Abstract
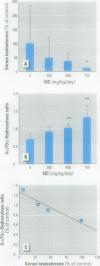
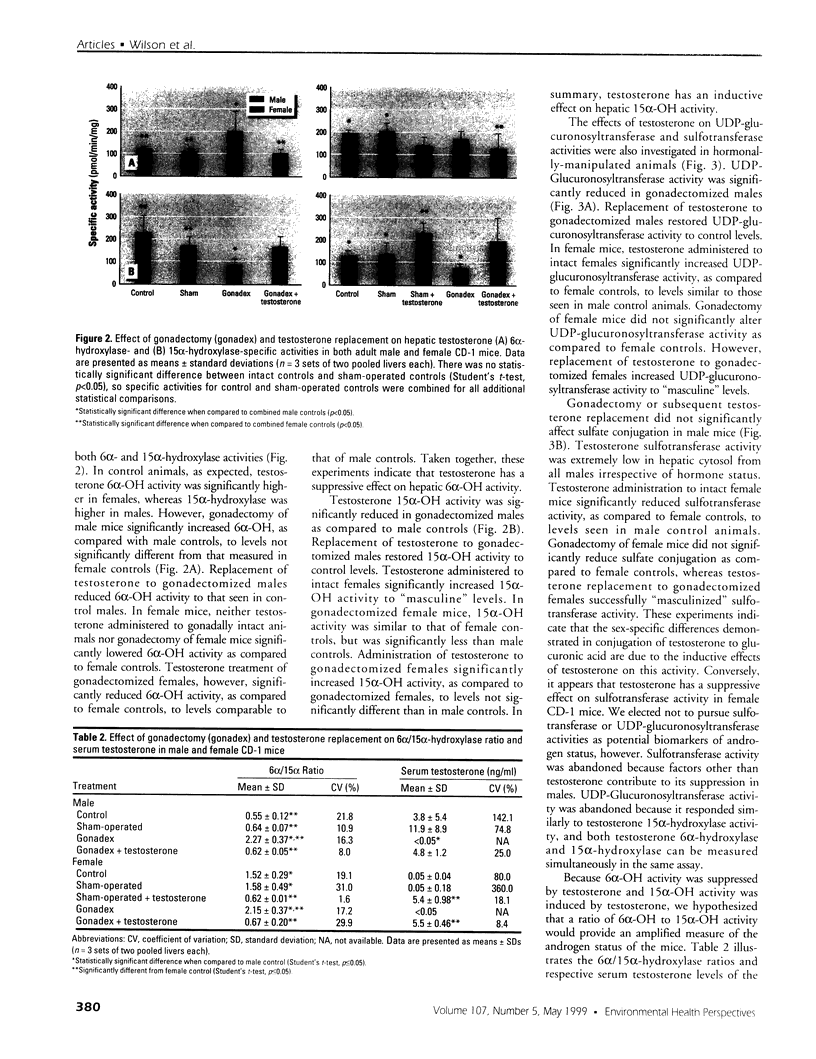
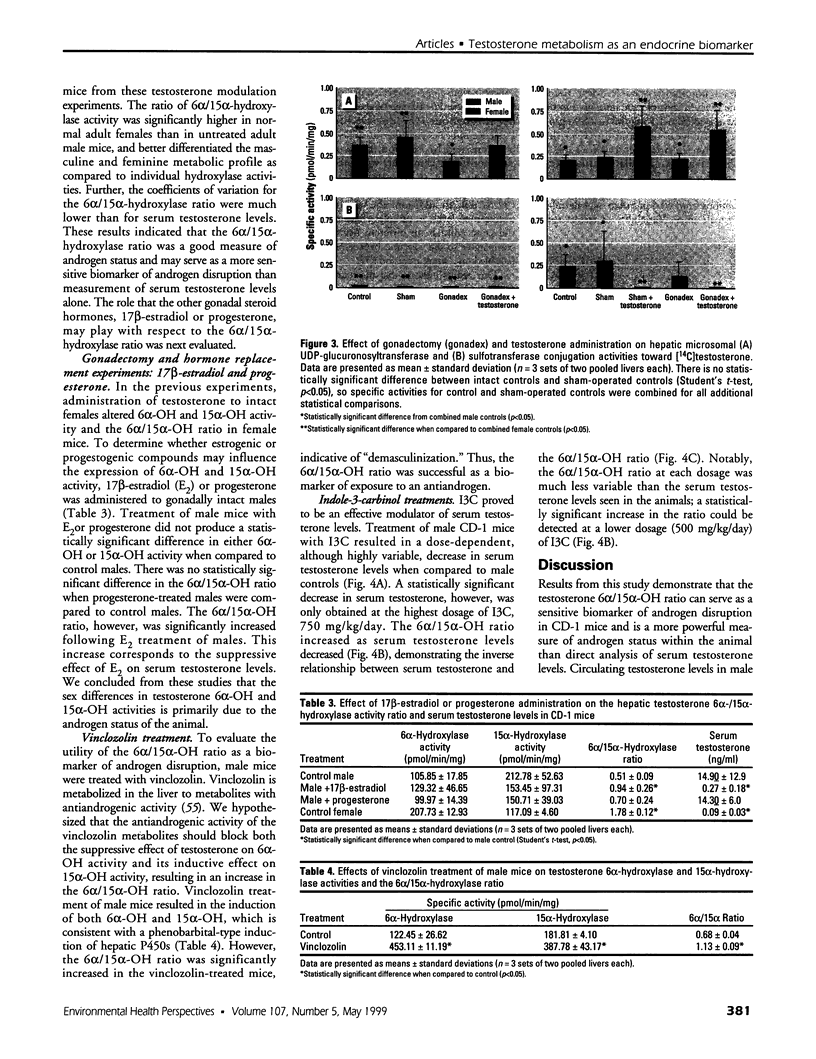
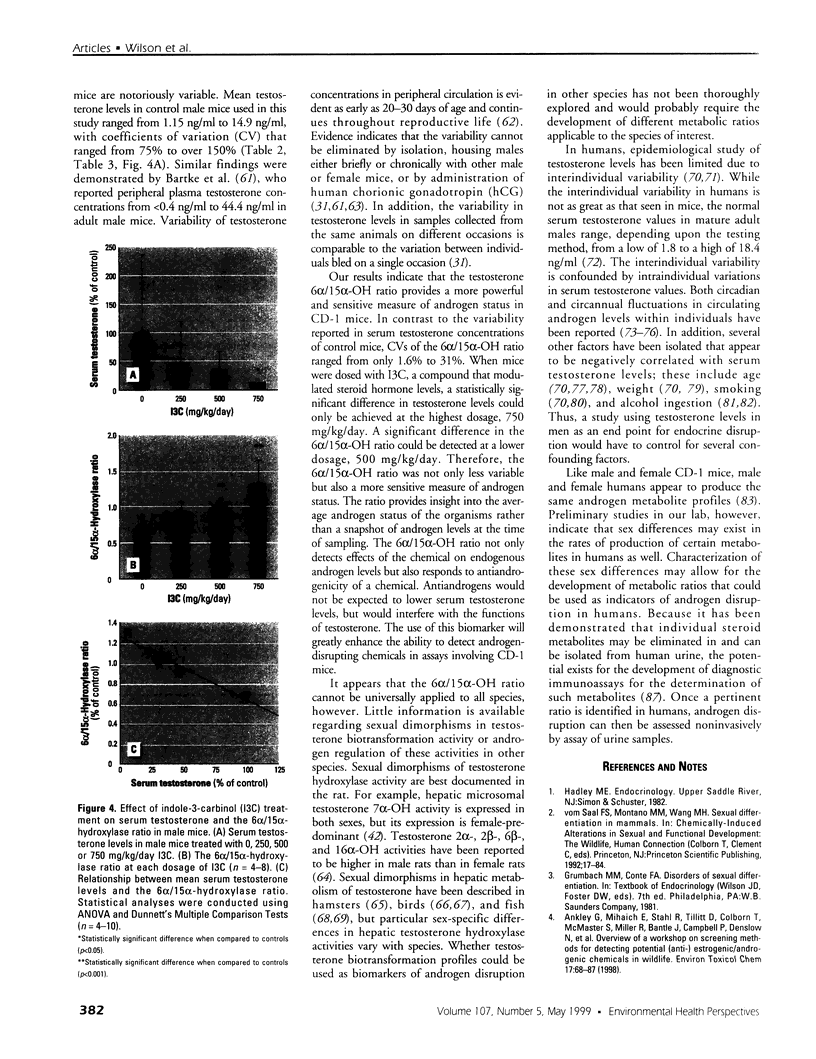
Assessment of the impact of environmental chemicals on androgen homeostasis in rodent models is confounded by high intraindividual and interindividual variability in circulating testosterone levels. Our goal was to evaluate changes in testosterone biotransformation processes as a measure of androgen homeostasis and as a biomarker of exposure to androgen-disrupting chemicals. Sex-specific differences in hepatic testosterone biotransformation enzyme activities were identified in CD-1 mice. Gonadectomy followed by replacement of individual steroid hormones identified specific sex differences in biotransformation profiles that were due to the inductive or suppressive effects of testosterone. Notably, significant androgen-dependent differences in testosterone 6[alpha]- and 15[alpha]-hydroxylase activities were demonstrated, and the ratio of 6[alpha]- and 15[alpha]-hydroxylase activities proved to be an excellent indicator of the androgen status within the animal. The male or "masculinized" testosterone 6[alpha]/15[alpha]-hydroxylase ratio was significantly less than the female or "feminized" ratio. Male mice were exposed to both an antiandrogen, vinclozolin, and to a compound that modulates serum androgen levels, indole-3-carbinol, to test the utility of this ratio as a biomarker of androgen disruption. Treatment with the antiandrogen vinclozolin significantly increased the 6[alpha]/15[alpha]-hydroxylase ratio. Indole-3-carbinol treatment resulted in a dose-dependent, but highly variable, decrease in serum testosterone levels. The 6[alpha]/15[alpha]-hydroxylase ratio increased as serum testosterone levels decreased in these animals. However, the increase in the ratio was much less variable and more sensitive than serum testosterone levels. These investigations demonstrate that the 6[alpha]/15[alpha]-hydroxylase ratio is a powerful measure of androgen modulation and a sensitive indicator of exposure to androgen-disrupting chemicals in CD-1 mice.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anylian G. H., Dorn J., Swerdlow J. The manifestations, aetiology and assessment of ethanol-induced hangover. S Afr Med J. 1978 Jul 29;54(5):193–198. [PubMed] [Google Scholar]
