Abstract
The aim of the present study is to characterize the role of the P2X receptor in spinal nociceptive processing in vivo. We investigated the mechanisms of the P2X receptor agonist α,β-methylene ATP (α,βmeATP)-induced modulation of acute nociceptive signalling in mouse spinal cord.
Intrathecal administration of α,βmeATP produced a significant and dose-dependent thermal hyperalgesic response. This response was completely blocked by intrathecal pretreatment with the non-selective P2 receptor antagonist, pyridoxal-phosphate-6-azophenyl-2′,4′-disulphonate (PPADS) and the selective P2X1, P2X3 and P2X2+3 receptor antagonist, 2′,3′-O-(2,4,6-trinitrophenyl)adenosine 5′-triphosphate (TNP-ATP). Pretreatment with α,βmeATP 15, 30 and 60 min prior to administration of a second dose of α,βmeATP diminished the α,βmeATP-induced thermal hyperalgesia.
A potent agonist for the P2X1 receptor, β,γ-methylene-L-ATP, did not show the hyperalgesic response, indicating that the P2X1 receptor is not involved in the spinal nociceptive pathway.
In fura-2 experiments using mouse dorsal root ganglion (DRG) neurons, α,βmeATP (100 μM) increased intracellular Ca2+ ([Ca2+]i). This was not produced by a second application of α,βmeATP. The same DRG neurons also showed a marked [Ca2+]i increase in response to capsaicin (3 μM).
Intrathecal pretreatment with the Ca2+-dependent exocytosis inhibitor, botulinum neurotoxin B, abolished the thermal hyperalgesia by α,βmeATP. Furthermore, thermal hyperalgesia was significantly inhibited by the N-methyl-D-aspartate (NMDA) receptor antagonists, 2-amino-5-phosphonopentanoate (APV), dizocilpine and ifenprodil.
These findings suggest that α,βmeATP-induced thermal hyperalgesia may be mediated by the spinal P2X3 receptor subtype that causes unresponsiveness by repetitive agonist applications, and that α,βmeATP (perhaps through P2X3 receptors) may evoke spinal glutamate release which, in turn, leads to the generation of thermal hyperalgesia via activation of NMDA receptors.
Keywords: α,βmeATP; P2X3 receptor subtype; thermal hyperalgesia; glutamate; NMDA receptor; mouse spinal cord
Introduction
Extracellular adenosine 5′-triphosphate (ATP) excites many neuronal preparations by activating ATP-gated cation channels called P2X receptors (Burnstock & Wood, 1996; Inoue et al., 1996; Ralevic & Burnstock, 1998). In sensory neurons, it is well known that ATP or its analogue evoke the inward current (Krishtal et al., 1983; 1988a,1998b; Bean, 1990; Lewis et al., 1995; Robertson et al., 1996; Cook et al., 1997; Rae et al., 1998; Ueno et al., 1999), proposing the role of ATP and its receptors in the generation or modulation of pain (Burnstock & Wood, 1996; Inoue et al., 1996; Ralevic & Burnstock, 1998). This idea is strongly supported by the interesting finding that mRNA of the P2X3 receptor subtype, which is one of seven cloned P2X receptor subtypes (P2X1–P2X7) (reviewed in Ralevic & Burnstock, 1998), is selectively expressed in capsaicin-sensitive small-diameter cell bodies of the trigeminal and dorsal root ganglion (DRG) (Chen et al., 1995). Using immunological methods, P2X3 receptors have been found in the nociceptive, but not non-nociceptive, sensory nerve endings and cell bodies (Cook et al., 1997). Recently, in addition to P2X3, the presence of both mRNA and protein of the other P2X receptor subtypes (P2X1,2,4–6) in sensory neurons has been shown (Collo et al., 1996; Vulchanova et al., 1997; Ueno et al., 1999; Xiang et al., 1998). Furthermore, the distribution of some of these P2X receptor subtypes in sensory neurons show different patterns (Ueno et al., 1999; Xiang et al., 1998), suggesting that there are several types of cell which express different P2X receptor subtypes. In fact, recent electrophysiological studies have revealed two types of ATP- and its analogue α,β-methylene ATP (α,βmeATP)-evoked inward currents in sensory neurons (Cook et al., 1997). We have further characterized the profiles of the ATP- and α,βmeATP-activated responses in DRG neurons based on current kinetic and capsaicin-sensitivity: capsaicin-sensitive DRG neurons have a rapidly desensitizing current, and capsaicin-insensitive medium-sized DRG neurons have a slowly desensitizing current (Ueno et al., 1999). Together with previous evidence (Ueno et al., 1998), it has been considered that the activation of the homomeric P2X3 receptor is responsible for a rapidly desensitizing current in capsaicin-sensitive DRG neurons and that the heteromultimeric P2X2+3 receptor is responsible for the slowly desensitizing current in capsaicin-insensitive medium-sized DRG neurons (Ueno et al., 1999). Therefore, these findings suggest the possibility that these P2X receptors may play a role in the signal for nociceptive processing in peripheral and/or central sites such as the spinal cord in vivo.
In vivo evidence of the role of P2X receptors at peripheral sites have been shown previously in several animal models for nociception (Bland-Ward & Humphrey, 1997; Sawynok & Reid, 1997; Dowd et al., 1998) and in humans (Bleehan & Keele, 1977; Bleehan, 1978; Coutts et al., 1981), and have suggested the involvement of P2X receptors on peripheral nerve endings of primary afferent neurons in acute nociceptive behaviour (Burnstock & Wood, 1996). As for the central site, in spite of recent studies concerning the localization of P2X receptors in the spinal cord (Collo et al., 1996; Vulchanova et al., 1997; 1998; LÊ et al., 1998; Llewellyn-Smith & Burnstock, 1998), there has been no further investigation since Driessen et al. (1994) demonstrated that intrathecal injection of α,βmeATP and 2-methylthio ATP decreased tail-flick latency. Thus, the nociceptive pathway of the P2X receptor-mediated pain-modulating effect in the spinal cord is still unknown. It seems worthy to investigate the in vivo mechanisms of P2X receptor-mediated modulation of nociceptive processing in the spinal cord because neuronal function in the spinal cord has been considered to play a pivotal role in pathological pain in the clinic (Coderre et al., 1993).
In the present study, to characterize the functional consequence of P2X receptor activation in the spinal nociceptive pathway in vivo, we studied (1) whether the intrathecal administration of α,βmeATP produces the increase in the thermal nociceptive response (thermal hyperalgesia); (2) which P2X receptor subtypes are involved in the α,βmeATP-induced thermal hyperalgesia and (3) whether α,βmeATP-induced thermal hyperalgesia is mediated by a signalling pathway through the activation of N-methyl-D-aspartate (NMDA) receptors in the spinal cord.
Methods
Animals
Male ddY mice (20–23 g) were obtained from the Shizuoka Laboratory Center (Shizuoka, Japan). The animals were housed at a temperature of 22±1°C with a 12 h light–dark cycle (light on 08 30 to 20 30). Food and water were available ad libitum.
