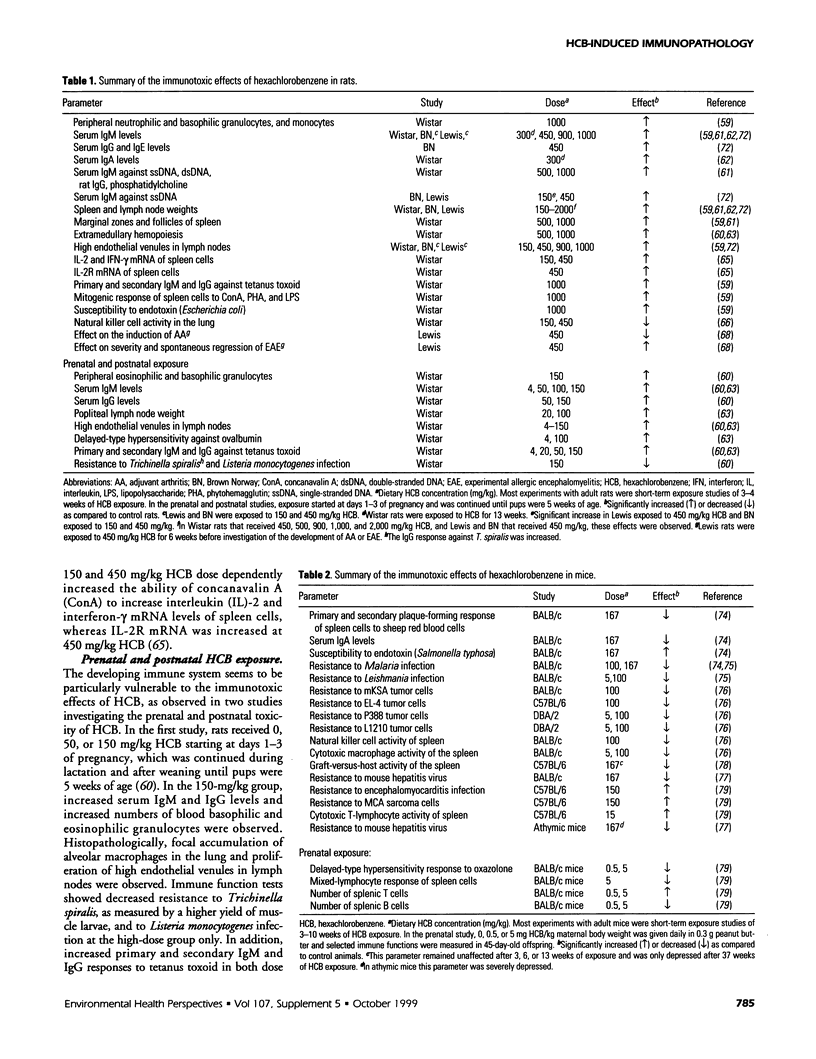
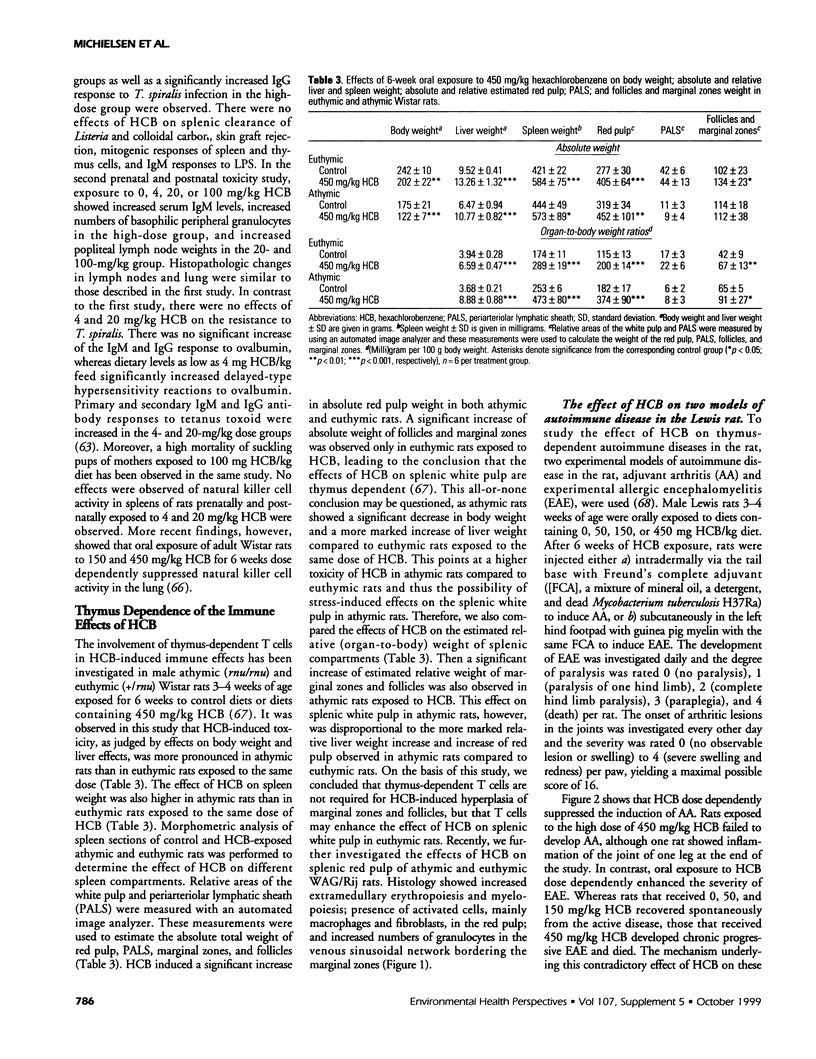
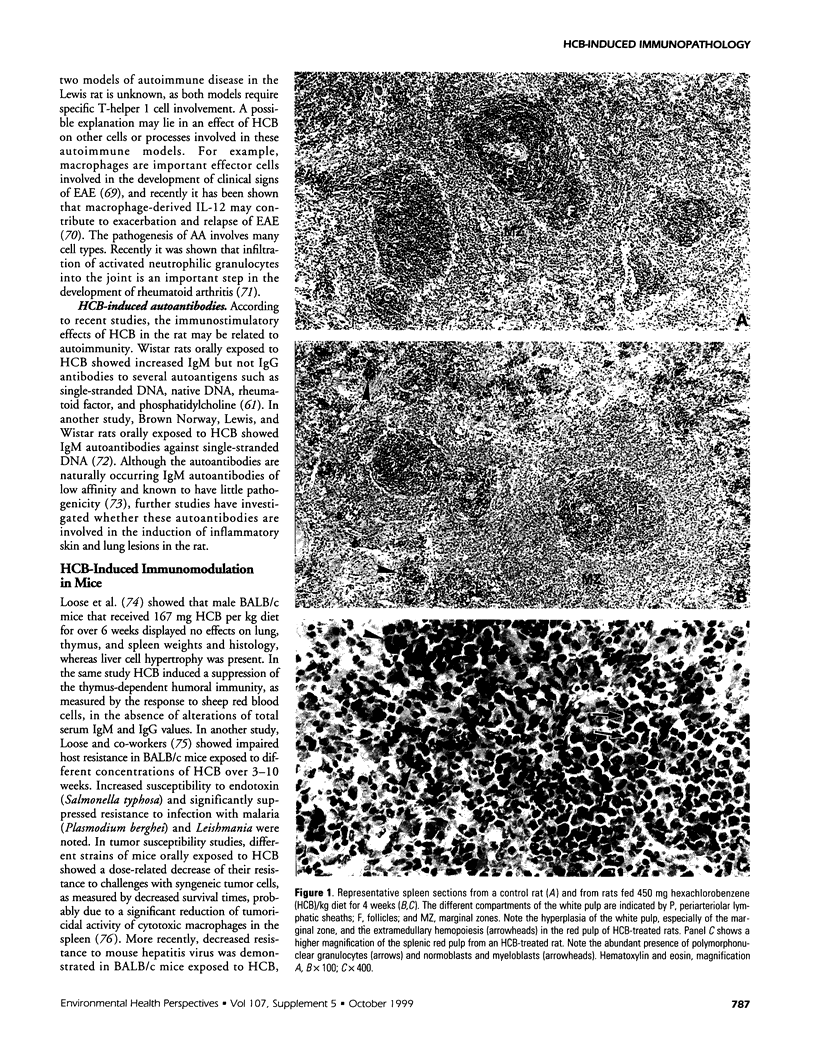
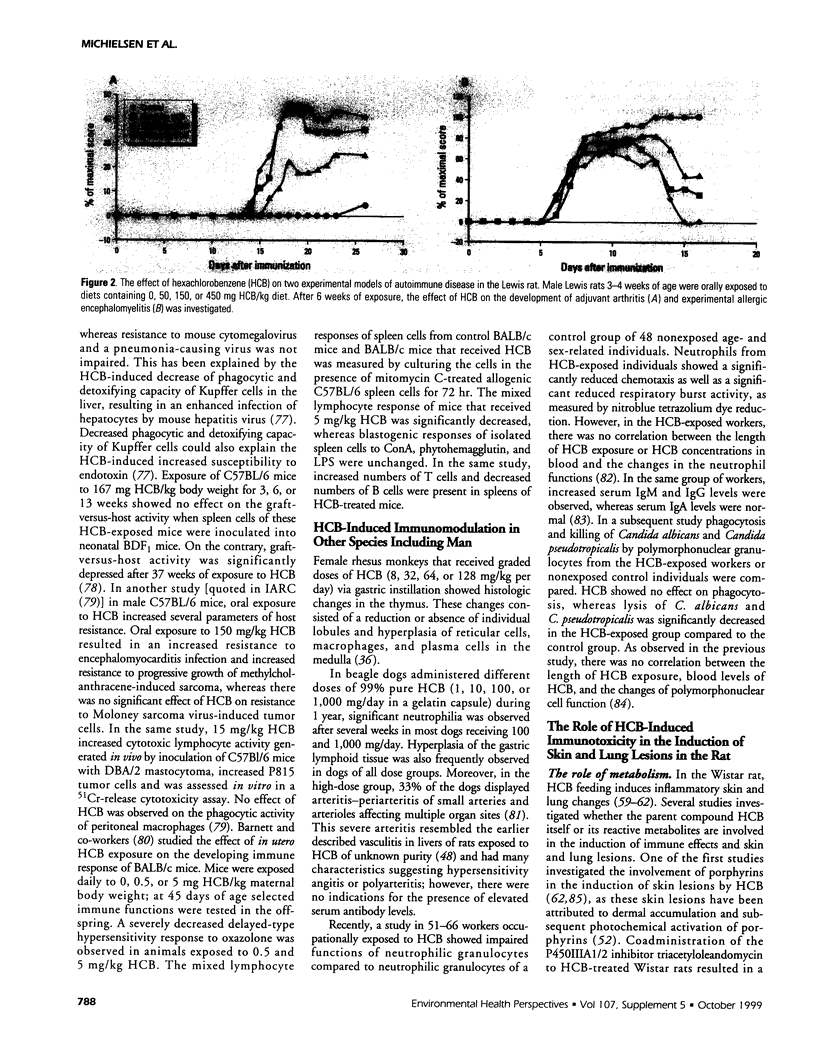
Abstract
Hexachlorobenzene (HCB) is a persistent environmental pollutant. The toxicity of HCB has been extensively studied after an accidental human poisoning in Turkey and more recently it has been shown that HCB has immunotoxic properties in laboratory animals and probably also in man. Oral exposure of rats to HCB showed stimulatory effects on spleen and lymph node weights and histology, increased serum IgM levels, and an enhancement of several parameters of immune function. Moreover, more recent studies indicate that HCB-induced effects in the rat may be related to autoimmunity. In Wistar rats exposed to HCB, IgM antibodies against several autoantigens were elevated; in the Lewis rat, HCB differently modulated two experimental models of autoimmune disease. Oral exposure of rats to HCB induces skin and lung pathology in the rat. Recently several studies have been conducted to investigate whether these skin and lung lesions can be related to HCB-induced immunomodulation, and these studies will be discussed in this review. HCB-induced skin and lung lesions probably have a different etiology; pronounced strain differences and correlation of skin lesions with immune parameters suggest a specific involvement of the immune system in HCB-induced skin lesions. The induction of lung lesions by HCB was thymus independent. Thymus-dependent T cells were not likely to be required for the induction of skin lesions, although T cells enhanced the rate of induction and the progression of the skin lesions. No deposition of autoantibodies was observed in nonlesional or lesional skin of HCB-treated rats. Therefore, we concluded that it is unlikely that the mechanism by which most allergic or autoimmunogenic chemicals work, i.e., by binding to macromolecules of the body and subsequent T- and B-cell activation, is involved in the HCB-induced immunopathology in the rat. Such a thymus-independent immunopathology is remarkable, as HCB strongly modulates T-cell-mediated immune parameters. This points at a very complex mechanism and possible involvement of multiple factors in the immunopathology of HCB.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett J. B., Barfield L., Walls R., Joyner R., Owens R., Soderberg L. S. The effect of in utero exposure to hexachlorobenzene on the developing immune response of BALB/c mice. Toxicol Lett. 1987 Dec;39(2-3):263–274. doi: 10.1016/0378-4274(87)90242-6. [DOI] [PubMed] [Google Scholar]