- Arai Y., Mori T., Suzuki Y., Bern H. A. Long-term effects of perinatal exposure to sex steroids and diethylstilbestrol on the reproductive system of male mammals. Int Rev Cytol. 1983;84:235–268. doi: 10.1016/s0074-7696(08)61019-0. [DOI] [PubMed] [Google Scholar]
- Baker H. W., Burger H. G., de Kretser D. M., Hudson B., O'Connor S., Wang C., Mirovics A., Court J., Dunlop M., Rennie G. C. Changes in the pituitary-testicular system with age. Clin Endocrinol (Oxf) 1976 Jul;5(4):349–372. doi: 10.1111/j.1365-2265.1976.tb01964.x. [DOI] [PubMed] [Google Scholar]
- Baldwin W. S., LeBlanc G. A. The anti-carcinogenic plant compound indole-3-carbinol differentially modulates P450-mediated steroid hydroxylase activities in mice. Chem Biol Interact. 1992 Aug 14;83(2):155–169. doi: 10.1016/0009-2797(92)90043-k. [DOI] [PubMed] [Google Scholar]
- Bartke A., Dalterio S. Evidence for episodic secretion of testosterone in laboratory mice. Steroids. 1975 Dec;26(6):749–756. doi: 10.1016/0039-128x(75)90107-5. [DOI] [PubMed] [Google Scholar]
- Bartke A., Steele R. E., Musto N., Caldwell B. V. Fluctuations in plasma testosterone levels in adult male rats and mice. Endocrinology. 1973 Apr;92(4):1223–1228. doi: 10.1210/endo-92-4-1223. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brown L. M., Pottern L. M., Hoover R. N., Devesa S. S., Aselton P., Flannery J. T. Testicular cancer in the United States: trends in incidence and mortality. Int J Epidemiol. 1986 Jun;15(2):164–170. doi: 10.1093/ije/15.2.164. [DOI] [PubMed] [Google Scholar]
- Carlsen E., Giwercman A., Keiding N., Skakkebaek N. E. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992 Sep 12;305(6854):609–613. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantilis S., Dombroski R., Shackleton C. H., Casey M. L., MacDonald P. C. Metabolism of 5 alpha-dihydroprogesterone in women and men: 3 beta- and 3 alpha-,6 alpha-dihydroxy-5 alpha-pregnan-20-ones are major urinary metabolites. J Clin Endocrinol Metab. 1996 Oct;81(10):3644–3649. doi: 10.1210/jcem.81.10.8855816. [DOI] [PubMed] [Google Scholar]