Assessment of nociception
To assess the thermally evoked paw withdrawal response, we measured the paw withdrawal latency to a radiant heat stimulus. Mice were gently held by hand with their right hindpaw positioned in an apparatus (Ugo Basile, Italy) for radiant heat stimulation on the plantar surface. Intensity of heat stimulus was adjusted for paw withdrawal latency of 9–10 s in normal animals. The latency of paw withdrawal response in control mice averaged 9.3 s over the course of the experiments. The thermal hyperalgesia was expressed as change in paw withdrawal latency (latency of paw withdrawal response after α,βmeATP injection minus latency before that). A cut-off time was set at 20 s to avoid injury to the hindpaw.
Intrathecal injection
An intrathecal administration was performed according to the procedure described by Hylden & Wilcox (1980) using a 25-μl Hamilton syringe with 28-gauge needle. The paw withdrawal response was measured 5 min after intrathecal injection of α,βmeATP. Pyridoxal-phosphate-6-azophenyl-2′,4′-disulphonate (PPADS: 1.0–5.0 μg per mouse) and 2′,3′-O-(2,4,6-trinitrophenyl)adenosine 5′-triphosphate (TNP-ATP: 0.25–1.0 μg per mouse) were intrathecally injected 10 min before α,βmeATP injection. Botulinum neurotoxin type B (BoNT/B: 0.1–10 ng per mouse) was intrathecally injected 12 h before α,βmeATP injection. The schedule for treatment with BoNT/B was as reported previously (Pierce & Kalivas, 1997). Mice were injected intrathecally with 2-amino-5-phosphonopentanate (APV: 0.1–1.0 μg per mouse), dizocilpine (0.1–0.4 μg per mouse) or ifenprodil (5–20 μg per mouse) 10 min before α,βmeATP injection.
Measurement of [Ca2+]i in acutely dissociated mouse DRG neuron
Male ddY mice were decapitated under ether anaesthesia and the DRG were removed from the L4–6 segments. The DRG were treated first with 20 unit ml−1 papain (Worshington Biochemical Co., NJ, U.S.A.) dissolved in Tyrode's solution for 10 min at 37°C. Tissue was then treated with 4 mg ml−1 collagenase type II (CLS2; Worshington Biochemical Co.) and 2.5 unit ml−1 Dispace (Calbiochem, CA, U.S.A.) dissolved in Tyrode's solution for 30 min at 37°C. At the end of this treatment, the enzyme solution was removed and the cells were then mechanically dissociated by trituration through a pasteur pipette. Cells were plated on poly-L-lysine (Sigma, MO, U.S.A.)-coated glass coverslips with silicon rubber walls (Flexiperm, W.C. GmbH, Germany). The increase in [Ca2+]i in single cells was measured by the fura-2 technique (Grynkiewicz et al., 1985) with minor modifications (Koizumi et al., 1994). The cells were incubated with 5 μM fura-2 acetoxymethylester (fura-2 AM; Dojindo, Kumamoto, Japan) for 30 min in balanced salt solution (BSS; composition in mM: NaCl 150, KCl 5, CaCl2 1.2, MgCl2 1.2, D-glucose 10 and N-2-hydroxyethylpiperazine-N′-2-ethanesulphonic acid (HEPES) 25; pH 7.4). Then, the cells were washed with BSS and mounted on an inverted fluorescence microscope (TMD-300, Nikon, Tokyo, Japan) equipped with a Xenon-lamp and band-pass filters of 340 nm wavelength and 360 nm wavelength. The emission fluorescence was measured at 510 nm. Image data, recorded by a high-sensitivity silicon intensifier target camera (C-2741-08, Hamamatsu Photonics, Hamamatsu, Japan), were processed by a Ca2+-analysing system (Furusawa Lab. Appliance Co., Kawagoe, Japan). α,βmeATP (100 μM) and capsaicin (3 μM) were applied to the cells for 15 s with each application.
Drugs
α,β-Methylene ATP (α,βmeATP) and β,γ-methylene-L-ATP (β,γ me-L-ATP) (Sigma, MO, U.S.A.) were dissolved in phosphate-buffered saline (PBS; composition in mM: NaCl 137, KCl 2.7, KH2PO4 1.5, NaH2PO4 8.1; pH 7.4). Pyridoxal-phosphate-6-azophenyl-2′,4′-disulphonate tetrasodium (PPADS: RBI, MA, U.S.A.), 2′,3′-O-(2,4,6-trinitrophenyl)adenosine 5′-triphosphate (TNP-ATP: Molecular Probes, OR, U.S.A.), 2-amino-5-phosphonopentanoic acid (APV: RBI, MA, U.S.A.), dizocilpine (RBI, MA, U.S.A.) and botulinum neurotoxin type B (BoNT/B: Calbiochem-Novabiochem, CA, U.S.A.) were dissolved and diluted in saline. Ifenprodil (RBI, MA, U.S.A.) was dissolved in 1% dimethyl sulphoxide (DMSO) in saline.
Statistical analysis
The latency of paw withdrawal responses was evaluated statistically using the Student's t-test or Wilcoxon test.
Results
Intrathecal administration of α,βmeATP (1.0–5.0 μg per mouse) produced a significant and dose-dependent thermal hyperalgesic response (the decrease in the paw withdrawal latency to noxious heat stimulus) (Figure 1a). The thermal hyperalgesic response was relatively short acting: at 5.0 μg per mouse of α,βmeATP, hyperalgesia peaked at 5 min after intrathecal injection of α,βmeATP (P<0.01) and recovered after 15 min (Figure 1b). In Figure 2, antagonistic effects of non-selective and selective antagonists of P2 receptors were examined. The α,βmeATP-induced thermal hyperalgesia was blocked in a dose dependent fashion by intrathecal pretreatment with the non-selective P2 receptor antagonist, PPADS (1.0–5.0 μg per mouse; P<0.01). Furthermore, pretreatment with the selective P2X1, P2X3 and P2X2+3 receptor antagonist, TNP-ATP (Virginio et al., 1998) at doses of 0.25, 0.5 and 1.0 μg per mouse inhibited the α,βmeATP-induced thermal hyperalgesia in a dose-dependent manner (P<0.01). The two largest doses of TNP-ATP completely blocked the α,βmeATP-induced thermal hyperalgesic response, suggesting that this response is mediated by P2X1, P2X3 or P2X2+3 receptors in the spinal cord. Paw withdrawal latency was not changed by each antagonist alone (control: 9.5±0.5, PPADS 5.0 μg per mouse: 9.3±0.5 s, TNP-ATP 1.0 μg per mouse: 9.8±0.5 s). Intrathecal administration of β,γ me-L-ATP (5.0 and 10.0 μg per mouse), which is a potent agonist for P2X1 receptors, did not affect the paw withdrawal latency (Figure 3). Therefore, it is not likely that the P2X1 receptor subtype is involved in the α,βmeATP-induced thermal hyperalgesia. As shown in Figure 4, the thermal hyperalgesic response was significantly reduced by intrathecal pretreatment with α,βmeATP (5.0 μg per mouse). The prior injection of α,βmeATP at 15 and 30 min extended the paw withdrawal latency as compared with non-treatment value, indicating that the P2X receptor involved in thermal hyperalgesic response is inactivated by pretreatment with α,β-meATP. The inactivation by pretreatment with α,βmeATP recovered slowly and lasted for 120 min.
Figure 1.