- Bickers D. R. The dermatologic manifestations of human porphyria. Ann N Y Acad Sci. 1987;514:261–267. doi: 10.1111/j.1749-6632.1987.tb48781.x. [DOI] [PubMed] [Google Scholar]
- Bleavins M. R., Aulerich R. J., Ringer R. K. Effects of chronic dietary hexachlorobenzene exposure on the reproductive performance and survivability of mink and European ferrets. Arch Environ Contam Toxicol. 1984 May;13(3):357–365. doi: 10.1007/BF01055287. [DOI] [PubMed] [Google Scholar]
- Bloksma N., Kubicka-Muranyi M., Schuppe H. C., Gleichmann E., Gleichmann H. Predictive immunotoxicological test systems: suitability of the popliteal lymph node assay in mice and rats. Crit Rev Toxicol. 1995;25(5):369–396. doi: 10.3109/10408449509049338. [DOI] [PubMed] [Google Scholar]
- CAM C. [A new epidemic dermatosis of children]. Ann Dermatol Syphiligr (Paris) 1960 Jul-Aug;87:393–397. [PubMed] [Google Scholar]
- CAMPBELL J. A. PATHOLOGICAL ASPECTS OF HEXACHLOROBENZENE FEEDING IN RATS. S Afr J Lab Clin Med. 1963 Dec;14:203–206. [PubMed] [Google Scholar]
- Cabral J. R., Mollner T., Raitano F., Shubik P. Carcinogenesis of hexachlorobenzene in mice. Int J Cancer. 1979 Jan 15;23(1):47–51. doi: 10.1002/ijc.2910230110. [DOI] [PubMed] [Google Scholar]
- Cabral J. R., Shubik P. Carcinogenic activity of hexachlorobenzene in mice and hamsters. IARC Sci Publ. 1986;(77):411–416. [PubMed] [Google Scholar]
- Cabral J. R., Shubik P., Mollner T., Raitano F. Carcinogenic activity of hexacholorobenzene in hamsters. Nature. 1977 Oct 6;269(5628):510–511. doi: 10.1038/269510a0. [DOI] [PubMed] [Google Scholar]
- Camps M., Planas J., Gómez-Catalán J., Sabroso M., To-Figueras J., Corbella J. Organochlorine residues in human adipose tissue in Spain: study of an agrarian area. Bull Environ Contam Toxicol. 1989 Feb;42(2):195–201. doi: 10.1007/BF01699400. [DOI] [PubMed] [Google Scholar]
- Carthew P., Edwards R. E., Smith A. G. Immunotoxic effects of hexachlorobenzene on the pathogenesis of systemic, pneumonic and hepatic virus infections in the mouse. Hum Exp Toxicol. 1990 Nov;9(6):403–411. doi: 10.1177/096032719000900608. [DOI] [PubMed] [Google Scholar]
- Carthew P., Smith A. G. Pathological mechanisms of hepatic tumour formation in rats exposed chronically to dietary hexachlorobenzene. J Appl Toxicol. 1994 Nov-Dec;14(6):447–452. doi: 10.1002/jat.2550140610. [DOI] [PubMed] [Google Scholar]
