303:63–73. doi: 10.1016/0005-2760(96)00077-x. [DOI] [PubMed] [Google Scholar]
- BERGMEYER H.U., BERNT E.Lactate dehydrogenase: UV assay with pyruvate and NADH Methods of enzymatic analysis 1974New York: Academic Press; 2nd Edn. ed. Bergmeyer, H.U. [Google Scholar]
- BOLOGNESE B., MCCORD M., MARSHALL L.A. Differential regulation of elicited-peritoneal macrophage 14 kDa and 85 kDa phospholipase A2(s) by transforming growth factor-β. Biochim. Biophys. Acta. 1995;1256:201–209. doi: 10.1016/0005-2760(95)00023-6. [DOI] [PubMed] [Google Scholar]
- BOMALASKI J.S., CLARK M.A. Phospholipase A2 and arthritis. Arthritis Rheum. 1993;36:190–198. doi: 10.1002/art.1780360208. [DOI] [PubMed] [Google Scholar]
- BONDESON J., SUNDLER R. Differential effects of tenidap on the zymosan- and lipopolysaccharide-induced expression of mRNA for proinflammatory cytokines in macrophages. Biochem. Pharmacol. 1996;52:35–42. doi: 10.1016/0006-2952(96)00136-0. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- BUSTOS G., FERRANDIZ M.L., SANZ M.J., PAYA M., ALCARAZ M.J. A study of the novel anti-inflammatory agent florifenine. Topical anti-inflammatory activity and influence on arachidonic acid metabolism and neutrophil functions. Naunyn-Schmiedeberg's Arch. Pharmacol. 1995;351:298–304. doi: 10.1007/BF00233250. [DOI] [PubMed] [Google Scholar]
- CARLSON R.P., O'NEILL-DAVIS L., CHANG J., LEWIS A.J. Modulation of mouse ear edema by cyclooxygenase and lipoxygenase inhibitors and other pharmacologic agents. Agents Actions. 1985;17:197–204. doi: 10.1007/BF01966592. [DOI] [PubMed] [Google Scholar]
- CAUGHEY G.E., POULIOT M., CLELAND L.G., JAMES M.J. Regulation of tumor necrosis factor-α and IL-1β synthesis by thromboxane A2 in nonadherent human monocytes. J. Immunol. 1997;158:351–358. [PubMed] [Google Scholar]
- CHEN Q.R., MIYAURA C., HIGASHI S., MURAKAMI M., KUDO I., SAITO S., HIRAIDE T., SHIBASAKI Y., SUDA T. Activation of cytosolic phospholipase A2 by platelet-derived growth factor is essential for cyclooxygenase-2-dependent prostaglandin E2 synthesis in mouse osteoblasts cultured with interleukin-1. J. Biol. Chem. 1997;272:5952–5958. doi: 10.1074/jbc.272.9.5952. [DOI] [PubMed] [Google Scholar]
- CIFONE M.G., RONCAIOLI P., CIRONI L., FESTUCCIA C., MECCIA A., D'ALO S., BOTTI D., SANTONI A. NKR-P1A stimulation of arachidonate-generating enzymes in rat NK cells is associated with granule release and cytotoxic activity. J. Immunol. 1997;159:309–317. [PubMed] [Google Scholar]
- CIRINO G., CICALA C., SORRENTINO L., MAIELLO F.M., BROWNING J.L. Recombinant secreted nonpancreatic phospholipase A2 induces a synovitis-like inflammation in the rat air pouch. J. Rheumatol. 1994;21:824–829. [PubMed] [Google Scholar]
- CLARK J.D., MILONA N., KNOPF J.L. Purification of a 110-kilodalton cytosolic phospholipase A2 from the human monocytic cell line U937. Proc. Natl. Acad. Sci. U.S.A. 1990;87:7708–7712. doi: 10.1073/pnas.87.19.7708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONNOR J.R., MANNING P.T., SETTLE S.L., MOORE W.M., JEROME G.M., WEBBER R.K., TJOENG F.S., CURRIE M.G. Suppression of adjuvant-induced arthritis by selective inhibition of inducible nitric oxide synthase. Eur. J. Pharmacol. 1995;273:15–24. doi: 10.1016/0014-2999(94)00672-t. [DOI] [PubMed] [Google Scholar]
