Abstract
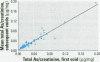
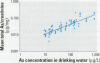
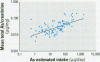
Urinary arsenic (As) concentrations were evaluated as a biomarker of exposure in a U.S. population chronically exposed to inorganic As (InAs) in their drinking water. Ninety-six individuals who consumed drinking water with As concentrations of 8-620 microg/L provided first morning urine voids for up to 5 consecutive days. The study population was 56% male, and 44% was younger than 18 years of age. On one day of the study period, all voided urines were collected over a 24-hr period. Arsenic intake from drinking water was estimated from daily food diaries. Comparison between the concentration of As in individual urine voids with that in the 24-hr urine collection indicated that the concentration of As in urine was stable throughout the day. Comparison of the concentration of As in each first morning urine void over the 5-day study period indicated that there was little day-to-day variation in the concentration of As in urine. The concentration of As in drinking water was a better predictor of the concentration of As in urine than was the estimated intake of As from drinking water. The concentration of As in urine did not vary by gender. An age-dependent difference in the concentration of As in urine may be attributed to the higher As dosage rate per unit body weight in children than in adults. These findings suggest that the analysis of a small number of urine samples may be adequate to estimate an individual's exposure to InAs from drinking water and that the determination of the concentration of InAs in a drinking water supply may be a useful surrogate for estimating exposure to this metalloid.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achari R., Mayersohn M., Conrad K. A. HPLC analysis of creatinine in human plasma and urine. J Chromatogr Sci. 1983 Jun;21(6):278–281. doi: 10.1093/chromsci/21.6.278. [DOI] [PubMed] [Google Scholar]
- Bates M. N., Smith A. H., Hopenhayn-Rich C. Arsenic ingestion and internal cancers: a review. Am J Epidemiol. 1992 Mar 1;135(5):462–476. doi: 10.1093/oxfordjournals.aje.a116313. [DOI] [PubMed] [Google Scholar]
- Brown K. G., Chen C. J. Significance of exposure assessment to analysis of cancer risk from inorganic arsenic in drinking water in Taiwan. Risk Anal. 1995 Aug;15(4):475–484. doi: 10.1111/j.1539-6924.1995.tb00340.x. [DOI] [PubMed] [Google Scholar]
- Chen C. J., Chen C. W., Wu M. M., Kuo T. L. Cancer potential in liver, lung, bladder and kidney due to ingested inorganic arsenic in drinking water. Br J Cancer. 1992 Nov;66(5):888–892. doi: 10.1038/bjc.1992.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. J., Chiou H. Y., Chiang M. H., Lin L. J., Tai T. Y. Dose-response relationship between ischemic heart disease mortality and long-term arsenic exposure. Arterioscler Thromb Vasc Biol. 1996 Apr;16(4):504–510. doi: 10.1161/01.atv.16.4.504. [DOI] [PubMed] [Google Scholar]
- Chen C. J., Hsueh Y. M., Lai M. S., Shyu M. P., Chen S. Y., Wu M. M., Kuo T. L., Tai T. Y. Increased prevalence of hypertension and long-term arsenic exposure. Hypertension. 1995 Jan;25(1):53–60. [PubMed] [Google Scholar]
- Chiou H. Y., Hsueh Y. M., Liaw K. F., Horng S. F., Chiang M. H., Pu Y. S., Lin J. S., Huang C. H., Chen C. J. Incidence of internal cancers and ingested inorganic arsenic: a seven-year follow-up study in Taiwan. Cancer Res. 1995 Mar 15;55(6):1296–1300. [PubMed] [Google Scholar]
- Chiou H. Y., Huang W. I., Su C. L., Chang S. F., Hsu Y. H., Chen C. J. Dose-response relationship between prevalence of cerebrovascular disease and ingested inorganic arsenic. Stroke. 1997 Sep;28(9):1717–1723. doi: 10.1161/01.str.28.9.1717. [DOI] [PubMed] [Google Scholar]
- Concha G., Nermell B., Vahter M. V. Metabolism of inorganic arsenic in children with chronic high arsenic exposure in northern Argentina. Environ Health Perspect. 1998 Jun;106(6):355–359. doi: 10.1289/ehp.98106355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabeka R. W., McKenzie A. D., Lacroix G. M., Cleroux C., Bowe S., Graham R. A., Conacher H. B., Verdier P. Survey of arsenic in total diet food composites and estimation of the dietary intake of arsenic by Canadian adults and children. J AOAC Int. 1993 Jan-Feb;76(1):14–25. [PubMed] [Google Scholar]
- Del Razo L. M., García-Vargas G. G., Vargas H., Albores A., Gonsebatt M. E., Montero R., Ostrosky-Wegman P., Kelsh M., Cebrián M. E. Altered profile of urinary arsenic metabolites in adults with chronic arsenicism. A pilot study. Arch Toxicol. 1997;71(4):211–217. doi: 10.1007/s002040050378. [DOI] [PubMed] [Google Scholar]
- Hopenhayn-Rich C., Smith A. H., Goeden H. M. Human studies do not support the methylation threshold hypothesis for the toxicity of inorganic arsenic. Environ Res. 1993 Feb;60(2):161–177. doi: 10.1006/enrs.1993.1024. [DOI] [PubMed] [Google Scholar]
- Ihrig M. M., Shalat S. L., Baynes C. A hospital-based case-control study of stillbirths and environmental exposure to arsenic using an atmospheric dispersion model linked to a geographical information system. Epidemiology. 1998 May;9(3):290–294. [PubMed] [Google Scholar]
- Lai M. S., Hsueh Y. M., Chen C. J., Shyu M. P., Chen S. Y., Kuo T. L., Wu M. M., Tai T. Y. Ingested inorganic arsenic and prevalence of diabetes mellitus. Am J Epidemiol. 1994 Mar 1;139(5):484–492. doi: 10.1093/oxfordjournals.aje.a117031. [DOI] [PubMed] [Google Scholar]
- Le X. C., Ma M. Short-column liquid chromatography with hydride generation atomic fluorescence detection for the speciation of arsenic. Anal Chem. 1998 May 1;70(9):1926–1933. doi: 10.1021/ac971247q. [DOI] [PubMed] [Google Scholar]
- Ma M., Le X. C. Effect of arsenosugar ingestion on urinary arsenic speciation. Clin Chem. 1998 Mar;44(3):539–550. [PubMed] [Google Scholar]
- Rahman M., Tondel M., Ahmad S. A., Axelson O. Diabetes mellitus associated with arsenic exposure in Bangladesh. Am J Epidemiol. 1998 Jul 15;148(2):198–203. doi: 10.1093/oxfordjournals.aje.a009624. [DOI] [PubMed] [Google Scholar]
- Roseberry A. M., Burmaster D. E. Lognormal distributions for water intake by children and adults. Risk Anal. 1992 Mar;12(1):99–104. doi: 10.1111/j.1539-6924.1992.tb01312.x. [DOI] [PubMed] [Google Scholar]
- Tseng C. H., Chong C. K., Chen C. J., Lin B. J., Tai T. Y. Abnormal peripheral microcirculation in seemingly normal subjects living in blackfoot-disease-hyperendemic villages in Taiwan. Int J Microcirc Clin Exp. 1995 Jan-Feb;15(1):21–27. doi: 10.1159/000178945. [DOI] [PubMed] [Google Scholar]