Abstract
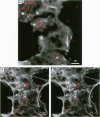
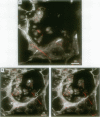
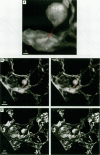
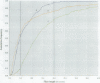
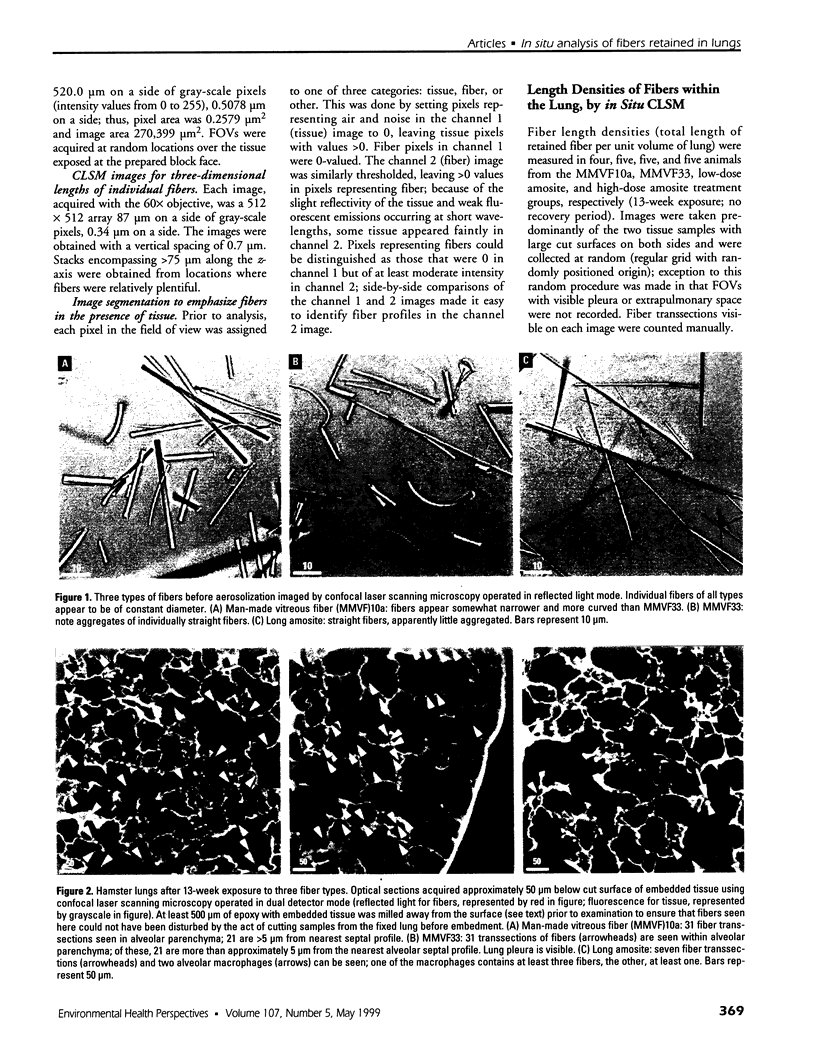
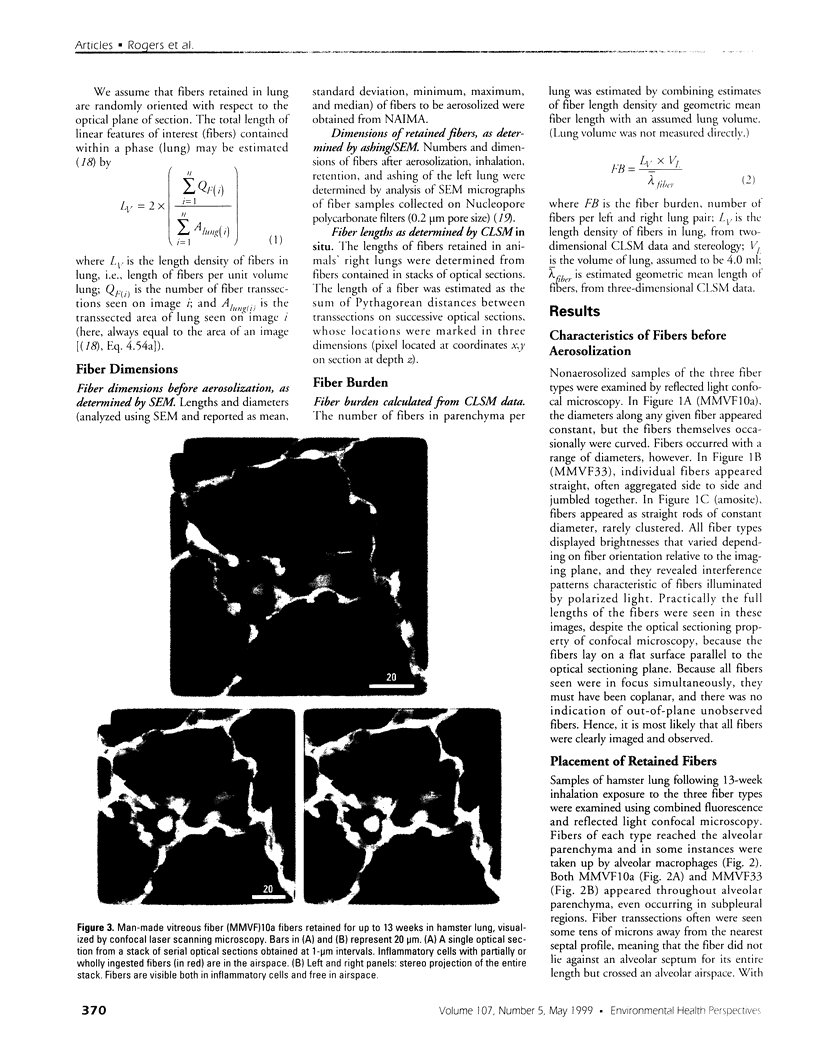
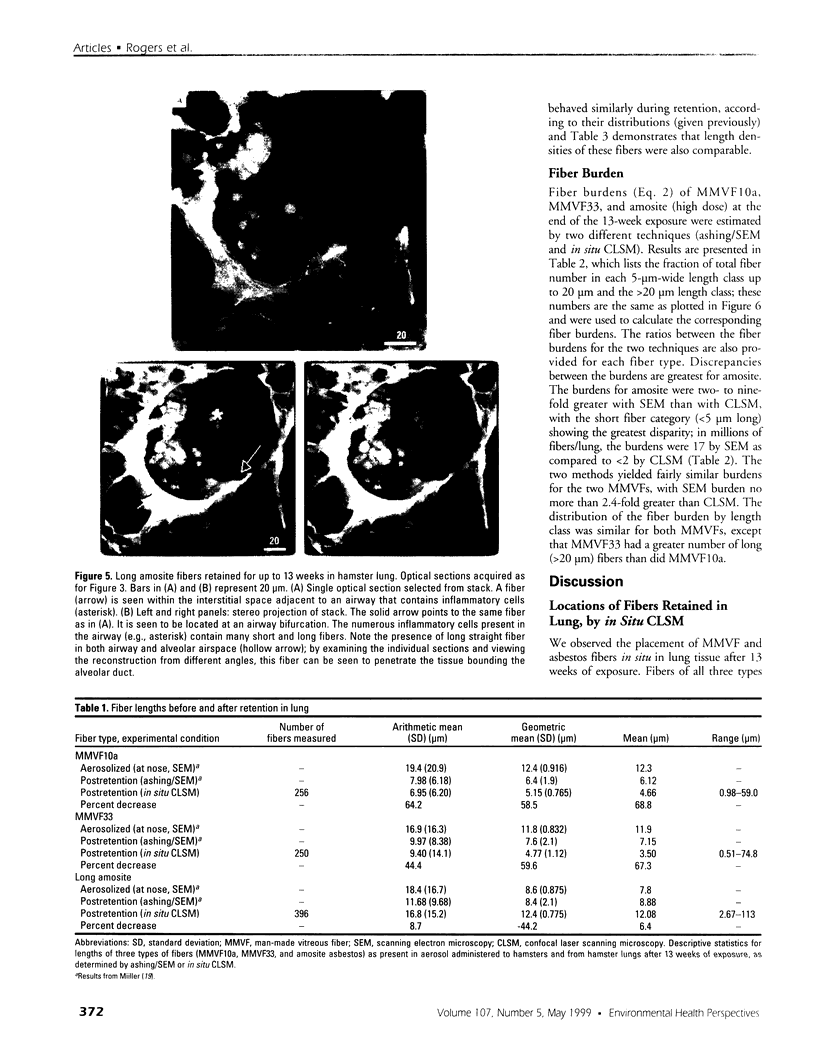
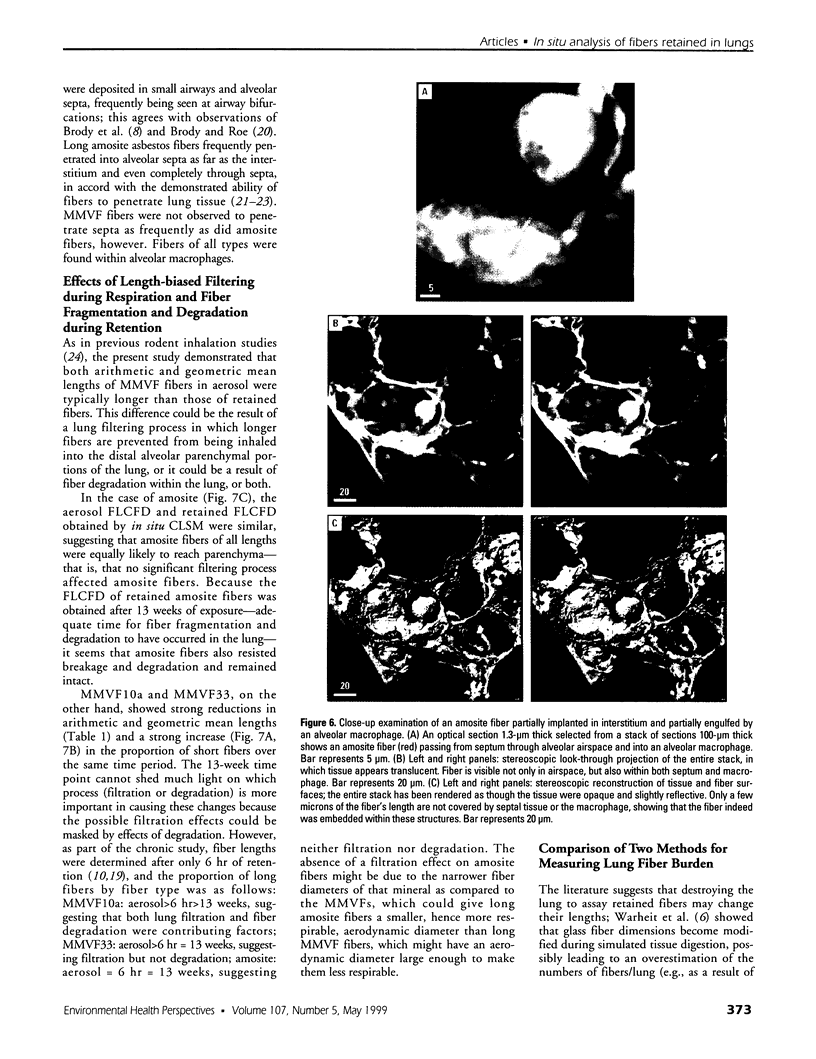
Hamsters breathed, nose-only, for 13 weeks, 5 days/week, 6 hr/day, either man-made vitreous fiber (MMVF)10a, MMVF33, or long amosite asbestos at approximately 300 World Health Organization (WHO) fibers/cc or long amosite at 25 WHO fibers/cc. [World Health Organization fibers are longer than 5 microm and thicker than 3 microm, with aspect ratio >3.] After sacrifice, fiber burden was estimated (left lungs) by ashing and scanning electron microscopy (ashing/SEM) or (right middle lobes) by confocal laser scanning microscopy (CLSM) in situ. In situ CLSM also provided three-dimensional views of fibers retained, undisturbed, in lung tissue. Fibers of each type were lodged in alveoli and small airways, especially at airway bifurcations, and were seen fully or partly engulfed by alveolar macrophages. Amosite fibers penetrated into and through alveolar septa. Length densities of fibers in parenchyma (total length of fiber per unit volume of lung) were estimated stereologically from fiber transsections counted on two-dimensional optical sections and were 30.5, 25.3, 20.0, and 81.6 mm/mm3 for MMVF10a, MMVF33, and low- and high-dose amosite, respectively. Lengths