- Courtney K. D. Hexachlorobenzene (HCB): a review. Environ Res. 1979 Dec;20(2):225–266. doi: 10.1016/0013-9351(79)90001-x. [DOI] [PubMed] [Google Scholar]
- Cripps D. J., Peters H. A., Gocmen A., Dogramici I. Porphyria turcica due to hexachlorobenzene: a 20 to 30 year follow-up study on 204 patients. Br J Dermatol. 1984 Oct;111(4):413–422. doi: 10.1111/j.1365-2133.1984.tb06603.x. [DOI] [PubMed] [Google Scholar]
- DE MATTEIS F., PRIOR B. E., RIMINGTON C. Nervous and biochemical disturbances following hexachlorobenzene intoxication. Nature. 1961 Jul 22;191:363–366. doi: 10.1038/191363a0. [DOI] [PubMed] [Google Scholar]
- DOGRAMACI I. PORPHYRIAS AND PORPHYRIN METABOLISM, WITH SPECIAL REFERENCE TO PORPHYRIA IN CHILDHOOD. Adv Pediatr. 1964;13:11–63. [PubMed] [Google Scholar]
- Driscoll K. E., Lindenschmidt R. C., Maurer J. K., Higgins J. M., Ridder G. Pulmonary response to silica or titanium dioxide: inflammatory cells, alveolar macrophage-derived cytokines, and histopathology. Am J Respir Cell Mol Biol. 1990 Apr;2(4):381–390. doi: 10.1165/ajrcmb/2.4.381. [DOI] [PubMed] [Google Scholar]
- Elder G. H., Evans J. O., Matlin S. A. The effect of the porphyrogenic compound, hexachlorobenzene, on the activity of hepatic uroporphyrinogen decarboxylase in the rat. Clin Sci Mol Med. 1976 Jul;51(1):71–80. doi: 10.1042/cs0510071. [DOI] [PubMed] [Google Scholar]
- Foster W. G., Pentick J. A., McMahon A., Lecavalier P. R. Ovarian toxicity of hexachlorobenzene (HCB) in the superovulated female rat. J Biochem Toxicol. 1992 Spring;7(1):1–4. doi: 10.1002/jbt.2570070102. [DOI] [PubMed] [Google Scholar]
- GAJDOS A., GAJDOS-TOROK M. [Experimental prophyria observed in the white rat following intoxication with hexachlorobenzene]. Rev Fr Etud Clin Biol. 1961 Jun-Jul;6:549–552. [PubMed] [Google Scholar]
- Goldstein J. A., Friesen M., Scotti T. M., Hickman P., Hass J. R., Bergman H. Assessment of the contribution of chlorinated dibenzo-p-dioxins and dibenzofurans to hexachlorobenzene-induced toxicity, porphyria, changes in mixed function oxygenases, and histopathological changes. Toxicol Appl Pharmacol. 1978 Dec;46(3):633–649. doi: 10.1016/0041-008x(78)90309-5. [DOI] [PubMed] [Google Scholar]