- CROXTALL J.D., CHOUDHURY Q., TOKUMOTO H., FLOWER R.J. Lipocortin-1 and the control of arachidonic acid release in cell signalling. Glucocorticoids inhibit G protein-dependent activation of cPLA2 activity. Biochem. Pharmacol. 1995;50:465–474. doi: 10.1016/0006-2952(95)00156-t. [DOI] [PubMed] [Google Scholar]
- DE ROSA S., CRISPINO A., DE GIULIO A., IODICE C., PRONZATO R., ZAVODNIK N. Cacospongionolide B, a new sesterterpene from the sponge Fasciospongia cavernosa. J. Nat. Prod. 1995;58:1776–1780. doi: 10.1021/np50125a024. [DOI] [PubMed] [Google Scholar]
- DE YOUNG L.M., KHEIFETS J.B., BALLARON S.J., YOUNG J.M. Edema and cell infiltration in the phorbol ester-treated mouse ear are temporally separate and can be differentially modulated by pharmacologic agents. Agents Actions. 1989;26:335–341. doi: 10.1007/BF01967298. [DOI] [PubMed] [Google Scholar]
- DENNIS E.A. The growing phospholipase A2 superfamily of signal transduction enzymes. Trends Biochem. Sci. 1997;22:1–2. doi: 10.1016/s0968-0004(96)20031-3. [DOI] [PubMed] [Google Scholar]
- ECKEY R., MENSCHIKOWSKI M., LATTKE P., JAROSS W. Minimal oxidation and storage of low density lipoproteins result in an increased susceptibility to phospholipid hydrolysis by phospholipase A2. Atherosclerosis. 1997;132:165–176. doi: 10.1016/s0021-9150(97)00088-9. [DOI] [PubMed] [Google Scholar]
- EDWARDS J.C.W., SEDGWICK A.D., WILLOUGHBY D.A. The formation of a structure with the features of synovial lining by subcutaneous injection of air: an in vivo tissue culture system. J. Pathol. 1981;134:147–156. doi: 10.1002/path.1711340205. [DOI] [PubMed] [Google Scholar]
- FONTEH A.N., BASS D.A., MARSHALL L.A., SEEDS M., SAMET J.M., CHILTON F.H. Evidence that secretory phospholipase A2 plays a role in arachidonic acid release and eicosanoid biosynthesis by mast cells. J. Immunol. 1994;152:5438–5446. [PubMed] [Google Scholar]
- FRANSON R., PATRIARCA P., ELSBACH P. Phospholipid metabolism by phagocytic cells. Phospholipases A2 associated with rabbit polymorphonuclear leukocyte granules. J. Lipid Res. 1974;15:380–388. [PubMed] [Google Scholar]
- GEIGER T., RORDORF C., COSENTI-VARGAS A., FERRINI P.G., WIDLER L., GLATT M., VOSBECK K. CGP 47969A: Effect on collagen induced arthritis in DBA/1 mice. J. Rheumatol. 1994;21:1992–1997. [PubMed] [Google Scholar]
- GLASER K.B., DE CARVALHO M.S., JACOBS R.S., KERNAN M.R., FAULKNER D.J. Manoalide: structure-activity studies and definition of the pharmacophore for phospholipase A2 inactivation. Mol. Pharmacol. 1989;36:782–788. [PubMed] [Google Scholar]
- GOPPELT-STRUEBE M. Molecular mechanisms involved in the regulation of prostaglandin biosynthesis by glucocorticoids. Biochem. Pharmacol. 1997;53:1389–1395. doi: 10.1016/s0006-2952(97)00018-x. [DOI] [PubMed] [Google Scholar]
- GROSS S.S., LEVI R. Tetrahydrobiopterin synthesis. An absolute requirement for cytokine-induced nitric oxide generation by vascular smooth muscle. J. Biol. Chem. 1992;267:25722–25729. [PubMed] [Google Scholar]
- GROSSMAN C.J., WISEMAN J., LUCAS F.S., TREVETHICK M.A., BIRCH P.J. Inhibition of constitutive and inducible cyclooxygenase activity in human platelets and mononuclear cells by NSAIDS and Cox 2 inhibitors. Inflamm. Res. 1995;44:253–257. doi: 10.1007/BF01782978. [DOI] [PubMed] [Google Scholar]