of individual fibers were measured in three dimensions by tracking individual fibers through series of optical sections. Length distributions of amosite fibers aerosolized, but before inhalation versus after retention in the lung were similar, whether determined by ashing/SEM or in situ CLSM. In contrast, the fraction of short MMVF10a and MMVF33 fibers increased and the geometric mean fiber lengths of both MMVFs decreased by approximately 60% during retention. Most likely due to fiber deposition pattern and differences in sampling, fiber burdens [MMVF10a, MMVF33, and amosite (high dose; 269 WHO fibers/cc)] determined by ashing/SEM were 1.4, 1. 5, and 3.5 times greater, respectively, than those calculated from in situ CLSM data. In situ CLSM is able to provide detailed information about the anatomic sites of fiber retention and also fiber lengths and burdens in good agreement with ashing/SEM results.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein D. M., Drew R. T., Kuschner M. Experimental approaches for exposure to sized glass fibers. Environ Health Perspect. 1980 Feb;34:47–57. doi: 10.1289/ehp.803447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody A. R., Hill L. H., Adkins B., Jr, O'Connor R. W. Chrysotile asbestos inhalation in rats: deposition pattern and reaction of alveolar epithelium and pulmonary macrophages. Am Rev Respir Dis. 1981 Jun;123(6):670–679. doi: 10.1164/arrd.1981.123.6.670. [DOI] [PubMed] [Google Scholar]
