Abstract
This study examined work-related chronic abnormality in pulmonary function and work-related acute irritant symptoms associated with exposure to borate dust in mining and processing operations. Chronic effects were examined by pulmonary function at the beginning and end of a 7-year interval. Time-specific estimates of sodium borate particulate exposures were used to estimate cumulative exposure during the study interval. Change in pulmonary function over the 7 years was found unrelated to the estimate of cumulative exposure during that interval. Exposure-response associations also were examined with respect to short-term peak exposures and incidence of five symptoms of acute respiratory irritation. Hourly measures of health outcome and continuous measures of particulate exposure were made on each subject throughout the day. Whenever a subject reported one of the irritant symptoms, a symptom intensity score was also recorded along with the approximate time of onset. The findings indicated that exposure-response relationships were present for each of the specific symptoms at several symptom intensity levels. The associations were present when exposure was estimated by both day-long and short-term (15-min) time-weighted average exposures. Associations persisted after taking account of smoking, age, and the presence of a common cold. No significant difference in response rate was found between workers exposed to different types of sodium borate dusts.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. D., Schoeffel R. E., Finney M. Evaluation of ultrasonically nebulised solutions for provocation testing in patients with asthma. Thorax. 1983 Apr;38(4):284–291. doi: 10.1136/thx.38.4.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcher N. G., Rees P. J., Clark T. J., Lee T. H. A comparison of the refractory periods induced by hypertonic airway challenge and exercise in bronchial asthma. Am Rev Respir Dis. 1987 Apr;135(4):822–825. doi: 10.1164/arrd.1987.135.4.822. [DOI] [PubMed] [Google Scholar]
- Borg G. A. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–381. [PubMed] [Google Scholar]