- HAAPAMAKI M.M., GRONROOS J.M., NURMI H., ALANEN K., KALLAJOKI M., NEVALAINEN T.J. Gene expression of group II phospholipase A2 in intestine in ulcerative colitis. Gut. 1997;40:95–101. doi: 10.1136/gut.40.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARA S., KUDO I., INOUE K. Augmentation of prostaglandin E2 production by mammalian phospholipase A2 added exogenously. J. Biochem. Tokyo. 1991;110:163–165. doi: 10.1093/oxfordjournals.jbchem.a123550. [DOI] [PubMed] [Google Scholar]
- HARWIG S.S.L., TAN L., QU X.-D., CHO Y., EISENHAUER P.B., LEHRER R.I. Bactericidal properties of murine intestinal phospholipase A2. J. Clin. Invest. 1995;95:603–610. doi: 10.1172/JCI117704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HULKOWER K.I., WERTHEIMER S.J., LEVIN W., COFFEY J.W., ANDERSON C.M., CHEN T., DEWITT D.L., CROWL R.M., HOPE W.C., MORGAN D.W. Interleukin-1β induces cytosolic phospholipase A2 and prostaglandin H synthase in rheumatoid synovial fibroblasts. Arthritis Rheum. 1994;37:653–661. doi: 10.1002/art.1780370508. [DOI] [PubMed] [Google Scholar]
- JACOBSON P.B., MARSHALL L.A., SUNG A., JACOBS R.S. Inactivation of human synovial fluid phospholipase A2 by the marine natural product, manoalide. Biochem. Pharmacol. 1990;39:1557–1564. doi: 10.1016/0006-2952(90)90521-l. [DOI] [PubMed] [Google Scholar]
- JACOBSON P.B., SCHRIER D.J. Regulation of CD11b/CD18 expression in human neutrophils by phospholipase A2. J. Immunol. 1993;151:5639–5652. [PubMed] [Google Scholar]
- JACQUES C., BEREZIAT G., HUMBERT L., OLIVIER J.L., CORVOL M.T., MASLIAH J., BERENBAUM F. Posttranscriptional effect of insulin like growth factor i on interleukin 1 beta induced type II secreted phospholipase A2 gene expression in rabbit articular chondrocytes. J. Clin. Invest. 1997;99:1864–1872. doi: 10.1172/JCI119353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIANG C., TING A.T., SEED B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- KNOWLES R.G., MERRETT M., SALTER M., MONCADA S. Differential induction of brain, lung and liver nitric oxide synthase by endotoxin in the rat. Biochem. J. 1990;270:833–836. doi: 10.1042/bj2700833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRAMER R.M., CHECANI G.C., DEYKIN D. Stimulation of Ca2+-activated human platelet phospholipase -A2 by diacylglycerol. Biochem. J. 1987;248:779–783. doi: 10.1042/bj2480779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARSHALL L.A., BOLOGNESE B., ROSHAK A. Characterization of phospholipase A2 release by elicited-peritoneal macrophage and its relationship to eicosanoid production. J. Lipid Mediat. Cell Signal. 1994;10:295–313. [PubMed] [Google Scholar]
- MARSHALL L.A., BOLOGNESE B., WINKLER J.D., ROSHAK A. Depletion of human monocyte 85-kDa phospholipase A2 does not alter leukotriene formation. J. Biol. Chem. 1997;272:759–765. doi: 10.1074/jbc.272.2.759. [DOI] [PubMed] [Google Scholar]
- MITCHELL J.A., SHENG H., FÖRSTERMANN U., MURAD F. Characterization of nitric oxide synthases in non-adrenergic non-cholinergic nerve containing tissue from the rat anococcygeus muscle. Br. J. Pharmacol. 1991;104:289–291. doi: 10.1111/j.1476-5381.1991.tb12422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIYAKE A., YAMAMOTO H., ENOMORI T., KAWASHIMA H. Exogenous group II phospholipase A2 induces prostaglandin E2 production in mouse peritoneal macrophages. Eur. J. Pharmacol. 1994;253:155–161. doi: 10.1016/0014-2999(94)90770-6. [DOI] [PubMed] [Google Scholar]