- Brody A. R., Roe M. W. Deposition pattern of inorganic particles at the alveolar level in the lungs of rats and mice. Am Rev Respir Dis. 1983 Oct;128(4):724–729. doi: 10.1164/arrd.1983.128.4.724. [DOI] [PubMed] [Google Scholar]
- Coin P. G., Roggli V. L., Brody A. R. Persistence of long, thin chrysotile asbestos fibers in the lungs of rats. Environ Health Perspect. 1994 Oct;102 (Suppl 5):197–199. doi: 10.1289/ehp.94102s5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. M., Addison J., Bolton R. E., Donaldson K., Jones A. D., Smith T. The pathogenicity of long versus short fibre samples of amosite asbestos administered to rats by inhalation and intraperitoneal injection. Br J Exp Pathol. 1986 Jun;67(3):415–430. [PMC free article] [PubMed] [Google Scholar]
- De Vuyst P., Dumortier P., Swaen G. M., Pairon J. C., Brochard P. Respiratory health effects of man-made vitreous (mineral) fibres. Eur Respir J. 1995 Dec;8(12):2149–2173. doi: 10.1183/09031936.95.08122149. [DOI] [PubMed] [Google Scholar]
- Hesterberg T. W., Axten C., McConnell E. E., Oberdörster G., Everitt J., Miiller W. C., Chevalier J., Chase G. R., Thevenaz P. Chronic inhalation study of fiber glass and amosite asbestos in hamsters: twelve-month preliminary results. Environ Health Perspect. 1997 Sep;105 (Suppl 5):1223–1229. doi: 10.1289/ehp.97105s51223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesterberg T. W., Chase G., Axten C., Miller W. C., Musselman R. P., Kamstrup O., Hadley J., Morscheidt C., Bernstein D. M., Thevenaz P. Biopersistence of synthetic vitreous fibers and amosite asbestos in the rat lung following inhalation. Toxicol Appl Pharmacol. 