- Colborn T. Environmental estrogens: health implications for humans and wildlife. Environ Health Perspect. 1995 Oct;103 (Suppl 7):135–136. doi: 10.1289/ehp.95103s7135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colborn T., vom Saal F. S., Soto A. M. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect. 1993 Oct;101(5):378–384. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couwenbergs C., Knussmann R., Christiansen K. Comparisons of the intra- and inter-individual variability in sex hormone levels of men. Ann Hum Biol. 1986 Jan-Feb;13(1):63–72. doi: 10.1080/03014468600008201. [DOI] [PubMed] [Google Scholar]
- Cryptorchidism: a prospective study of 7500 consecutive male births, 1984-8. John Radcliffe Hospital Cryptorchidism Study Group. Arch Dis Child. 1992 Jul;67(7):892–899. doi: 10.1136/adc.67.7.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeizel A. Increasing trends in congenital malformations of male external genitalia. Lancet. 1985 Feb 23;1(8426):462–463. doi: 10.1016/s0140-6736(85)91185-7. [DOI] [PubMed] [Google Scholar]
- Dai W. S., Kuller L. H., LaPorte R. E., Gutai J. P., Falvo-Gerard L., Caggiula A. The epidemiology of plasma testosterone levels in middle-aged men. Am J Epidemiol. 1981 Dec;114(6):804–816. doi: 10.1093/oxfordjournals.aje.a113251. [DOI] [PubMed] [Google Scholar]
- Daston G. P., Gooch J. W., Breslin W. J., Shuey D. L., Nikiforov A. I., Fico T. A., Gorsuch J. W. Environmental estrogens and reproductive health: a discussion of the human and environmental data. Reprod Toxicol. 1997 Jul-Aug;11(4):465–481. doi: 10.1016/s0890-6238(97)00014-2. [DOI] [PubMed] [Google Scholar]
- Dehennin L., Delgado A., Pérès G. Urinary profile of androgen metabolites at different stages of pubertal development in a population of sporting male subjects. Eur J Endocrinol. 1994 Jan;130(1):53–59. doi: 10.1530/eje.0.1300053. [DOI] [PubMed] [Google Scholar]
- Divoll M., Greenblatt D. J., Harmatz J. S., Shader R. I. Effect of age and gender on disposition of temazepam. J Pharm Sci. 1981 Oct;70(10):1104–1107. doi: 10.1002/jps.2600701004. [DOI] [PubMed] [Google Scholar]
- Doering C. H., Kraemer H. C., Brodie H. K., Hamburg D. A. A cycle of plasma testosterone in the human male. J Clin Endocrinol Metab. 1975 Mar;40(3):492–500. doi: 10.1210/jcem-40-3-492. [DOI] [PubMed] [Google Scholar]
- Dotson L. E., Robertson L. S., Tuchfeld B. Plasma alcohol, smoking, hormone concentrations and self-reported aggression. A study in a social-drinking situation. J Stud Alcohol. 1975 May;36(5):578–586. doi: 10.15288/jsa.1975.36.578. [DOI] [PubMed] [Google Scholar]