- MORONEY M.A., ALCARAZ M.J., FORDER R.A., CAREY F., HOULT J.R.S. Selectivity of neutrophil 5-lipoxygenase and cyclo-oxygenase inhibition by an anti-inflammatory flavonoid glycoside and related aglycone flavonoids. J. Pharm. Pharmacol. 1988;40:787–792. doi: 10.1111/j.2042-7158.1988.tb05173.x. [DOI] [PubMed] [Google Scholar]
- MURAKAMI M., KUDO I., INOUE K. Molecular nature of phospholipases A2 involved in prostaglandin I2 synthesis in human umbilical vein endothelial cells. Possible participation of cytosolic and extracellular type II phospholipases A2. J. Biol. Chem. 1993;268:839–844. [PubMed] [Google Scholar]
- NARABA H., MURAKAMI M., MATSUMOTO H., SHIMBARA S., UENO A., KUDO I., OH-ISHI S. Segregated coupling of phospholipase A2, cyclooxygenases, and terminal prostanoid synthases in different phases of prostanoid biosynthesis in rat peritoneal macrophages. J. Immunol. 1998;160:2974–2982. [PubMed] [Google Scholar]
- PALMER S., HUGHES K.T., LEE D.Y., WAKELAM M.J.O. Development of a novel, Ins(1,4,5)P3-specific binding assay. Its use to determine the intracellular concentration of Ins(1,4,5)P3 in unstimulated and vasopressin-stimulated rat hepatocytes. Cell. Signal. 1989;1:147–156. doi: 10.1016/0898-6568(89)90004-1. [DOI] [PubMed] [Google Scholar]
- PAYA M., TERENCIO M.C., FERRANDIZ M.L., ALCARAZ M.J. Involvement of secretory phospholipase A2 activity in the zymosan rat air pouch model of inflammation. Br. J. Pharmacol. 1996;117:1773–1779. doi: 10.1111/j.1476-5381.1996.tb15353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PFEILSCHIFTER J., SCHALKWIJK C., BRINER V.A., VAN DEN BOSCH H. Cytokine-stimulated secretion of group II phospholipase A2 by rat mesangial cells. Its contribution to arachidonic acid release and prostaglandin synthesis by cultured rat glomerular cells. J. Clin. Invest. 1993;92:2516–2523. doi: 10.1172/JCI116860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POTTS B.C.M., FAULKNER D.J., JACOBS R.S. Phospholipase A2 inhibitors from marine organisms. J. Nat. Prod. 1992;55:1701–1717. doi: 10.1021/np50090a001. [DOI] [PubMed] [Google Scholar]
- PRUZANSKI W., KEYSTONE E.C., BOMBARDIER C., SNOW K.M., VADAS P. Phospholipase A2 correlates with disease activity in rheumatoid arthritis. Arthritis Rheum. 1987;30 Suppl.:S-114. [PubMed] [Google Scholar]
- QIU Z.-H., LESLIE C.C. Protein kinase C-dependent and -independent pathways of mitogen-activated protein kinase activation in macrophages by stimuli that activate phospholipase A2. J. Biol. Chem. 1994;269:19480–19487. [PubMed] [Google Scholar]
- RIENDEAU D., GUAY J., WEECH P.K., LALIBERTÉ F., YERGEY J., LI C., DESMARAIS S., PERRIER H., LIU S., NICOLL-GRIFFITH D., STREET I.P. Arachidonyl trifluoromethyl ketone, a potent inhibitor of 85-kDa phospholipase A2, blocks production of arachidonate and 12-hydroxyeicosatetraenoic acid by calcium ionophore-challenged platelets. J. Biol. Chem. 1994;269:15619–15624. [PubMed] [Google Scholar]
- ROLA-PLESZCZYNSKI M., LEMAIRE I. Leukotrienes augment interleukin 1 production by human monocytes. J. Immunol. 1985;135:3958–3961. [PubMed] [Google Scholar]
- SEGEL I.H. Behavior and analysis of rapid equilibrium and steady-state enzyme systems. New York: John Wiley & Sons; 1975. Enzyme kinetics; pp. 127–128. [Google Scholar]
- SERHAN C.N., HAEGGSTRÖM J.Z., LESLIE C.C. Lipid mediator networks in cell signaling: update and impact of cytokines. FASEB J. 1996;10:1147–1158. doi: 10.1096/fasebj.10.10.8751717. [DOI] [PubMed] [Google Scholar]