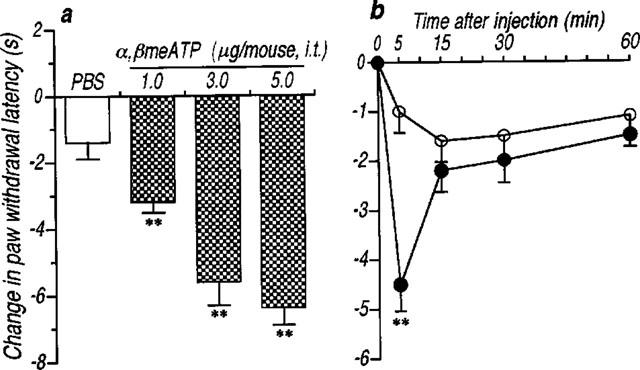
An intrathecal administration of α,βmeATP caused the thermal hyperalgesic response in mice. (a) Dose-response and (b) time-course of the thermal hyperalgesic response by intrathecal α,βmeATP injection. (a) Paw withdrawal response was measured 5 min after intrathecal injection of α,βmeATP (1.0–5.0 μg per mouse; dotted columns) or PBS (5 μl; open column). (b) Paw withdrawal response was measured 0, 5, 15, 30 and 60 min after intrathecal injection of α,βmeATP (5.0 μg per mouse; closed circle) or PBS (5 μl; open circle). Ordinate: change in paw withdrawal latency (s; latency of paw withdrawal response after α,βmeATP or PBS injection minus latency before that). Each point and column represent the mean±s.e.mean of 10 mice. **P<0.01 vs PBS-treated group.
Figure 2.
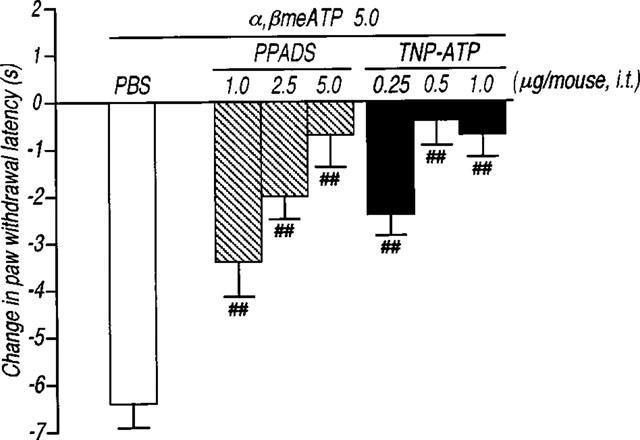
Effect of the antagonists for the non-selective P2 receptor PPADS and the selective P2X1, P2X3 and P2X2+3 receptor TNP-ATP on the thermal hyperalgesic response by intrathecal injection of α,βmeATP in mice. Mice were injected intrathecal with PPADS (1.0–5.0 μg per mouse; hatched columns) or TNP-ATP (0.25–1.0 μg per mouse; closed columns) 10 min prior to intrathecal injection of α,βmeATP (5.0 μg per mouse; open column). Paw withdrawal response was measured 5 min after injection with α,βmeATP. Ordinate: change in paw withdrawal latency(s). Each column represents the mean±s.e.mean of 7–11 mice. ##P<0.01 vs α,βmeATP (5.0 μg per mouse)-injected control group.
Figure 3.
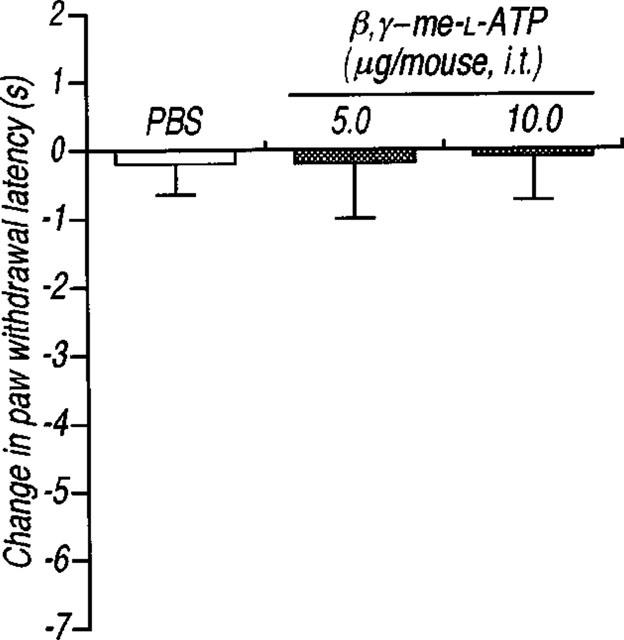
Effect of the intrathecal injection of β,γme-L-ATP on the paw withdrawal latency in mice. The paw withdrawal response was measured 5 min after intrathecal injection of β,γ me-L-ATP (5.0 and 10.0 μg per mouse; dotted columns) or PBS (5 μl; open column). Ordinate: change in paw withdrawal latency(s). Each column represents the mean±s.e.mean of 5–7 mice.
Figure 4.
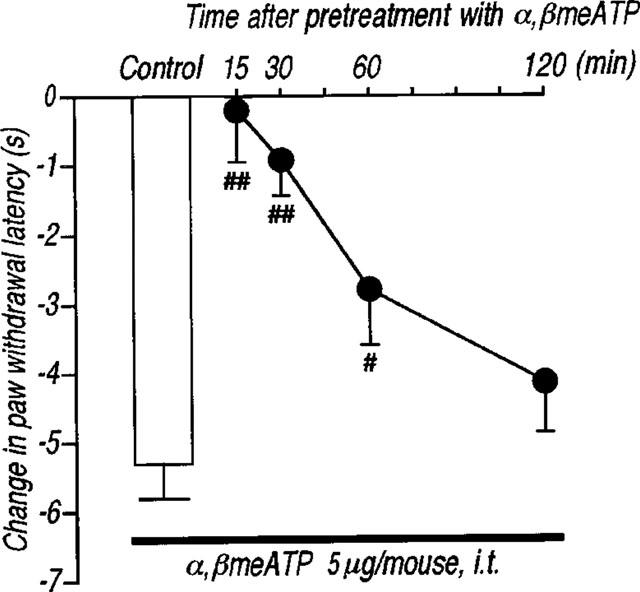
Effect of the intrathecal pretreatment with α,βmeATP on the thermal hyperalgesic response by intrathecal injection of α,βmeATP in mice. Mice were injected intrathecal with α,βmeATP (5.0 μg per mouse) 15, 30, 60 and 120 min prior to intrathecal injection of α,βmeATP (5.0 μg per mouse) (closed circles). Paw withdrawal response was measured 5 min after injection with α,βmeATP. Ordinate: change in paw withdrawal latency(s). Each column represents the mean±s.e.mean of 8–12 mice. #P<0.05, ##P<0.01 vs α,βmeATP (5.0 μg per mouse)-injected control group.