- Emi Y., Ikushiro S., Iyanagi T. Xenobiotic responsive element-mediated transcriptional activation in the UDP-glucuronosyltransferase family 1 gene complex. J Biol Chem. 1996 Feb 16;271(7):3952–3958. doi: 10.1074/jbc.271.7.3952. [DOI] [PubMed] [Google Scholar]
- Falls J. G., Ryu D. Y., Cao Y., Levi P. E., Hodgson E. Regulation of mouse liver flavin-containing monooxygenases 1 and 3 by sex steroids. Arch Biochem Biophys. 1997 Jun 15;342(2):212–223. doi: 10.1006/abbi.1997.9965. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J. The molecular biology of cytochrome P450s. Pharmacol Rev. 1988 Dec;40(4):243–288. [PubMed] [Google Scholar]
- Gray E. S., Woodin B. R., Stegeman J. J. Sex differences in hepatic monooxygenases in winter flounder (Pseudopleuronectes americanus) and scup (Stenotomus chrysops) and regulation of P450 forms by estradiol. J Exp Zool. 1991 Sep;259(3):330–342. doi: 10.1002/jez.1402590308. [DOI] [PubMed] [Google Scholar]
- Greenblatt D. J., Divoll M., Harmatz J. S., Shader R. I. Oxazepam kinetics: effects of age and sex. J Pharmacol Exp Ther. 1980 Oct;215(1):86–91. [PubMed] [Google Scholar]
- Guengerich F. P., Shimada T. Oxidation of toxic and carcinogenic chemicals by human cytochrome P-450 enzymes. Chem Res Toxicol. 1991 Jul-Aug;4(4):391–407. doi: 10.1021/tx00022a001. [DOI] [PubMed] [Google Scholar]
- Guillette L. J., Jr, Gross T. S., Masson G. R., Matter J. M., Percival H. F., Woodward A. R. Developmental abnormalities of the gonad and abnormal sex hormone concentrations in juvenile alligators from contaminated and control lakes in Florida. Environ Health Perspect. 1994 Aug;102(8):680–688. doi: 10.1289/ehp.94102680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean-Faucher C., Berger M., de Turckheim M., Veyssiere G., Jean C. Developmental patterns of plasma and testicular testosterone in mice from birth to adulthood. Acta Endocrinol (Copenh) 1978 Dec;89(4):780–788. doi: 10.1530/acta.0.0890780. [DOI] [PubMed] [Google Scholar]
- Kavlock R. J., Daston G. P., DeRosa C., Fenner-Crisp P., Gray L. E., Kaattari S., Lucier G., Luster M., Mac M. J., Maczka C. Research needs for the risk assessment of health and environmental effects of endocrine disruptors: a report of the U.S. EPA-sponsored workshop. Environ Health Perspect. 1996 Aug;104 (Suppl 4):715–740. doi: 10.1289/ehp.96104s4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelce W. R., Monosson E., Gamcsik M. P., Laws S. C., Gray L. E., Jr Environmental hormone disruptors: evidence that vinclozolin developmental toxicity is mediated by antiandrogenic metabolites. Toxicol Appl Pharmacol. 1994 Jun;126(2):276–285. doi: 10.1006/taap.1994.1117. [DOI] [PubMed] [Google Scholar]