- Gralla E. J., Fleischman R. W., Luthra Y. K., Hagopian M., Baker J. R., Esber H., Marcus W. Toxic effects of hexachlorobenzene after daily administration to beagle dogs for one year. Toxicol Appl Pharmacol. 1977 May;40(2):227–239. doi: 10.1016/0041-008x(77)90093-x. [DOI] [PubMed] [Google Scholar]
- Grant D. L., Phillips W. E., Hatina G. V. Effect of hexachlorobenzene on reproduction in the rat. Arch Environ Contam Toxicol. 1977;5(2):207–216. doi: 10.1007/BF02220904. [DOI] [PubMed] [Google Scholar]
- Grimalt J. O., Sunyer J., Moreno V., Amaral O. C., Sala M., Rosell A., Anto J. M., Albaiges J. Risk excess of soft-tissue sarcoma and thyroid cancer in a community exposed to airborne organochlorinated compound mixtures with a high hexachlorobenzene content. Int J Cancer. 1994 Jan 15;56(2):200–203. doi: 10.1002/ijc.2910560209. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Honda M., Herzenberg L. A., Steinberg A. D., Herzenberg L. A. Ly-1 B cells: functionally distinct lymphocytes that secrete IgM autoantibodies. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2494–2498. doi: 10.1073/pnas.81.8.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirji N., Lin T. J., Befus A. D. A novel CD8 molecule expressed by alveolar and peritoneal macrophages stimulates nitric oxide production. J Immunol. 1997 Feb 15;158(4):1833–1840. [PubMed] [Google Scholar]
- Hirji N., Lin T. J., Bissonnette E., Belosevic M., Befus A. D. Mechanisms of macrophage stimulation through CD8: macrophage CD8alpha and CD8beta induce nitric oxide production and associated killing of the parasite Leishmania major. J Immunol. 1998 Jun 15;160(12):6004–6011. [PubMed] [Google Scholar]
- Huitinga I., Ruuls S. R., Jung S., Van Rooijen N., Hartung H. P., Dijkstra C. D. Macrophages in T cell line-mediated, demyelinating, and chronic relapsing experimental autoimmune encephalomyelitis in Lewis rats. Clin Exp Immunol. 1995 May;100(2):344–351. doi: 10.1111/j.1365-2249.1995.tb03675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iatropoulos M. J., Hobson W., Knauf V., Adams H. P. Morphological effects of hexachlorobenzene toxicity in female rhesus monkeys. Toxicol Appl Pharmacol. 1976 Sep;37(3):433–444. doi: 10.1016/0041-008x(76)90205-2. [DOI] [PubMed] [Google Scholar]
- Jacoff F. S., Scarberry R., Rosa D. Source assessment of hexachlorobenzene from the organic chemical manufacturing industry. IARC Sci Publ. 1986;(77):31–37. [PubMed] [Google Scholar]