Since ATP and related compounds have been previously shown to stimulate P2X receptors located on sensory neurons, causing intense pain, the present study explored the possibility that α,βmeATP-induced thermal hyperalgesia is produced by induction of neurotransmitter release in the spinal cord. First, we examined whether α,βmeATP could increase the Ca2+ influx that is necessary to evoke the neurotransmitter release. Using the fura-2 technique, α,βmeATP (100 μM) induced a transient [Ca2+]i increase in acutely dissociated mouse DRG neurons (Figure 5). The [Ca2+]i increase by α,βmeATP was not observed following a second application of α,βmeATP (100 μM). Treatment with capsaicin (3 μM) also caused an increase in [Ca2+]i in this neuron, indicating that activation of the P2X receptors located on capsaicin-sensitive DRG neurons triggers the increase in the intracellular Ca2+ concentration. Second, we examined the involvement of neurotransmitters release in α,βmeATP-induced thermal hyperalgesia using mice which had been injected with BoNT/B which inhibits the Ca2+-dependent exocytosis of neurotransmitters. As shown in Figure 6, the α,βmeATP-induced thermal hyperalgesia was markedly inhibited by pretreatment with BoNT/B [0.1 (P<0.05), 0.5, 1, 5 and 10 ng per mouse (P<0.01)] in a dose-dependent manner. Pretreatment with BoNT/B alone (control: 10.7±0.7 s, BoNT/B 10 ng per mouse: 12.0±0.4 s), caused a small increase in paw withdrawal latency but this was not statistically significant. These results suggest that the evoked release of certain neurotransmitters by intrathecal injection of α,βmeATP may be involved in α,βmeATP-induced thermal hyperalgesia. Thus, we investigated the role of NMDA receptors in the spinal cord. The α,βmeATP-induced thermal hyperalgesia was abolished by pretreatment with the competitive NMDA receptor antagonist APV (0.1–1.0 μg per mouse: P<0.01) (Figure 7). Furthermore, the non-competitive NMDA receptor antagonists dizocilpine and ifenprodil significantly inhibited the thermal hyperalgesic response (dizocilpine 0.1–0.4 μg per mouse: P<0.01, ifenprodil 20 μg per mouse: P<0.01), indicating the involvement of spinal NMDA receptors. In contrast to the effect of APV and dizocilpine which completely inhibited the α,βmeATP-induced thermal hyperalgesia, the inhibitory effect of ifenprodil was weaker than that of APV and dizocilpine. In addition, the latency of paw withdrawal response was not changed by each NMDA receptor antagonist alone [control: 9.3±0.6 s (n=8), APV 1.0 μg per mouse: 10.5±0.5 s (n=10), dizocilpine 0.4 μg per mouse: 10.5±0.6 s (n=10), ifenprodil 20 μg per mouse: 9.6±0.7 s (n=11)].
Figure 5.
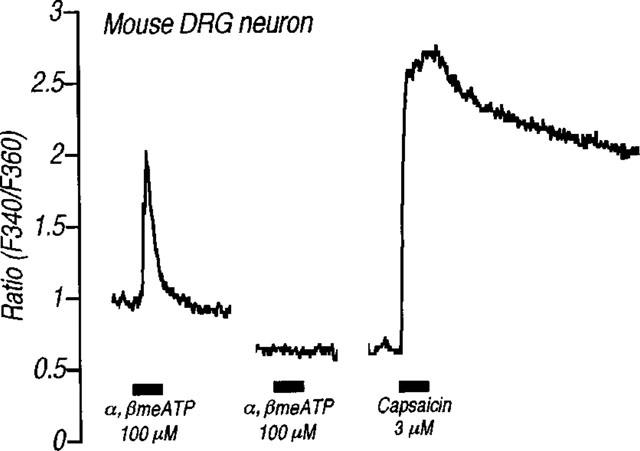
Effects of α,βmeATP on [Ca2+] in acutely dissociated DRG neuron from adult mouse. Horizontal solid bars show the applications of α,βmeATP (100 μM) and capsaicin (3 μM) for 15 s. A second application of α,βmeATP (100 μM) was applied 5 min after the first application.
Figure 6.
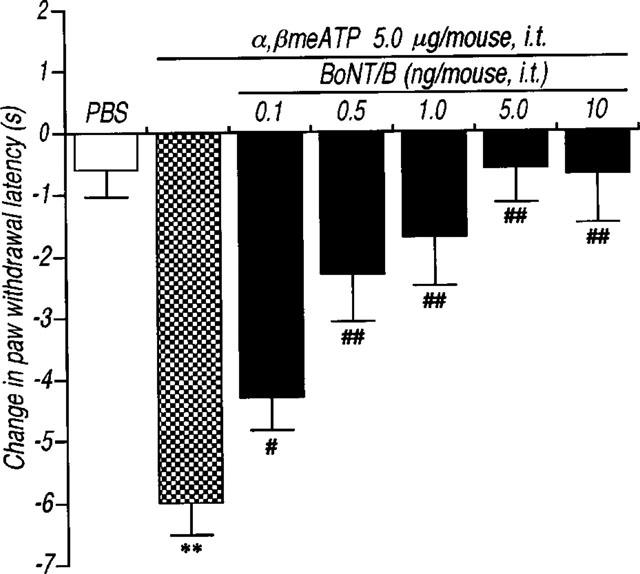
Effects of pretreatment with botulinum neurotoxin type B (BoNT/B) on the thermal hyperalgesic response by intrathecal injection of α,βmeATP in mice. Mice were injected intrathecal with BoNT/B (0.1–10.0 ng per mouse; closed columns) 12 h prior to intrathecal injection of α,βmeATP (5.0 μg per mouse). Paw withdrawal response was measured 5 min after the injection of α,βmeATP or PBS. Ordinate: change in paw withdrawal latency(s). Each column represents the mean±s.e.mean of 7–12 mice. **P<0.01 vs PBS-injected group; #P<0.05, ##P<0.01 vs α,βmeATP (5.0 μg per mouse)-injected control group.
Figure 7.
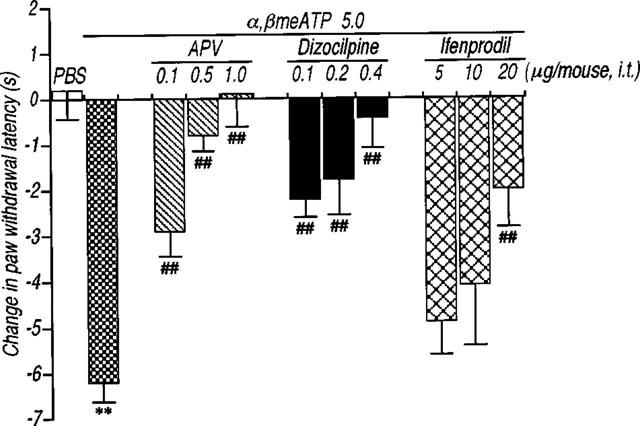
Effects of the pretreatment with NMDA receptor antagonists on the thermal hyperalgesic response by intrathecal injection of α,βmeATP in mice. Mice were injected intrathecal with APV (0.1–1.0 μg per mouse: hatched columns), dizocilpine (0.1–0.4 μg per mouse; closed columns) and ifenprodil (5–20 μg per mouse; cross hatched columns) 10 min prior to the intrathecal injection of α,βmeATP (5.0 μg per mouse). Paw withdrawal response was measured 5 min after the injection of α,βmeATP or PBS. Ordinate: change in paw withdrawal latency(s). Each column represents the mean±s.e.mean of 6–18 mice. **P<0.01 vs PBS-injected group; ##P<0.01 vs α,βmeATP (5.0 μg per mouse)-injected control group.