- Kley H. K., Solbach H. G., McKinnan J. C., Krüskemper H. L. Testosterone decrease and oestrogen increase in male patients with obesity. Acta Endocrinol (Copenh) 1979 Jul;91(3):553–563. doi: 10.1530/acta.0.0910553. [DOI] [PubMed] [Google Scholar]
- Kohen F., De Boever J., Kim J. B. Recent advances in chemiluminescence-based immunoassays for steroid hormones. J Steroid Biochem. 1987;27(1-3):71–79. doi: 10.1016/0022-4731(87)90296-2. [DOI] [PubMed] [Google Scholar]
- Kulkarni A. P., Hodgson E. The metabolism of insecticides: the role of monooxygenase enzymes. Annu Rev Pharmacol Toxicol. 1984;24:19–42. doi: 10.1146/annurev.pa.24.040184.000315. [DOI] [PubMed] [Google Scholar]
- Källén B., Winberg J. An epidemiological study of hypospadias in Sweden. Acta Paediatr Scand Suppl. 1982;293:1–21. doi: 10.1111/j.1651-2227.1982.tb09577.x. [DOI] [PubMed] [Google Scholar]
- Lau I. F., Saksena S. K., Chang M. C. Effects of hCG on serum levels of testosterone, dihydrotestosterone and androstenedione in male mice. Horm Res. 1978;9(3):169–175. doi: 10.1159/000178910. [DOI] [PubMed] [Google Scholar]
- LeBlanc G. A., Waxman D. J. Feminization of rat hepatic P-450 expression by cisplatin. Evidence for perturbations in the hormonal regulation of steroid-metabolizing enzymes. J Biol Chem. 1988 Oct 25;263(30):15732–15739. [PubMed] [Google Scholar]
- Liu L., Klaassen C. D. Regulation of hepatic sulfotransferases by steroidal chemicals in rats. Drug Metab Dispos. 1996 Aug;24(8):854–858. [PubMed] [Google Scholar]
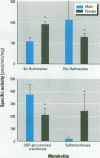
- Lund J., Zaphiropoulos P. G., Mode A., Warner M., Gustafsson J. A. Hormonal regulation of cytochrome P-450 gene expression. Adv Pharmacol. 1991;22:325–354. doi: 10.1016/s1054-3589(08)60040-x. [DOI] [PubMed] [Google Scholar]
- Macdonald J. I., Herman R. J., Verbeeck R. K. Sex-difference and the effects of smoking and oral contraceptive steroids on the kinetics of diflunisal. Eur J Clin Pharmacol. 1990;38(2):175–179. doi: 10.1007/BF00265980. [DOI] [PubMed] [Google Scholar]