- Jarrell J. F., McMahon A., Villeneuve D., Franklin C., Singh A., Valli V. E., Bartlett S. Hexachlorobenzene toxicity in the monkey primordial germ cell without induced porphyria. Reprod Toxicol. 1993;7(1):41–47. doi: 10.1016/0890-6238(93)90008-u. [DOI] [PubMed] [Google Scholar]
- Jarrell J., Gocmen A., Foster W., Brant R., Chan S., Sevcik M. Evaluation of reproductive outcomes in women inadvertently exposed to hexachlorobenzene in southeastern Turkey in the 1950s. Reprod Toxicol. 1998 Jul-Aug;12(4):469–476. doi: 10.1016/s0890-6238(98)00019-7. [DOI] [PubMed] [Google Scholar]
- Kitchin K. T., Linder R. E., Scotti T. M., Walsh D., Curley A. O., Svendsgaard D. Offspring mortality and maternal lung pathology in female rats fed hexachlorobenzene. Toxicology. 1982;23(1):33–39. doi: 10.1016/0300-483x(82)90039-7. [DOI] [PubMed] [Google Scholar]
- Koss G., Koransky W., Steinbach K. Studies on the toxicology of hexachlorobenzene. II. Identification and determination of metabolites. Arch Toxicol. 1976 Mar 11;35(2):107–114. doi: 10.1007/BF00372764. [DOI] [PubMed] [Google Scholar]
- Koss G., Seubert S., Seubert A., Koransky W., Ippen H. Studies on the toxicology of hexachlorobenzene. III. Observations in a long-term experiment. Arch Toxicol. 1978 Aug 9;40(4):285–294. doi: 10.1007/BF00310334. [DOI] [PubMed] [Google Scholar]
- Kuiper-Goodman T., Grant D. L., Moodie C. A., Korsrud G. O., Munro I. C. Subacute toxicity of hexachlorobenzene in the rat. Toxicol Appl Pharmacol. 1977 Jun;40(3):529–549. doi: 10.1016/0041-008x(77)90078-3. [DOI] [PubMed] [Google Scholar]
- Loose L. D., Pittman K. A., Benitz K. F., Silkworth J. B. Polychlorinated biphenyl and hexachlorobenzene induced humoral immunosuppression. J Reticuloendothel Soc. 1977 Sep;22(3):253–271. [PubMed] [Google Scholar]
- Loose L. D., Silkworth J. B., Charbonneau T., Blumenstock F. Environmental chemical-induced macrophage dysfunction. Environ Health Perspect. 1981 Jun;39:79–92. doi: 10.1289/ehp.813979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loose L. D., Silkworth J. B., Pittman K. A., Benitz K. F., Mueller W. Impaired host resistance to endotoxin and malaria in polychlorinated biphenyl- and hexachlorobenzene-treated mice. Infect Immun. 1978 Apr;20(1):30–35. doi: 10.1128/iai.20.1.30-35.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michielsen C. P., Bloksma N., Ultee A., van Mil F., Vos J. G. Hexachlorobenzene-induced immunomodulation and skin and lung lesions: a comparison between brown Norway, Lewis, and Wistar rats. Toxicol Appl Pharmacol. 1997 May;144(1):12–26. doi: 10.1006/taap.1997.8104. [DOI] [PubMed] [Google Scholar]