Discussion
Recent in vitro studies have provided evidence that strongly supports the proposal that P2X receptors could play a role in the signal for nociceptive processing in the spinal cord (see Introduction). However, the in vivo mechanisms of P2X-mediated modulation of nociceptive processing in the spinal cord are unclear. The present study first demonstrated that intrathecal administration of α,βmeATP significantly and dose-dependently evokes thermal hyperalgesia in mice. This is consistent with a previous finding that intrathecal injection of α,βmeATP produces hyperalgesia using the tail-flick test in rats (Driessen et al., 1994), confirming the presence of a pain-modulating effect by α,βmeATP in the spinal cord. The α,βmeATP-induced thermal hyperalgesic response appears to be mediated by the activation of P2X receptors because the thermal hyperalgesic response was completely blocked by the non-selective P2 receptor antagonist PPADS and by the selective P2X1, P2X3, and P2X2+3 receptor antagonist TNP-ATP (Figure 2). Furthermore, intrathecal injection of β,γme-L-ATP, which is a potent agonist for P2X1 receptors (Evans et al., 1995; Trezise et al., 1995; Chen et al., 1995), failed to produce a thermal hyperalgesic response (Figure 3). These data indicate that the P2X3 and/or P2X2+3 receptor subtypes in the spinal cord may be involved in the signalling system of nociception. In agreement, our previous electrophysiological study has shown that the kinetics of α,βmeATP-evoked inward current in rat DRG neurons (Ueno et al., 1998b) are not the same as those in cells transfected with P2X1 receptors (Werner et al., 1996; Parker, 1998). Furthermore, ATP and β,γme-D-ATP (which is a potent agonist of P2X1 and P2X3 receptors) evoke a concentration-dependent inward current in rat DRG neurons while β,γme-L-ATP is less active (Rae et al., 1998). Therefore, it is concluded that the α,βmeATP-induced thermal hyperalgesia is mediated by the activation of P2X3 or P2X2+3 receptor subtypes in the spinal cord.
Under the present experimental conditions, determination of the involvement of P2X receptors (P2X3 or P2X2+3) in α,βmeATP-induced thermal hyperalgesia is difficult, however, we have presumed the importance of the P2X3 receptor subtype in the thermal hyperalgesia by α,βmeATP from the following observations. In capsaicin-sensitive small-sized DRG neurons that have been shown to be nociceptors (Simone et al., 1989; Holzer, 1991; Szallasi & Blumberg, 1996), α,βmeATP evokes a rapidly desensitizing inward current which is dramatically decreased by a second application of the compound (Ueno et al., 1999). This electrophysiological data appears to be similar to the present behavioural profile, in that α,βmeATP-induced thermal hyperalgesia was short-lived and produced a lesser response in mice that had previously received α,βmeATP intrathecally. The α,βmeATP-activated response in capsaicin-sensitive DRG neurons has been thought to be mediated by P2X3 receptors (Ueno et al., 1999). In fact, the P2X3 receptor subtype is observed in capsaicin-sensitive small-sized DRG neurons (Chen et al., 1995; Ueno et al., 1999; Vulchanova et al., 1998), and is found in greater numbers than other P2X receptor subtypes (Llewellyn-Smith & Burnstock, 1998). In contrast, capsaicin-insensitive, medium-sized DRG neurons possess the α,β-meATP-evoked slowly desensitizing current that retains its responsiveness after first application, and this type of current has been thought to be mediated via heteromeric P2X2+3 receptors (Ueno et al., 1999). The specific localization of the P2X3 receptor subtype in small-sized DRG neurons is of particular interest in the light of the demonstration that small-, but not large-, diameter DRG neurons evoke the inward current by application of noxious heat stimulation (Reiching & Levine, 1997). The activation of small DRG neurons by noxious heat has been proposed to mediate the heat-evoked nociceptive response in vivo (Cesara & McNaughton, 1996; Caterina et al., 1997; Kirschstein et al., 1997; Reichling & Levine, 1997; Tominaga et al., 1998). On the basis of these observations, it is reasonable to suggest that the thermal hyperalgesia by intrathecal injection of α,βmeATP may be associated with the spinal P2X3 receptor activation-mediated enhancement of the signalling of noxious heat stimulation in heat-sensitive small-diameter DRG neurons, although the possible involvement of other P2X subtypes can not be completely excluded. This possibility will be clarified by the development of new selective compounds for the P2X receptor subtypes.
Immunohistochemical studies have revealed that P2X3 receptors in the spinal cord are localized in the central presynaptic terminal sites of primary afferent neurons, but not in the cell bodies of superficial dorsal horn neurons (Cook et al., 1997; Vulchanova et al., 1997; 1998; Llewellyn-Smith & Burnstock, 1998). This evidence would suggest that activation of P2X3 receptors leads to the release of certain neurotransmitters in the spinal cord, and that these released neurotransmitters play an important role in α,β-meATP-induced thermal hyperalgesia. We have undertaken three experiments to clarify this possibility. Since Ca2+ has been shown to be important in the release of neurotransmitters, we first examined the ability of α,βmeATP to increase intracellular Ca2+ concentration in adult mouse DRG neuron using the fura-2 method. In the present study, α,βmeATP produced an increase in the [Ca2+]i in acutely dissociated adult mouse DRG neuron. This is in agreement with previous findings in neurons cultured from neonatal rat DRG (Bouvier et al., 1991), confirming that α,βmeATP causes the Ca2+ influx into DRG neurons. Furthermore, this α,βmeATP-induced increase [Ca2+]i was not produced by a second application of α,βmeATP and in addition, capsaicin treatment dramatically increased the [Ca2+]i. These findings lead to the suggestion that activation of P2X (perhaps P2X3) receptors located in the central terminal of capsaicin-sensitive DRG neurons may trigger the Ca2+ influx. In the second experiment we used BoNT/B that specially cleaves the synaptic vesicle protein synaptobrevin. This cleavage event has been proposed to be part of the fusion machinery involved in the fusion between synaptic vesicles and the presynaptic plasma membrane (Schiavo et al., 1992; Poulain et al., 1993; Burgoyne & Morgan, 1995; Almeida et al., 1997), which inhibits Ca2+-dependent neurotransmitter release both in vitro (Schiavo et al., 1992; McMahon et al., 1992; Poulain et al., 1993) and in vivo (Pierce & Kalivas, 1997). We found in the present behavioural study that intrathecal pretreatment with BoNT/B caused a dose-dependent blockade of the