Abstract
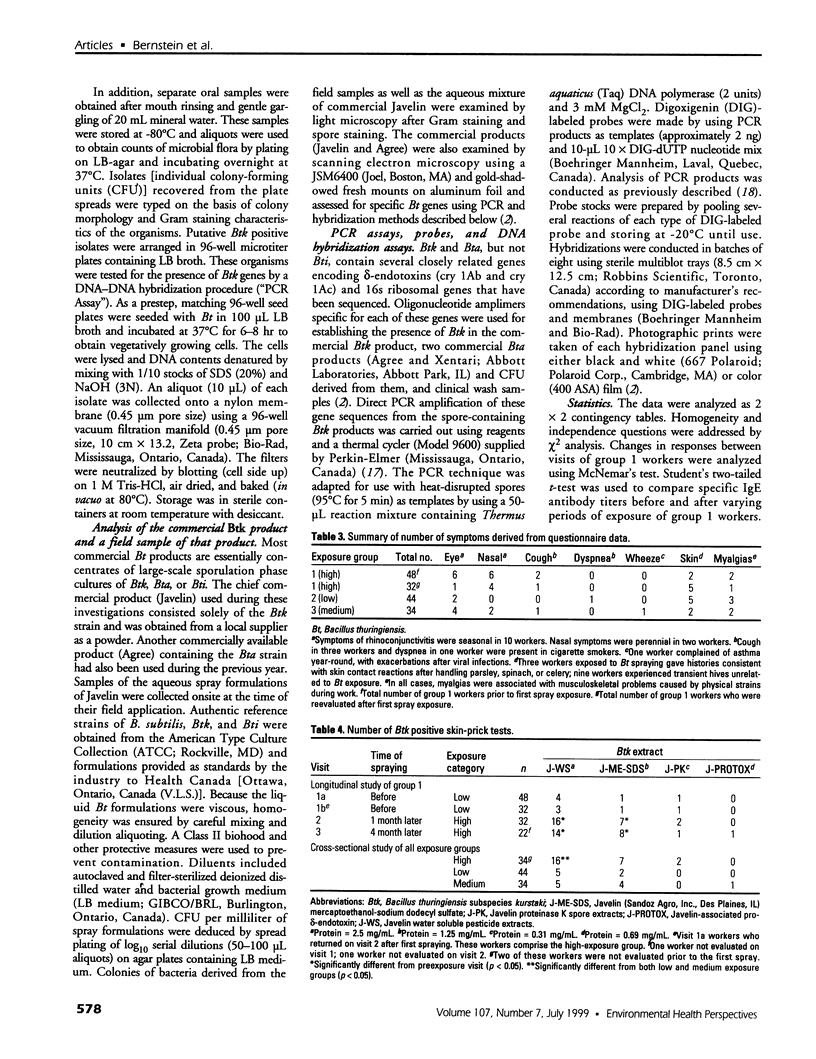
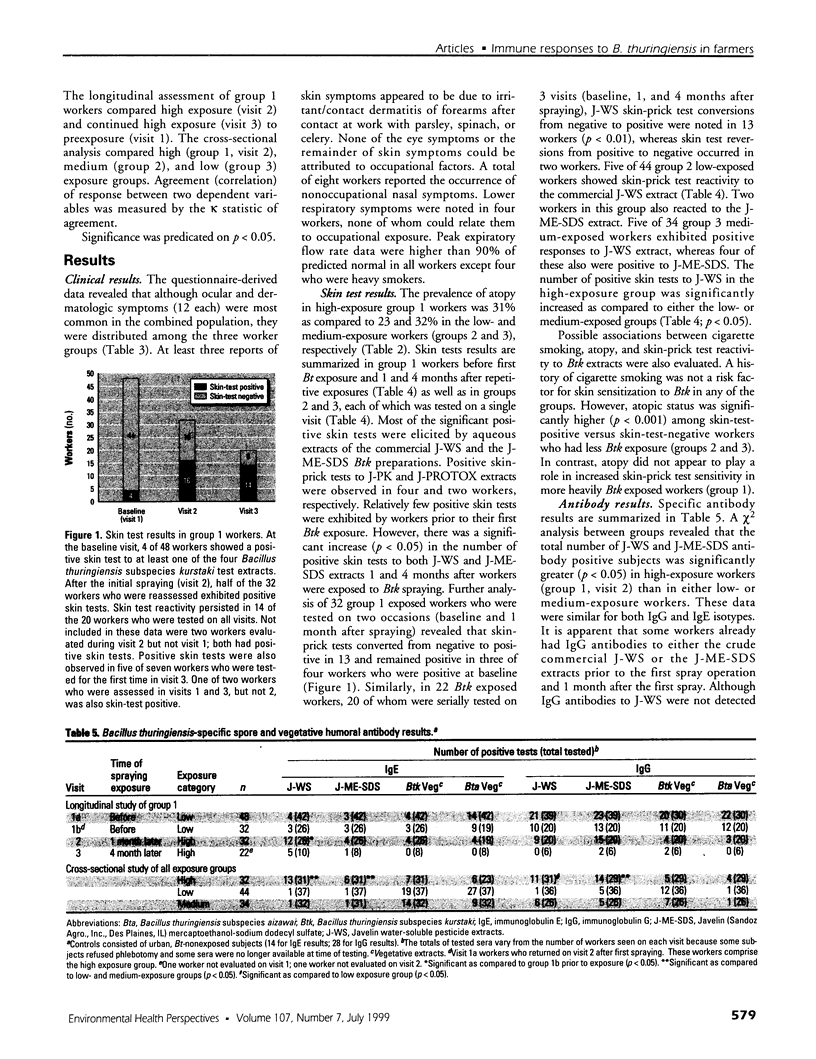
Although health risks to pesticides containing Bacillus thuringiensis (Bt) have been minimal, the potential allergenicity of these organisms has not been evaluated. Therefore, a health survey was conducted in farm workers before and after exposure to Bt pesticides. Farm workers who picked vegetables that required Bt pesticide spraying were evaluated before the initial spraying operation (n = 48) and 1 and 4 months after (n = 32 and 20, respectively). Two groups of low- (n = 44) and medium- (n = 34) exposure workers not directly exposed to Bt spraying were also assessed. The investigation included questionnaires, nasal/mouth lavages, ventilatory function assessment, and skin tests to indigenous aeroallergens and to a variety of Bt spore and vegetative preparations. To authenticate exposure to the organism present in the commercial preparation, isolates from lavage specimens were tested for Bt genes by DNA-DNA hybridization. Humoral immunoglobulin G (IgG) and immunoglobulin E (IgE) antibody responses to spore and vegetative Bt extracts were assayed. There was no evidence of occupationally related respiratory symptoms. Positive skin-prick tests to several spore extracts were seen chiefly in exposed workers. In particular, there was a significant (p < 0.05) increase in the number of positive skin tests to spore extracts 1 and 4 months after exposure to Bt spray. The number of positive skin test responses was also significantly higher in high (p < 0.05) than in low- or medium-exposure workers. The majority of nasal lavage cultures from exposed workers was positive for the commercial Bt organism, as demonstrated by specific molecular genetic probes. Specific IgE antibodies were present in more high-exposure workers (p < 0.05) than in the low and medium groups. Specific IgG antibodies occurred more in the high (p < 0.05) than in the low-exposure group. Specific IgG and IgE antibodies to vegetative organisms were present in all groups of workers. Exposure to Bt sprays may lead to allergic skin sensitization and induction of IgE and IgG antibodies, or both.