1998 Aug;151(2):262–275. doi: 10.1006/taap.1998.8472. [DOI] [PubMed] [Google Scholar]
- Hesterberg T. W., Miiller W. C., McConnell E. E., Chevalier J., Hadley J. G., Bernstein D. M., Thevenaz P., Anderson R. Chronic inhalation toxicity of size-separated glass fibers in Fischer 344 rats. Fundam Appl Toxicol. 1993 May;20(4):464–476. doi: 10.1006/faat.1993.1057. [DOI] [PubMed] [Google Scholar]
- Lee K. P., Barras C. E., Griffith F. D., Waritz R. S. Pulmonary response to glass fiber by inhalation exposure. Lab Invest. 1979 Feb;40(2):123–133. [PubMed] [Google Scholar]
- Lee K. P., Kelly D. P., Kennedy G. L., Jr Pulmonary response to inhaled Kevlar aramid synthetic fibers in rats. Toxicol Appl Pharmacol. 1983 Nov;71(2):242–253. doi: 10.1016/0041-008x(83)90341-1. [DOI] [PubMed] [Google Scholar]
- Timbrell V. Deposition and retention of fibres in the human lung. Ann Occup Hyg. 1982;26(1-4):347–369. [PubMed] [Google Scholar]
- Wagner J. C., Berry G., Skidmore J. W., Timbrell V. The effects of the inhalation of asbestos in rats. Br J Cancer. 1974 Mar;29(3):252–269. doi: 10.1038/bjc.1974.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warheit D. B., Hwang H. C., Achinko L. Assessments of lung digestion methods for recovery of fibers. Environ Res. 1991 Apr;54(2):183–193. doi: 10.1016/s0013-9351(05)80100-8. [DOI] [PubMed] [Google Scholar]