Abstract
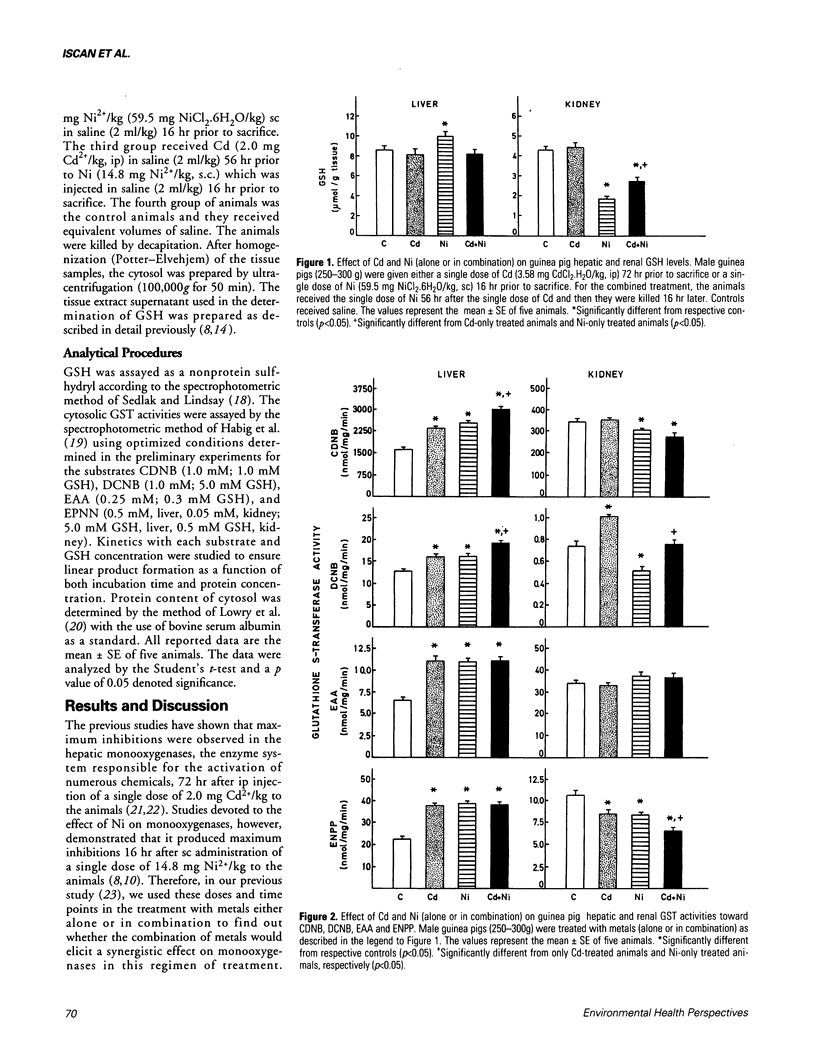
When male guinea pigs were given a single dose of Cd (2.0 mg Cd2+/kg, ip) 72 hr prior to sacrifice, the hepatic reduced glutathione (GSH) level did not change although glutathione S-transferase (GST) activities toward the substrates 1-chloro-2,4-dinitrobenzene (CDNB), 1,2-dichloro-4-nitrobenzene (DCNB), ethacrynic acid (EAA), and 1,2-epoxy-3-(p-nitrophenoxy) propane (ENPP) increased significantly as compared to controls. Cd did not change the renal GSH level and GST activities toward CDNB and EAA. However, significant increase was observed in the GST activity for DCNB whereas GST activity for ENPP was significantly inhibited by Cd. When the animals were given a single dose of Ni (14.8 mg Ni2+/kg, sc) 16 hr prior to sacrifice, significant increases were observed in hepatic GSH level and GST activities toward CDNB, DCNB, EAA and ENPP. Ni, however, depressed the renal GSH level and GST activities toward CDNB, DCNB and ENPP significantly. The renal GST activity toward EAA remained unaltered. For the combined treatment, guinea pigs received the single dose of Ni 56 hr after the single dose of Cd and then they were killed 16 hr later. In these animals, no significant alteration was observed in the hepatic GSH level. The augmentation of elevation was observed in hepatic GST activities toward CDNB and DCNB. Combined metal treatment did not potentiate the elevation of hepatic GST activities toward EAA and ENPP to any greater degree. The depression of renal GSH level was significantly ameliorated by the combined treatment. Combination treatment potentiated the depression of renal GST activity for ENPP but not for CDNB.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chasseaud L. F. The role of glutathione and glutathione S-transferases in the metabolism of chemical carcinogens and other electrophilic agents. Adv Cancer Res. 1979;29:175–274. doi: 10.1016/s0065-230x(08)60848-9. [DOI] [PubMed] [Google Scholar]
- Davies M. H., Schnell R. C. Comparison of basal glutathione S-transferase activities and of the influence of phenobarbital, butylated hydroxy-anisole or 5,5'-diphenylhydantoin on enzyme activity in male rodents. Comp Biochem Physiol C. 1987;88(1):91–93. doi: 10.1016/0742-8413(87)90051-x. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Hales B. J., Neims A. H. Induction of rat hepatic glutathione S-transferase B by phenobarbital and 3-methylcholanthrene. Biochem Pharmacol. 1977 Mar 15;26(6):555–556. doi: 10.1016/0006-2952(77)90337-9. [DOI] [PubMed] [Google Scholar]
- Igarashi T., Tomihari N., Ohmori S., Ueno K., Kitagawa H., Satoh T. Comparison of glutathione S-transferases in mouse, guinea pig, rabbit and hamster liver cytosol to those in rat liver. Biochem Int. 1986 Oct;13(4):641–648. [PubMed] [Google Scholar]