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein D. I., Korbee L., Stauder T., Bernstein J. A., Scinto J., Herd Z. L., Bernstein I. L. The low prevalence of occupational asthma and antibody-dependent sensitization to diphenylmethane diisocyanate in a plant engineered for minimal exposure to diisocyanates. J Allergy Clin Immunol. 1993 Sep;92(3):387–396. doi: 10.1016/0091-6749(93)90117-x. [DOI] [PubMed] [Google Scholar]
- Bernstein I. L. Enzyme allergy in populations exposed to long-term, low-level concentrations of household laundry products. J Allergy Clin Immunol. 1972 Apr;49(4):219–237. doi: 10.1016/0091-6749(72)90085-1. [DOI] [PubMed] [Google Scholar]
- Brarda O. A., Vanella L. M., Boudet R. V. Anti-Staphylococcus aureus, anti-Streptococcus pneumoniae and anti-Moraxella catarrhalis specific IgE in asthmatic children. J Investig Allergol Clin Immunol. 1996 Jul-Aug;6(4):266–269. [PubMed] [Google Scholar]
- Damgaard P. H., Larsen H. D., Hansen B. M., Bresciani J., Jørgensen K. Enterotoxin-producing strains of Bacillus thuringiensis isolated from food. Lett Appl Microbiol. 1996 Sep;23(3):146–150. doi: 10.1111/j.1472-765x.1996.tb00051.x. [DOI] [PubMed] [Google Scholar]
- Drobniewski F. A., Ellar D. J. Purification and properties of a 28-kilodalton hemolytic and mosquitocidal protein toxin of Bacillus thuringiensis subsp. darmstadiensis 73-E10-2. J Bacteriol. 1989 Jun;171(6):3060–3067. doi: 10.1128/jb.171.6.3060-3067.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz T., McMurrain K. D., Brooks S., Bernstein I. L. Clinical, immunologic, and physiologic observations in factory workers exposed to B. subtilis enzyme dust. J Allergy. 1971 Mar;47(3):170–180. doi: 10.1016/s0091-6749(71)80295-6. [DOI] [PubMed] [Google Scholar]
- Goodman R. M., Hauptli H., Crossway A., Knauf V. C. Gene transfer in crop improvement. Science. 1987 Apr 3;236(4797):48–54. doi: 10.1126/science.236.4797.48. [DOI] [PubMed] [Google Scholar]
- Green M., Heumann M., Sokolow R., Foster L. R., Bryant R., Skeels M. Public health implications of the microbial pesticide Bacillus thuringiensis: an epidemiological study, Oregon, 1985-86. Am J Public Health. 1990 Jul;80(7):848–852. doi: 10.2105/ajph.80.7.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C. L., Bernstein I. L., Gallagher J. S., Bonventre P. F., Brooks S. M. Familial hypersensitivity pneumonitis induced by Bacillus subtilis. Am Rev Respir Dis. 1980 Aug;122(2):339–348. doi: 10.1164/arrd.1980.122.2.339. [DOI] [PubMed] [Google Scholar]