- Mill T., Haag W. The environmental fate of hexachlorobenzene. IARC Sci Publ. 1986;(77):61–66. [PubMed] [Google Scholar]
- OCKNER R. K., SCHMID R. Acquired porphyria in man and rat due to hexachlorobenzene intoxication. Nature. 1961 Feb 11;189:499–499. doi: 10.1038/189499a0. [DOI] [PubMed] [Google Scholar]
- Peters H. A., Johnson S. A., Cam S., Müftü Y., Oral S., Ergene T. Hexachlorobenzene-induced porphyria: effect of chelation on the disease, porphyrin and metal metabolism. Am J Med Sci. 1966 Mar;251(3):314–322. doi: 10.1097/00000441-196603000-00010. [DOI] [PubMed] [Google Scholar]
- Queiroz M. L., Bincoletto C., Perlingeiro R. C., Quadros M. R., Souza C. A. Immunoglobulin levels in workers exposed to hexachlorobenzene. Hum Exp Toxicol. 1998 Mar;17(3):172–175. doi: 10.1177/096032719801700308. [DOI] [PubMed] [Google Scholar]
- Queiroz M. L., Bincoletto C., Perlingeiro R. C., Souza C. A., Toledo H. Defective neutrophil function in workers occupationally exposed to hexachlorobenzene. Hum Exp Toxicol. 1997 Jun;16(6):322–326. doi: 10.1177/096032719701600605. [DOI] [PubMed] [Google Scholar]
- Queiroz M. L., Quadros M. R., Valadares M. C., Silveira J. P. Polymorphonuclear phagocytosis and killing in workers occupationally exposed to hexachlorobenzene. Immunopharmacol Immunotoxicol. 1998 Aug;20(3):447–454. doi: 10.3109/08923979809034826. [DOI] [PubMed] [Google Scholar]
- Renner G. Biotransformation of the fungicides hexachlorobenzene and pentachloronitrobenzene. Xenobiotica. 1981 Jul;11(7):435–446. doi: 10.3109/00498258109045854. [DOI] [PubMed] [Google Scholar]
- Rozman K., Rozman T., Greim H. Enhanced fecal elimination of stored hexachlorobenzene from rats and rhesus monkeys by hexadecane or mineral oil. Toxicology. 1981;22(1):33–44. doi: 10.1016/0300-483x(81)90005-6. [DOI] [PubMed] [Google Scholar]
- Rush G. F., Smith J. H., Maita K., Bleavins M., Aulerich R. J., Ringer R. K., Hook J. B. Perinatal hexachlorobenzene toxicity in the mink. Environ Res. 1983 Jun;31(1):116–124. doi: 10.1016/0013-9351(83)90068-3. [DOI] [PubMed] [Google Scholar]
- Santos L. L., Morand E. F., Hutchinson P., Boyce N. W., Holdsworth S. R. Anti-neutrophil monoclonal antibody therapy inhibits the development of adjuvant arthritis. Clin Exp Immunol. 1997 Feb;107(2):248–253. doi: 10.1111/j.1365-2249.1997.263-ce1154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schielen P., Den Besten C., Vos J. G., Van Bladeren P. J., Seinen W., Bloksma N. Immune effects of hexachlorobenzene in the rat: role of metabolism in a 13-week feeding study. Toxicol Appl Pharmacol. 1995 Mar;131(1):37–43. doi: 10.1006/taap.1995.1044. [DOI] [PubMed] [Google Scholar]