α,βmeATP-induced thermal hyperalgesia. This behavioural finding using BoNT/B strongly supports our theory and suggests that the Ca2+-dependent exocytosis of synaptic vesicular storing certain neurotransmitters would be involved in the generation of thermal hyperalgesic response by α,βmeATP. For several neurotransmitters that have their release prevented by BoNT/B (Schiavo et al., 1992; McMahon et al., 1992; Poulain et al., 1993; Pierce & Kalivas, 1997), we presumed that one potential candidate involved in thermal hyperalgesia is glutamate. Glutamate is a major excitatory neurotransmitter in the central nervous system including the spinal cord and is located in the presynaptic termini of the spinal cord (De Biasi & Rustioni, 1988). The release of glutamate from these termini results in hyperalgesia via activation of NMDA receptors (Aanonsen & Wilcox, 1987; Coderre & Melzack, 1992; Malmberg & Yaksh, 1995; Ren et al., 1992; Dickenson et al., 1997). In the third experiment, we determined whether several NMDA receptor antagonists inhibit the α,βmeATP-induced thermal hyperalgesia. The competitive antagonist APV and the non-competitive antagonists dizocilpine and ifenprodil blocked the α,βmeATP-induced thermal hyperalgesia, indica-ting the involvement of spinal NMDA receptors in the α,βmeATP-induced thermal hyperalgesia. However, the inhibitory effect of ifenprodil on α,βmeATP-induced thermal hyperalgesia was weaker than that of APV and dizocilpine. Ifenprodil is known to be a selective antagonist for NMDAR2B (NR2B) subunit-containing NMDA receptors (Williams, 1993; Molinoff et al., 1994). In spinal lamina I–III, antibodies for NR2A and NR2B subunits stain with fair intensity (Petralia et al., 1996). Thus, the inability of the complete blockade of the hyperalgesic response by ifenprodil is likely to be due to the residual action via NR2A-containing NMDA receptors in the spinal cord. The putative release of glutamate by α,βmeATP in the present study is strongly supported by the study of Gu & MacDermott that shows that ATP evokes the glutamate release from cultured sensory neuron synapses via the activation of P2X receptor that are localized at presynaptic termini of DRG neurons (Gu & MacDermott, 1997). Similar to the presynaptic localization of P2X3 receptors (Llewellyn-Smith & Burnstock, 1998), immunoreactivity for glutamate is also observed in many dark scalloped terminal in substantial gelatinosa (De Biasi & Rustioni, 1988). Furthermore, glutamate is released by capsaicin from capsaicin-sensitive primary afferent termini (Ueda et al., 1993) that are known to express P2X3 receptors (Vulchanova et al., 1998), and this glutamate release by capsaicin has been considered to play a role in capsaicin-evoked nociception in vivo (Sakurada et al., 1998). Moreover, P2X3 receptors are presynaptically localized in the inner portion of lamina II in the dorsal horn of the spinal cord (Vulchanova et al, 1998), and interestingly, dense immuno-reactivity of NR1, the essential subunit for the NMDA receptor, is also found particularly in the inner lamina II (Liu et al., 1994). Although there is no direct evidence concerning the localization of either P2X3 receptors, glutamate or NMDA receptors using immunohistochemical studies, the close relationship of P2X3 receptors with the glutamate-NMDA receptor pathway may be considered. Besides glutamate, sensory neurons contain neuropeptides such as substance P and somatostatin that play a pivotal role in the signal transduction of pain. However, recent studies have shown the very limited localization of substance P and somatostatin in P2X3-immunoreactive positive termini in the dorsal horn (Vulchanova et al, 1997; 1998). From the present findings in vivo together with previous evidence, it is, therefore, suggested that the glutamate-NMDA receptor pathway in the spinal cord may be involved mainly in the generation of α,βmeATP-induced thermal hyperalgesia via the P2X3 receptor subtype.
In summary, the present study using a behavioural approach has shown that thermal hyperalgesia by the intrathecal injection of α,βmeATP may be mediated by activation of the P2X3 receptor subtype in the spinal cord, and that intrathecal injection of α,βmeATP may evoke spinal glutamate release which, in turn, leads to the generation of thermal hyperalgesia via NMDA receptors. These findings provide the first in vivo evidence for the involvement of glutamate-NMDA receptors pathway in the P2X receptors-mediated modulation of acute nociceptive signalling in the spinal cord. In addition to the peripheral site, this pain-modulating pathway, mediated by P2X receptors in the spinal cord, may lead to the discovery of a new class of compounds that suppress pain and help to elucidate the role of ATP and its receptors in pathological pain.
Abbreviations
- α,βmeATP
α,β-methylene ATP
- APV
2-amino-5-phosphonopentanoate
- β,γme-L-ATP
β,γ-methylene-L-ATP
- BoTNT/B
botulinum neurotoxin B
- DRG
dorsal root ganglia
- NMDA
N-methyl-D-aspartate
- PBS
phosphate-buffered saline
- PPADS
pyridoxal-phosphate-6-azophenyl-2′,4′-disulphonate
- TNP-ATP
2′,3′-O-(2,4,6-trinitrophenyl)adenosine 5′-triphosphate
References
- AANONSEN L.M., WILCOX G.L. Nociceptive action of excitatory amino acids in the mouse: effects of spinally administered opioids, phencylidine and sigma agonists. J. Pharmacol. Exp. Ther. 1987;243:9–19. [PubMed] [Google Scholar]
- ALMEIDA M.T., RAMALHO-SANTOS J., OLIVEIRA C.R., PEDROSO DE LIMA M.C. Evidence that synaptobrevin is involved in fusion between synaptic vesicle and synaptic plasma membrane vesicle. Biochem. Biophys. Res. Comm. 1997;236:184–188. doi: 10.1006/bbrc.1997.6928. [DOI] [PubMed] [Google Scholar]
- BEAN B.P. ATP-activated channels in rat and bullfrog sensory neurons: concentration dependence and kinetics. J. Neurosci. 1990;10:1–10. doi: 10.1523/JNEUROSCI.10-01-00001.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLAND-WARD P.A., HUMPHREY P.P.A. Acute nociception mediated by hindpaw P2X receptor activation in the rat. Br. J. Pharmacol. 1997;122:365–371. doi: 10.1038/sj.bjp.0701371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLEEHAN T. The effects of adenine nucleotides on cutaneous afferent nerve activity. Br. J. Pharmacol. 1978;62:573–577. doi: 10.1111/j.1476-5381.1978.tb07764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLEEHAN T., KEELE C.A. Observations on the algogenic actions of adenosine compounds on the human blister base preparation. Pain. 1977;3:267–277. doi: 10.1016/0304-3959(77)90066-5. [DOI] [PubMed] [Google Scholar]
- BOUVIER M.M., EVANS M.L., BENHAM C.D. Calcium influx induced by stimulation of ATP receptors on neurons cultured from rat dorsal root ganglia. Eur. J. Neurosci. 1991;3:285–291. doi: 10.1111/j.1460-9568.1991.tb00090.x. [DOI] [PubMed] [Google Scholar]