- Matlai P., Beral V. Trends in congenital malformations of external genitalia. Lancet. 1985 Jan 12;1(8420):108–108. doi: 10.1016/s0140-6736(85)91999-3. [DOI] [PubMed] [Google Scholar]
- Mendelson J. H., Mello N. K., Ellingboe J. Effects of acute alcohol intake on pituitary-gonadal hormones in normal human males. J Pharmacol Exp Ther. 1977 Sep;202(3):676–682. [PubMed] [Google Scholar]
- Merrill R. M., Brawley O. W. Prostate cancer incidence and mortality rates among white and black men. Epidemiology. 1997 Mar;8(2):126–131. doi: 10.1097/00001648-199703000-00001. [DOI] [PubMed] [Google Scholar]
- Micheli A., Muti P., Pisani P., Secreto G., Recchione C., Totis A., Fissi R., Cavalleri A., Panico S., Berrino F. Repeated serum and urinary androgen measurements in premenopausal and postmenopausal women. J Clin Epidemiol. 1991;44(10):1055–1061. doi: 10.1016/0895-4356(91)90007-v. [DOI] [PubMed] [Google Scholar]
- Miners J. O., Attwood J., Birkett D. J. Influence of sex and oral contraceptive steroids on paracetamol metabolism. Br J Clin Pharmacol. 1983 Nov;16(5):503–509. doi: 10.1111/j.1365-2125.1983.tb02207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miners J. O., Robson R. A., Birkett D. J. Gender and oral contraceptive steroids as determinants of drug glucuronidation: effects on clofibric acid elimination. Br J Clin Pharmacol. 1984 Aug;18(2):240–243. doi: 10.1111/j.1365-2125.1984.tb02461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery P. R., Berger L. G., Mitenko P. A., Sitar D. S. Salicylate metabolism: effects of age and sex in adults. Clin Pharmacol Ther. 1986 May;39(5):571–576. doi: 10.1038/clpt.1986.98. [DOI] [PubMed] [Google Scholar]
- Niwa T., Kaneko H., Naritomi Y., Togawa A., Shiraga T., Iwasaki K., Tozuka Z., Hata T. Species and sex differences of testosterone and nifedipine oxidation in liver microsomes of rat, dog and monkey. Xenobiotica. 1995 Nov;25(10):1041–1049. doi: 10.3109/00498259509061904. [DOI] [PubMed] [Google Scholar]
- Overpeck J. G., Colson S. H., Hohmann J. R., Applestine M. S., Reilly J. F. Concentrations of circulating steroids in normal prepubertal and adult male and female humans, chimpanzees, rhesus monkeys, rats, mice, and hamsters: a literature survey. J Toxicol Environ Health. 1978 Sep-Nov;4(5-6):785–803. doi: 10.1080/15287397809529700. [DOI] [PubMed] [Google Scholar]