- Işcan M., Karakaya A. Cadmium sensitivity differences between liver microsomal drug metabolizing enzyme systems of guinea-pig and rat. Comp Biochem Physiol C. 1988;90(1):101–105. doi: 10.1016/0742-8413(88)90105-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mannervik B., Danielson U. H. Glutathione transferases--structure and catalytic activity. CRC Crit Rev Biochem. 1988;23(3):283–337. doi: 10.3109/10409238809088226. [DOI] [PubMed] [Google Scholar]
- Misra M., Rodriguez R. E., Kasprzak K. S. Nickel induced lipid peroxidation in the rat: correlation with nickel effect on antioxidant defense systems. Toxicology. 1990 Oct;64(1):1–17. doi: 10.1016/0300-483x(90)90095-x. [DOI] [PubMed] [Google Scholar]
- Oshino R., Kamei K., Nishioka M., Shin M. Purification and characterization of glutathione S-transferases from guinea pig liver. J Biochem. 1990 Jan;107(1):105–110. doi: 10.1093/oxfordjournals.jbchem.a122991. [DOI] [PubMed] [Google Scholar]
- Planas-Bohne F., Elizalde M. Activity of glutathione-S-transferase in rat liver and kidneys after administration of lead or cadmium. Arch Toxicol. 1992;66(5):365–367. doi: 10.1007/BF01973633. [DOI] [PubMed] [Google Scholar]
- Schnell R. C. Cadmium-induced alteration of drug action. Fed Proc. 1978 Jan;37(1):28–34. [PubMed] [Google Scholar]
- Seagrave J., Hildebrand C. E., Enger M. D. Effects of cadmium on glutathione metabolism in cadmium sensitive and cadmium resistant Chinese hamster cell lines. Toxicology. 1983 Dec;29(1-2):101–107. doi: 10.1016/0300-483x(83)90042-2. [DOI] [PubMed] [Google Scholar]
- Sedlak J., Lindsay R. H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968 Oct 24;25(1):192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- Siegers C. P., Schenke M., Younes M. Influence of cadmium chloride, mercuric chloride, and sodium vanadate on the glutathione-conjugating enzyme system in liver, kidney, and brain of mice. J Toxicol Environ Health. 1987;22(2):141–148. doi: 10.1080/15287398709531058. [DOI] [PubMed] [Google Scholar]
- Sunderman F. W., Jr, Marzouk A., Hopfer S. M., Zaharia O., Reid M. C. Increased lipid peroxidation in tissues of nickel chloride-treated rats. Ann Clin Lab Sci. 1985 May-Jun;15(3):229–236. [PubMed] [Google Scholar]
- Sunderman F. W., Jr, Zaharia O., Reid M. C., Belliveau J. F., O'Leary G. P., Jr, Griffin H. Effects of diethyldithiocarbamate and nickel chloride on glutathione and trace metal concentrations in rat liver. Toxicology. 1984 Jul;32(1):11–21. doi: 10.1016/0300-483x(84)90030-1. [DOI] [PubMed] [Google Scholar]
- Vos R. M., Van Bladeren P. J. Glutathione S-transferases in relation to their role in the biotransformation of xenobiotics. Chem Biol Interact. 1990;75(3):241–265. doi: 10.1016/0009-2797(90)90069-y. [DOI] [PubMed] [Google Scholar]