- BURGOYNE R.D., MORGAN A. Ca2+ and secretory-vesicle dynamics. Trends Neurosci. 1995;18:191–196. doi: 10.1016/0166-2236(95)93900-i. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G., WOOD J.N. Purinergic receptors: their role in nociception and primary afferent neurotransmission. Curr. Opin. Neurobiol. 1996;6:526–532. doi: 10.1016/s0959-4388(96)80060-2. [DOI] [PubMed] [Google Scholar]
- CATERINA M.J., SCHUMACHER M.A., TOMINAGA M., ROSEN T.A., LEVINE J.D., JULIUS D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- CESARE P., MCNAUGHTON P. Novel heat-activated current in nociceptive neurons and its sensitization by bradykinin. Proc. Natl. Acad. Sci. U.S.A. 1996;93:15435–15439. doi: 10.1073/pnas.93.26.15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN C.C., AKOPIAN A.N., SIVILOTTI L., COLQUUHOUN D., BURNSTOCK G., WOOD J.N. A P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995;377:428–431. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- CODERRE T.J., KATZ J., VACCARINO A.L., MELZACK R. Contribution of central neuroplasticity to pathological pain: review of clinical and experimental evidence. Pain. 1993;52:259–285. doi: 10.1016/0304-3959(93)90161-H. [DOI] [PubMed] [Google Scholar]
- CODERRE T.J., MELZACK R. The contribution of excitatory amino acids to central sensitization and persistent nociception after formalin-induced tissue injury. J. Neurosci. 1992;12:3665–3670. doi: 10.1523/JNEUROSCI.12-09-03665.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLLO G., NORTH R.A., KAWASHIMA E., MERLO-PICH E., NEIDHART S., SURPRENANT A., BUELL G. Cloning of P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J. Neurosci. 1996;16:2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOK S.P., VULCHANOVA L., HARGREAVES K.M., ELDE R., MCCLESKEY E.W. Distinct ATP receptors on pain-sensing and stretch-sensing neurons. Nature. 1997;387:505–508. doi: 10.1038/387505a0. [DOI] [PubMed] [Google Scholar]
- COUTTS A.A., JORIZZO J.L., EADY R.J.A., GREAVES M.W., BURNSTOCK G. Adenosine triphosphate-evoked vascular changes ion human skin: mechanism of action. Eur. J. Pharmacol. 1981;76:391–401. doi: 10.1016/0014-2999(81)90110-2. [DOI] [PubMed] [Google Scholar]
- DE BIASI S., RUSTIONI A. Glutamate and substance P coexist in primary afferent terminals in the superficial laminae of spinal cord. Proc. Natl. Acad. Sci. U.S.A. 1988;85:7820–7824. doi: 10.1073/pnas.85.20.7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DICKENSON A.H., CHAPMAN V., GREEN G.M. The pharmacology of excitatory and inhibitory amino acid-mediated events in the transmission and modulation of pain in the spinal cord. Gen. Pharmacol. 1997;28:633–638. doi: 10.1016/s0306-3623(96)00359-x. [DOI] [PubMed] [Google Scholar]
- DOWD E., MCQUEEN D.S., CHESSELL I.P., HUMPHREY P.P.A. P2X receptor-mediated excitation of nociceptive afferents in the normal and arthritic rat knee joint. Br. J. Pharmacol. 1998;125:341–346. doi: 10.1038/sj.bjp.0702080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRIESSEN B., REIMANN W., SELVE N., FRIDERICHS E., BÜLTMANN R. Antinociceptive effect of intrathecally administered P2-purinoceptor antagonist in rats. Brain Res. 1994;666:182–188. doi: 10.1016/0006-8993(94)90770-6. [DOI] [PubMed] [Google Scholar]
- EVANS R.J., LEWIS C., BUELL G., VALERA S., NORTH R.A., SURPRENANT A. Pharmacological characterization of heterologously expressed ATP-gated cation channels (P2X purinoceptors) Mol. Pharmacol. 1995;48:178–183. [PubMed] [Google Scholar]
- GRYNKIEWICZ G., POENIE M., TSIEN R. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- GU J.G., MACDERMOTT A.B. Activation of ATP P2X receptors elicits glutamate release from sensory neuron synapses. Nature. 1997;389:749–753. doi: 10.1038/39639. [DOI] [PubMed] [Google Scholar]
- HOLZER P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol. Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- HYLDEN J.L.K., WILCOX G.L. Intrathecal morphine in mice, A new technique. Eur. J. Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- INOUE K., KOIZUMI S., UENO S. Implication of ATP receptors in brain functions. Prog. Neurobiol. 1996;50:483–492. doi: 10.1016/s0301-0082(96)00037-8. [DOI] [PubMed] [Google Scholar]
- KIRSCHSTEIN T., BUSSELBERG D., TREEDE R.-D. Coexpression of heat-evoked and capsaicin-evoked inward currents in acutely dissociated rat dorsal root ganglion neurons. Neurosci. Lett. 1997;231:33–36. doi: 10.1016/s0304-3940(97)00533-8. [DOI] [PubMed] [Google Scholar]
- KOIZUMI S., WATANO T., NAKAZAWA K., INOUE K. Potentiation by adenosine of ATP-evoked dopamine release via a pertussis toxin-sensitive mechanism in rat pheochromocytoma. Br. J. Pharmacol. 1994;112:992–997. doi: 10.1111/j.1476-5381.1994.tb13179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRISHTAL O.A., MARCHENKO S.M., OBYKHOV A.G. Cationic channels activated by extracellular ATP in rat sensory neurons. Neuroscience. 1988a;27:995–1000. doi: 10.1016/0306-4522(88)90203-5. [DOI] [PubMed] [Google Scholar]
- KRISHTAL O.A., MARCHENKO S.M., OBYKHOV A.G., VOLTOVA T.M. Receptors for ATP in rat sensory neurons: The structure function relationship for ligands. Br. J. Pharmacol. 1988b;95:1057–1062. doi: 10.1111/j.1476-5381.1988.tb11739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRISHTAL O.A., MARCHENKO S.M., PIDOPLICHKO V.I. Receptor for ATP in the membrane of mammalian sensory neurons. Neurosci. Lett. 1983;35:41–45. doi: 10.1016/0304-3940(83)90524-4. [DOI] [PubMed] [Google Scholar]
- LÊ K.-T., VILLENEUVE P., RAMJAUN A.R., MCPHERSON P.S., BEAUDET A., SÉGUÈLA P. Sensory presynaptic and widespread somatodendritic immunolocalization of central ionotropic P2X ATP receptors. Neuroscience. 1998;83:177–190. doi: 10.1016/s0306-4522(97)00365-5. [DOI] [PubMed] [Google Scholar]
- LEWIS C., NEIDHART S., HOLY C., NORTH R.A., BUELL G., SURPRENANT A. Co-expression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature. 1995;377:432–435. doi: 10.1038/377432a0. [DOI] [PubMed] [Google Scholar]
- LIU H., WANG H., SHENG H., JAN L.Y., BASBAUM A.I. Evidence for presynaptic N-methyl-D-aspartate autoreceptors in the spinal cord dorsal horn. Proc. Natl. Acad. Sci. U.S.A. 1994;91:8383–8387. doi: 10.1073/pnas.91.18.8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LLEWELLYN I.J., BURNSTOCK G. Ultrastructural localization of P2X3 receptors in rat sensory neurons. Neuro Report. 1998;9:2545–2550. doi: 10.1097/00001756-199808030-00022. [DOI] [PubMed] [Google Scholar]