- Pampori N. A., Shapiro B. H. Sexual dimorphism in avian hepatic monooxygenases. Biochem Pharmacol. 1993 Sep 1;46(5):885–890. doi: 10.1016/0006-2952(93)90498-l. [DOI] [PubMed] [Google Scholar]
- Pelkonen P., Lang M., Pasanen M. Tissue and sex-dependent differences in CYP2A activities in hamsters. Arch Toxicol. 1994;68(7):416–422. doi: 10.1007/s002040050091. [DOI] [PubMed] [Google Scholar]
- Quinn M., Allen E. Changes in incidence of and mortality from breast cancer in England and Wales since introduction of screening. United Kingdom Association of Cancer Registries. BMJ. 1995 Nov 25;311(7017):1391–1395. doi: 10.1136/bmj.311.7017.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinberg A., Lagoguey M., Chauffournier J. M., Cesselin F. Circannual and circadian rhythms in plasma testosterone in five healthy young Parisian males. Acta Endocrinol (Copenh) 1975 Dec;80(4):732–734. doi: 10.1530/acta.0.0800732. [DOI] [PubMed] [Google Scholar]
- Rose R. M., Kreuz L. E., Holaday J. W., Sulak K. J., Johnson C. E. Diurnal variation of plasma testosterone and cortisol. J Endocrinol. 1972 Jul;54(1):177–178. doi: 10.1677/joe.0.0540177. [DOI] [PubMed] [Google Scholar]
- Tedford C. E., Ruperto V. B., Barnett A. Characterization of a rat liver glucuronosyltransferase that glucuronidates the selective D1 antagonist, SCH 23390, and other benzazepines. Drug Metab Dispos. 1991 Nov-Dec;19(6):1152–1159. [PubMed] [Google Scholar]
- Toppari J., Larsen J. C., Christiansen P., Giwercman A., Grandjean P., Guillette L. J., Jr, Jégou B., Jensen T. K., Jouannet P., Keiding N. Male reproductive health and environmental xenoestrogens. Environ Health Perspect. 1996 Aug;104 (Suppl 4):741–803. doi: 10.1289/ehp.96104s4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venturelli E., Cavalleri A., Secreto G. Methods for urinary testosterone analysis. J Chromatogr B Biomed Appl. 1995 Sep 15;671(1-2):363–380. doi: 10.1016/0378-4347(95)00062-n. [DOI] [PubMed] [Google Scholar]
- Waxman D. J., Attisano C., Guengerich F. P., Lapenson D. P. Human liver microsomal steroid metabolism: identification of the major microsomal steroid hormone 6 beta-hydroxylase cytochrome P-450 enzyme. Arch Biochem Biophys. 1988 Jun;263(2):424–436. doi: 10.1016/0003-9861(88)90655-8. [DOI] [PubMed] [Google Scholar]