- Schielen P., Schoo W., Tekstra J., Oostermeijer H. H., Seinen W., Bloksma N. Autoimmune effects of hexachlorobenzene in the rat. Toxicol Appl Pharmacol. 1993 Oct;122(2):233–243. doi: 10.1006/taap.1993.1192. [DOI] [PubMed] [Google Scholar]
- Sherwood R. L., Thomas P. T., O'Shea W. J., Bradof J. N., Ratajczak H. V., Graham J. A., Aranyi C. Effects of inhaled hexachlorobenzene aerosols on rat pulmonary host defenses. Toxicol Ind Health. 1989 May;5(3):451–461. doi: 10.1177/074823378900500306. [DOI] [PubMed] [Google Scholar]
- Silkworth J. B., Loose L. D. Assessment of environmental contaminant-induced lymphocyte dysfunction. Environ Health Perspect. 1981 Jun;39:105–128. doi: 10.1289/ehp.8139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon G. S., Tardiff R. G., Borzelleca J. F. Failure of hexachlorobenzene to induce dominant lethal mutations in the rat. Toxicol Appl Pharmacol. 1979 Feb;47(2):415–419. doi: 10.1016/0041-008x(79)90337-5. [DOI] [PubMed] [Google Scholar]
- Smith A. G., Cabral J. R. Liver-cell tumours in rats fed hexachlorobenzene. Cancer Lett. 1980 Dec;11(2):169–172. doi: 10.1016/0304-3835(80)90108-1. [DOI] [PubMed] [Google Scholar]
- Smith T., Hewson A. K., Kingsley C. I., Leonard J. P., Cuzner M. L. Interleukin-12 induces relapse in experimental allergic encephalomyelitis in the Lewis rat. Am J Pathol. 1997 Jun;150(6):1909–1917. [PMC free article] [PubMed] [Google Scholar]
- Stewart F. P., Manson M. M., Cabral J. R., Smith A. G. Hexachlorobenzene as a promoter of diethylnitrosamine-initiated hepatocarcinogenesis in rats and comparison with induction of porphyria. Carcinogenesis. 1989 Jul;10(7):1225–1230. doi: 10.1093/carcin/10.7.1225. [DOI] [PubMed] [Google Scholar]
- Van Loveren H., Krajnc E. I., Rombout P. J., Blommaert F. A., Vos J. G. Effects of ozone, hexachlorobenzene, and bis(tri-n-butyltin)oxide on natural killer activity in the rat lung. Toxicol Appl Pharmacol. 1990 Jan;102(1):21–33. doi: 10.1016/0041-008x(90)90080-e. [DOI] [PubMed] [Google Scholar]