- Leung D. Y., Harbeck R., Bina P., Reiser R. F., Yang E., Norris D. A., Hanifin J. M., Sampson H. A. Presence of IgE antibodies to staphylococcal exotoxins on the skin of patients with atopic dermatitis. Evidence for a new group of allergens. J Clin Invest. 1993 Sep;92(3):1374–1380. doi: 10.1172/JCI116711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., Walker T., MacManus J. P., Seligy V. L. Differentiation of HT-29 human colonic adenocarcinoma cells correlates with increased expression of mitochondrial RNA: effects of trehalose on cell growth and maturation. Cancer Res. 1992 Jul 1;52(13):3718–3725. [PubMed] [Google Scholar]
- Nunn A. J., Gregg I. New regression equations for predicting peak expiratory flow in adults. BMJ. 1989 Apr 22;298(6680):1068–1070. doi: 10.1136/bmj.298.6680.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepys J., Longbottom J. L., Hargreave F. E., Faux J. Allergic reactions of the lungs to enzymes of Bacillus subtilis. Lancet. 1969 Jun 14;1(7607):1181–1184. doi: 10.1016/s0140-6736(69)92166-7. [DOI] [PubMed] [Google Scholar]
- Platts-Mills T. A., Carter M. C. Asthma and indoor exposure to allergens. N Engl J Med. 1997 May 8;336(19):1382–1384. doi: 10.1056/NEJM199705083361909. [DOI] [PubMed] [Google Scholar]
- Smith R. A., Couche G. A. The Phylloplane as a Source of Bacillus thuringiensis Variants. Appl Environ Microbiol. 1991 Jan;57(1):311–315. doi: 10.1128/aem.57.1.311-315.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelma G. N., Jr, Aronson A. I., Fitz-James P. Properties of Bacillus cereus temperature-sensitive mutants altered in spore coat formation. J Bacteriol. 1978 Jun;134(3):1157–1170. doi: 10.1128/jb.134.3.1157-1170.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayabali A. F., Seligy V. L. Cell integrity markers for in vitro evaluation of cytotoxic responses to bacteria-containing commercial insecticides. Ecotoxicol Environ Saf. 1997 Jul;37(2):152–162. doi: 10.1006/eesa.1997.1525. [DOI] [PubMed] [Google Scholar]