- SUGISHITA E., AMAGAYA S., OGIHARA Y. Anti-inflammatory testing methods: comparative evaluation of mice and rats. J. Pharmacobio-Dynam. 1981;4:565–575. doi: 10.1248/bpb1978.4.565. [DOI] [PubMed] [Google Scholar]
- TAKASAKI J., KAWAUCHI Y., YASUNAGA T., MASUHO Y. Human type II phospholipase A2 induced mac 1 expression on human neutrophils. J. Leukoc. Biol. 1996;60:174–180. doi: 10.1002/jlb.60.2.174. [DOI] [PubMed] [Google Scholar]
- TANAKA K., MATSUTANI S., MATSUMOTO K., YOSHIDA T. Effect of thielocin A1β on bee venom phospholipase A2-induced edema in mouse paw. Eur. J. Pharmacol. 1995;279:143–148. doi: 10.1016/0014-2999(95)00148-e. [DOI] [PubMed] [Google Scholar]
- TATESON J.E., RANDALL R.W., REYNOLDS C.H., JACKSON W.P., BHATTACHERJEE P., SALMON J.A., GARLAND L.G. Selective inhibition of arachidonate 5-lipoxygenase by novel acetohydroxamic acids: biochemical assessment in vitro and ex vivo. Br. J. Pharmacol. 1988;94:528–539. doi: 10.1111/j.1476-5381.1988.tb11557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAUROG J.D., ARGENTIERI D.C., MCREYNOLDS R.A. Adjuvant arthritis. Methods Enzymol. 1988;162:339–355. doi: 10.1016/0076-6879(88)62089-1. [DOI] [PubMed] [Google Scholar]
- TESLENKO V., ROGERS M., LEFKOWITH J.B. Macrophage arachidonate release via both the cytosolic Ca2+ dependent and independent phospholipases is necessary for cell spreading. Biochim. Biophys. Acta. 1997;1344:189–199. doi: 10.1016/s0005-2760(96)00137-3. [DOI] [PubMed] [Google Scholar]
- VISHWANATH B.S., FAWZY A.A., FRANSON R.C. Edema-inducing activity of phospholipase A2 purified from human synovial fluid and inhibition by aristolochic acid. Inflammation. 1988;12:549–561. doi: 10.1007/BF00914317. [DOI] [PubMed] [Google Scholar]
- WADA A., TOJO H., SUGIURA T., FUJIWARA Y., KAMADA T., UEDA N., OKAMOTO M. Group II phospholipase A2 as an autocrine growth factor mediating interleukin 1 action on mesangial cells. Biochim. Biophys. Acta. 1997;1345:99–108. doi: 10.1016/s0005-2760(96)00158-0. [DOI] [PubMed] [Google Scholar]
- WEINRAUCH Y., ELSBACH P., MADSEN L.M., FOREMAN A., WEISS J. The potent anti-Staphylococcus aureus activity of a sterile rabbit inflammatory fluid is due to a 14-kDa phospholipase A2. J. Clin. Invest. 1996;97:250–257. doi: 10.1172/JCI118399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISS J., INADA M., ELSBACH P., CROWL R.M. Structural determinants of the action against Escherichia coli of a human inflammatory fluid phospholipase A2 in concert with polymorphonuclear leukocytes. J. Biol. Chem. 1994;269:26331–26337. [PubMed] [Google Scholar]
- WIJKANDER J., O'FLAHERTY J.T., NIXON A.B., WYKLE R.L. 5-Lipoxygenase products modulate the activity of the 85-kDa phospholipase A2 in human neutrophils. J. Biol. Chem. 1995;270:26543–26549. doi: 10.1074/jbc.270.44.26543. [DOI] [PubMed] [Google Scholar]
- WU T., LEVINE S.J., COWAN M., LOGUN C., ANGUS C.W., SHELHAMER J.H. Antisense inhibition of 85-kDa cPLA2 blocks arachidonic acid release from airway epithelial cells. Am. J. Physiol. 1997;273:L331–L338. doi: 10.1152/ajplung.1997.273.2.L331. [DOI] [PubMed] [Google Scholar]
- ZHANG Y.Y., DEEMS R.A., DENNIS E.A. Lysophospholipases I and II from P388D1 macrophage-like cell line. Methods Enzymol. 1991;197:456–468. doi: 10.1016/0076-6879(91)97171-t. [DOI] [PubMed] [Google Scholar]