- Vandebriel R. J., Meredith C., Scott M. P., Roholl P. J., Van Loveren H. Effects of in vivo exposure to bis(tri-n-butyltin)oxide, hexachlorobenzene, and benzo(a)pyrene on cytokine (receptor) mRNA levels in cultured rat splenocytes and on IL-2 receptor protein levels. Toxicol Appl Pharmacol. 1998 Jan;148(1):126–136. doi: 10.1006/taap.1997.8294. [DOI] [PubMed] [Google Scholar]
- Vos J. G., van Logten M. J., Kreeftenberg J. G., Kruizinga W. Hexachlorobenzene-induced stimulation of the humoral immune response in rats. Ann N Y Acad Sci. 1979 May 31;320:535–550. [PubMed] [Google Scholar]
- Vos J. G., van Logten M. J., Kreeftenberg J. G., Steerenberg P. A., Kruizinga W. Effect of hexachlorobenzene on the immune system of rats following combined pre- and postnatal exposure. Drug Chem Toxicol. 1979;2(1-2):61–76. doi: 10.3109/01480547908993182. [DOI] [PubMed] [Google Scholar]
- Vos J. G., van der Maas H. L., Musch A., Ram E. Toxicity of hexachlorobenzene in Japanese quail with special reference to porphyria, liver damage, reproduction, and tissue residues. Toxicol Appl Pharmacol. 1971 Apr;18(4):944–957. doi: 10.1016/0041-008x(71)90240-7. [DOI] [PubMed] [Google Scholar]
- Weller P. F. The immunobiology of eosinophils. N Engl J Med. 1991 Apr 18;324(16):1110–1118. doi: 10.1056/NEJM199104183241607. [DOI] [PubMed] [Google Scholar]
- Weltzien H. U., Moulon C., Martin S., Padovan E., Hartmann U., Kohler J. T cell immune responses to haptens. Structural models for allergic and autoimmune reactions. Toxicology. 1996 Feb 22;107(2):141–151. doi: 10.1016/0300-483x(95)03253-c. [DOI] [PubMed] [Google Scholar]
- den Besten C., Bennik M. H., Bruggeman I., Schielen P., Kuper F., Brouwer A., Koeman J. H., Vos J. G., Van Bladeren P. J. The role of oxidative metabolism in hexachlorobenzene-induced porphyria and thyroid hormone homeostasis: a comparison with pentachlorobenzene in a 13-week feeding study. Toxicol Appl Pharmacol. 1993 Apr;119(2):181–194. doi: 10.1006/taap.1993.1059. [DOI] [PubMed] [Google Scholar]
- den Tonkelaar E. M., Verschuuren H. G., Bankovska J., de Vries T., Kroes R., van Esch G. J. Hexachlorobenzene toxicity in pigs. Toxicol Appl Pharmacol. 1978 Jan;43(1):137–145. doi: 10.1016/s0041-008x(78)80038-6. [DOI] [PubMed] [Google Scholar]
- van Ommen B., Hendriks W., Bessems J. G., Geesink G., Müller F., van Bladeren P. J. The relation between the oxidative biotransformation of hexachlorobenzene and its porphyrinogenic activity. Toxicol Appl Pharmacol. 1989 Sep 15;100(3):517–528. doi: 10.1016/0041-008x(89)90299-8. [DOI] [PubMed] [Google Scholar]