- Waxman D. J. Interactions of hepatic cytochromes P-450 with steroid hormones. Regioselectivity and stereospecificity of steroid metabolism and hormonal regulation of rat P-450 enzyme expression. Biochem Pharmacol. 1988 Jan 1;37(1):71–84. doi: 10.1016/0006-2952(88)90756-3. [DOI] [PubMed] [Google Scholar]
- Waxman D. J., Ko A., Walsh C. Regioselectivity and stereoselectivity of androgen hydroxylations catalyzed by cytochrome P-450 isozymes purified from phenobarbital-induced rat liver. J Biol Chem. 1983 Oct 10;258(19):11937–11947. [PubMed] [Google Scholar]
- Waxman D. J., Morrissey J. J., LeBlanc G. A. Female-predominant rat hepatic P-450 forms j (IIE1) and 3 (IIA1) are under hormonal regulatory controls distinct from those of the sex-specific P-450 forms. Endocrinology. 1989 Jun;124(6):2954–2966. doi: 10.1210/endo-124-6-2954. [DOI] [PubMed] [Google Scholar]
- Waxman D. J. Rat hepatic cytochrome P-450 isoenzyme 2c. Identification as a male-specific, developmentally induced steroid 16 alpha-hydroxylase and comparison to a female-specific cytochrome P-450 isoenzyme. J Biol Chem. 1984 Dec 25;259(24):15481–15490. [PubMed] [Google Scholar]
- Wilson V. S., LeBlanc G. A. Endosulfan elevates testosterone biotransformation and clearance in CD-1 mice. Toxicol Appl Pharmacol. 1998 Jan;148(1):158–168. doi: 10.1006/taap.1997.8319. [DOI] [PubMed] [Google Scholar]
- Wolff M. S., Toniolo P. G., Lee E. W., Rivera M., Dubin N. Blood levels of organochlorine residues and risk of breast cancer. J Natl Cancer Inst. 1993 Apr 21;85(8):648–652. doi: 10.1093/jnci/85.8.648. [DOI] [PubMed] [Google Scholar]
- Wong B. K., Fei P., Kong A. N. Differential induction of UDP-glucuronosyltransferase activity and gene expression in rat liver. Pharm Res. 1995 Jul;12(7):1105–1108. doi: 10.1023/a:1016291305564. [DOI] [PubMed] [Google Scholar]
- de Lacerda L., Kowarski A., Johanson A. J., Athanasiou R., Migeon C. J. Integrated concentration and circadian variation of plasma testosterone in normal men. J Clin Endocrinol Metab. 1973 Sep;37(3):366–371. doi: 10.1210/jcem-37-3-366. [DOI] [PubMed] [Google Scholar]
- van der Hoeven T. A., Coon M. J. Preparation and properties of partially purified cytochrome P-450 and reduced nicotinamide adenine dinucleotide phosphate-cytochrome P-450 reductase from rabbit liver microsomes. J Biol Chem. 1974 Oct 10;249(19):6302–6310. [PubMed] [Google Scholar]