- MALMBERG A.B., YAKSH T.L. The effect of morphine on formalin-evoked behavior and spinal release of excitatory amino acids and prostaglandin E2 using microdialysis in conscious rats. Br. J. Pharmacol. 1995;114:1069–1075. doi: 10.1111/j.1476-5381.1995.tb13315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCMAHON H.T., FORAN P., DOLLY J.O., VERHAGE M., WIEGANT V.M., NICHOLLS D.G. Tetanus toxin and botulinum toxins type A and B inhibit glutamate, γ-aminobutyric acid, aspartate, and Met-enkephalin release from synaptosomes. J. Biol. Chem. 1992;267:21338–21343. [PubMed] [Google Scholar]
- MOLINOFF P.B., WILLIAMS K., PRITCHETT D.B., ZHONG J. Molecular pharmacology of NMDA receptors: Modulatory role of NR2 subunits. Prog. Brain Res. 1994;100:39–45. doi: 10.1016/s0079-6123(08)60766-9. [DOI] [PubMed] [Google Scholar]
- PARKER K.E. Modulation of ATP-gated non-selective cation channel (P2X1 receptor) activation and desensitization by the actin cytoskeleton. J. Physiol. 1998;510:19–25. doi: 10.1111/j.1469-7793.1998.019bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETRALIA R.S., WANG Y.-X., WENTHOLD R.J. The NMDA receptor subunits NR2A and NR2B show histological and ultrastructural localization patterns similar to those of NR1. J. Neurosci. 1996;14:6102–6120. doi: 10.1523/JNEUROSCI.14-10-06102.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIERCE R.C., KALIVAS P.W. Repeated cocaine modifies the mechanism by which amphetamine releases dopamine. J. Neurosci. 1997;17:3254–3261. doi: 10.1523/JNEUROSCI.17-09-03254.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POULAIN B., ROSSETTO O., DELOYE F., SCHIAVO G., TAUC L., MONTECUCCO C. Antibodies against rat brain vesicle-associated membrane protein (synaptobrevin) prevent inhibition of acetylcholine release by tetanus toxin or botulinum neurotoxin type B. J. Neurochem. 1993;61:1175–1178. doi: 10.1111/j.1471-4159.1993.tb03640.x. [DOI] [PubMed] [Google Scholar]
- RAE M.G., ROWAN E.G., KENNEDY C. Pharmacological properties of P2X3-receptors present in neurones of the rat dorsal root ganglia. Br. J. Pharmacol. 1998;124:176–180. doi: 10.1038/sj.bjp.0701803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- REICHLING D.B., LEVINE J.D. Heat transduction in rat sensory neurons by calcium-dependent activation of a cation channel. Proc. Natl. Acad. Sci. U.S.A. 1997;94:7006–7011. doi: 10.1073/pnas.94.13.7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REN K., WILLIAMS G.M., HYLDEN L.K., RUDA M.A., DUBNER R. The intrathecal administration of excitatory amino-acid antagonists selectively attenuates carrageenan-induced behavioral hyperalgesia in rats. Eur. J. Pharmacol. 1992;219:235–243. doi: 10.1016/0014-2999(92)90301-j. [DOI] [PubMed] [Google Scholar]
- ROBERTSON S.J., RAE M.G., ROWAN E.G., KENNEDY C. Characterization of a P2X-purinoceptor in cultured neurones of the rat dorsal root ganglia. Br. J. Pharmacol. 1996;118:951–956. doi: 10.1111/j.1476-5381.1996.tb15491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAKURADA T., WAKO K., SUGIYAMA A., SAKURADA C., TAN-NO K., KISARA K. Involvement of spinal NMDA receptors in capsaicin-induced nociception. Pharmacol. Biochem. Behav. 1998;59:339–345. doi: 10.1016/s0091-3057(97)00423-1. [DOI] [PubMed] [Google Scholar]
- SAWYNOK J., REID A. Peripheral adenosine 5′-triphosphate enhances nociception in the formalin test via activation of a purinergic P2X receptor. Eur. J. Pharmacol. 1997;330:115–121. doi: 10.1016/s0014-2999(97)01001-7. [DOI] [PubMed] [Google Scholar]
- SCHIAVO G., BENFENATI F., POULAIN B., ROSSETTO O., POLVERINO DE LURETO P., DASGUPTA B.R., MONTECUCCO C. Tetanus and botulinum-B neurotoxin block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature. 1992;359:832–835. doi: 10.1038/359832a0. [DOI] [PubMed] [Google Scholar]
- SIMONE D.A., BAUMANN T.K., LAMOTTE R.H. Dose-dependent pain and mechanical hyperalgesia in human after intradermal injection of capsaicin. Pain. 1989;38:99–107. doi: 10.1016/0304-3959(89)90079-1. [DOI] [PubMed] [Google Scholar]
- SZALLASI A., BLUMBERG P.M. Vanilloid receptors: new insights enhance potential as a therapeutic target. Pain. 1996;68:195–208. doi: 10.1016/s0304-3959(96)03202-2. [DOI] [PubMed] [Google Scholar]
- TOMINAGA M., CATERINA M.J., MALMBERG A.B., ROSEN T.A., GILBERT H., SKINNER K., RAUMANN B.E., BASBAUM A.I., JULIUS D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- TREZISE D.J., MICHEL A.D., GRAHAMES C.B.A., KHAKH B.S., SURPRENANT A., HUMPHREY P.P.A. The selective P2X purinoceptor agonist, β,γ-methylene-L-adenosine 5′-triphosphate, discriminates between smooth muscle and neuronal P2X purinoceptors. Naunyn-Schmiedeberg's Arch. Pharmacol. 1995;351:603–609. doi: 10.1007/BF00170159. [DOI] [PubMed] [Google Scholar]
- UEDA M., KURAISHI Y., SATOH M. Detection of capsaicin-evoked release of glutamate from spinal dorsal horn slice of rat with on-line monitoring system. Neurosci. Lett. 1993;155:179–182. doi: 10.1016/0304-3940(93)90702-m. [DOI] [PubMed] [Google Scholar]
- UENO S., KOIZUMI S., INOUE K. Characterization of Ca2+ influx through recombinant P2X receptor in C6BU-1 cells. Br. J. Pharmacol. 1998;124:1484–1490. doi: 10.1038/sj.bjp.0701963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UENO S., TSUDA M., IWANAGA T., INOUE K. Cell type-specific ATP-activated responses in rat dorsal root ganglion neurons. Br. J. Pharmacol. 1999;126:429–436. doi: 10.1038/sj.bjp.0702319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VIRGINIO C., ROBERTSON G., SURPRENANT A., NORTH R.A. Trinitrophenyl-substituted nucleotides are potent antagonists selective for P2X1, P2X3 and heteromeric P2X2/3 receptors. Mol. Pharmacol. 1998;53:969–973. [PubMed] [Google Scholar]
- VULCHANOVA L., REIDL M.S., SHUSTER S.J., BUELL G., SURPRENANT A., NORTH R.A., ELDE R. Immunohistochemical study of the P2X2 and P2X3 receptor subunits in rat and monkey sensory neurons and their central terminals. Neuropharmacology. 1997;36:1229–1242. doi: 10.1016/s0028-3908(97)00126-3. [DOI] [PubMed] [Google Scholar]
- VULCHANOVA L., RIEDL M.S., SHUSTER S.J., STONE L.S., HARGREAVES K.M., BUELL G., SURPRENANT A., NORTH R.A., ELDE R. P2X3 is expressed by DRG neurons that terminate in inner lamina II. Eur. J. Neurosci. 1998;10:3470–3478. doi: 10.1046/j.1460-9568.1998.00355.x. [DOI] [PubMed] [Google Scholar]
- WERNER P., SEWARD P.W., BUELL G.N., NORTH R.A. Domains of P2X receptors involved in desensitization. Proc. Natl. Acad. Sci. U.S.A. 1996;93:15485–15490. doi: 10.1073/pnas.93.26.15485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS K. Ifenprodil discriminates subtypes of the N-methyl-D-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol. Pharmacol. 1993;44:851–859. [PubMed] [Google Scholar]
- XIANG Z., BO X., BURNSTOCK G. Localization of ATP-gated P2X receptor immunoreactivity in rat sensory and sympathetic ganglia. Neurosci. Lett. 1998;256:105–108. doi: 10.1016/s0304-3940(98)00774-5. [DOI] [PubMed] [Google Scholar]