- Borg G. Psychophysical scaling with applications in physical work and the perception of exertion. Scand J Work Environ Health. 1990;16 (Suppl 1):55–58. doi: 10.5271/sjweh.1815. [DOI] [PubMed] [Google Scholar]
- Boulet L. P., Legris C., Thibault L., Turcotte H. Comparative bronchial responses to hyperosmolar saline and methacholine in asthma. Thorax. 1987 Dec;42(12):953–958. doi: 10.1136/thx.42.12.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggleston P. A., Kagey-Sobotka A., Lichtenstein L. M. A comparison of the osmotic activation of basophils and human lung mast cells. Am Rev Respir Dis. 1987 May;135(5):1043–1048. doi: 10.1164/arrd.1987.135.5.1043. [DOI] [PubMed] [Google Scholar]
- Eisen E. A., Wegman D. H., Kriebel D., Woskie S. R., Hu X. An epidemiologic approach to the study of acute reversible health effects in the workplace. Epidemiology. 1991 Jul;2(4):263–270. doi: 10.1097/00001648-199107000-00005. [DOI] [PubMed] [Google Scholar]
- Eschenbacher W. L., Boushey H. A., Sheppard D. Alteration in osmolarity of inhaled aerosols cause bronchoconstriction and cough, but absence of a permeant anion causes cough alone. Am Rev Respir Dis. 1984 Feb;129(2):211–215. [PubMed] [Google Scholar]
- Findlay S. R., Dvorak A. M., Kagey-Sobotka A., Lichtenstein L. M. Hyperosmolar triggering of histamine release from human basophils. J Clin Invest. 1981 Jun;67(6):1604–1613. doi: 10.1172/JCI110195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney M. J., Anderson S. D., Black J. L. The effect of non-isotonic solutions on human isolated airway smooth muscle. Respir Physiol. 1987 Sep;69(3):277–286. doi: 10.1016/0034-5687(87)90082-x. [DOI] [PubMed] [Google Scholar]
- Garabrant D. H., Bernstein L., Peters J. M., Smith T. J., Wright W. E. Respiratory effects of borax dust. Br J Ind Med. 1985 Dec;42(12):831–837. doi: 10.1136/oem.42.12.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godden D. J., Borland C., Lowry R., Higenbottam T. W. Chemical specificity of coughing in man. Clin Sci (Lond) 1986 Mar;70(3):301–306. doi: 10.1042/cs0700301. [DOI] [PubMed] [Google Scholar]
- Harms-Ringdahl K., Carlsson A. M., Ekholm J., Raustorp A., Svensson T., Toresson H. G. Pain assessment with different intensity scales in response to loading of joint structures. Pain. 1986 Dec;27(3):401–411. doi: 10.1016/0304-3959(86)90163-6. [DOI] [PubMed] [Google Scholar]
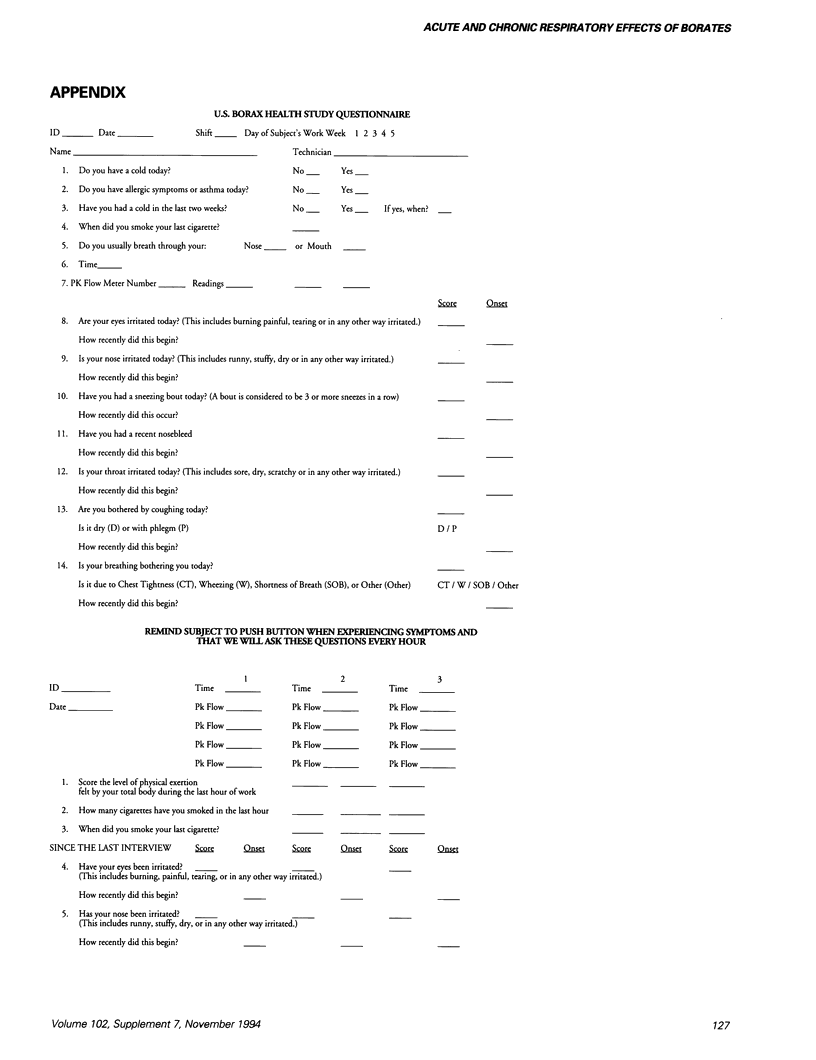
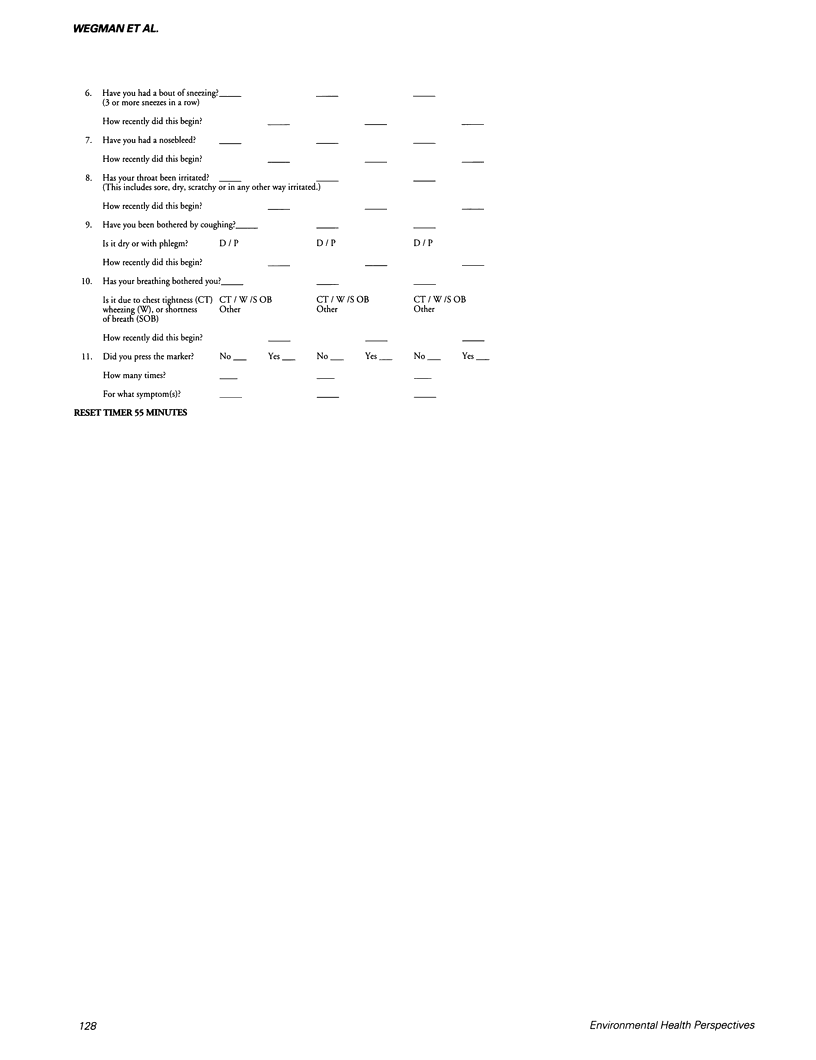
- Hu X., Wegman D. H., Eisen E. A., Woskie S. R., Smith R. G. Dose related acute irritant symptom responses to occupational exposure to sodium borate dusts. Br J Ind Med. 1992 Oct;49(10):706–713. doi: 10.1136/oem.49.10.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juniper E. F., Frith P. A., Dunnett C., Cockcroft D. W., Hargreave F. E. Reproducibility and comparison of responses to inhaled histamine and methacholine. Thorax. 1978 Dec;33(6):705–710. doi: 10.1136/thx.33.6.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivity S., Greif J., Reisner B., Fireman E., Topilsky M. Bronchial inhalation challenge with ultrasonically nebulized saline; comparison to exercise-induced asthma. Ann Allergy. 1986 Nov;57(5):355–358. [PubMed] [Google Scholar]
- Knudson R. J., Slatin R. C., Lebowitz M. D., Burrows B. The maximal expiratory flow-volume curve. Normal standards, variability, and effects of age. Am Rev Respir Dis. 1976 May;113(5):587–600. doi: 10.1164/arrd.1976.113.5.587. [DOI] [PubMed] [Google Scholar]
- Mygind N., Borum P., Secher C., Kirkegaard J. Nasal challenge. Eur J Respir Dis Suppl. 1986;143:31–34. [PubMed] [Google Scholar]
- Patterson R., Tomita Y., Oh S. H., Suszko I. M., Pruzansky J. J. Respiratory mast cells and basophiloid cells. I. Evidence that they are secreted into the bronchial lumen, morphology, degranulation and histamine release. Clin Exp Immunol. 1974 Feb;16(2):223–234. [PMC free article] [PubMed] [Google Scholar]
- Rubow K. L., Marple V. A., Olin J., McCawley M. A. A personal cascade impactor: design, evaluation and calibration. Am Ind Hyg Assoc J. 1987 Jun;48(6):532–538. doi: 10.1080/15298668791385174. [DOI] [PubMed] [Google Scholar]
- Sheppard D., Rizk N. W., Boushey H. A., Bethel R. A. Mechanism of cough and bronchoconstriction induced by distilled water aerosol. Am Rev Respir Dis. 1983 Jun;127(6):691–694. doi: 10.1164/arrd.1983.127.6.691. [DOI] [PubMed] [Google Scholar]


