Abstract
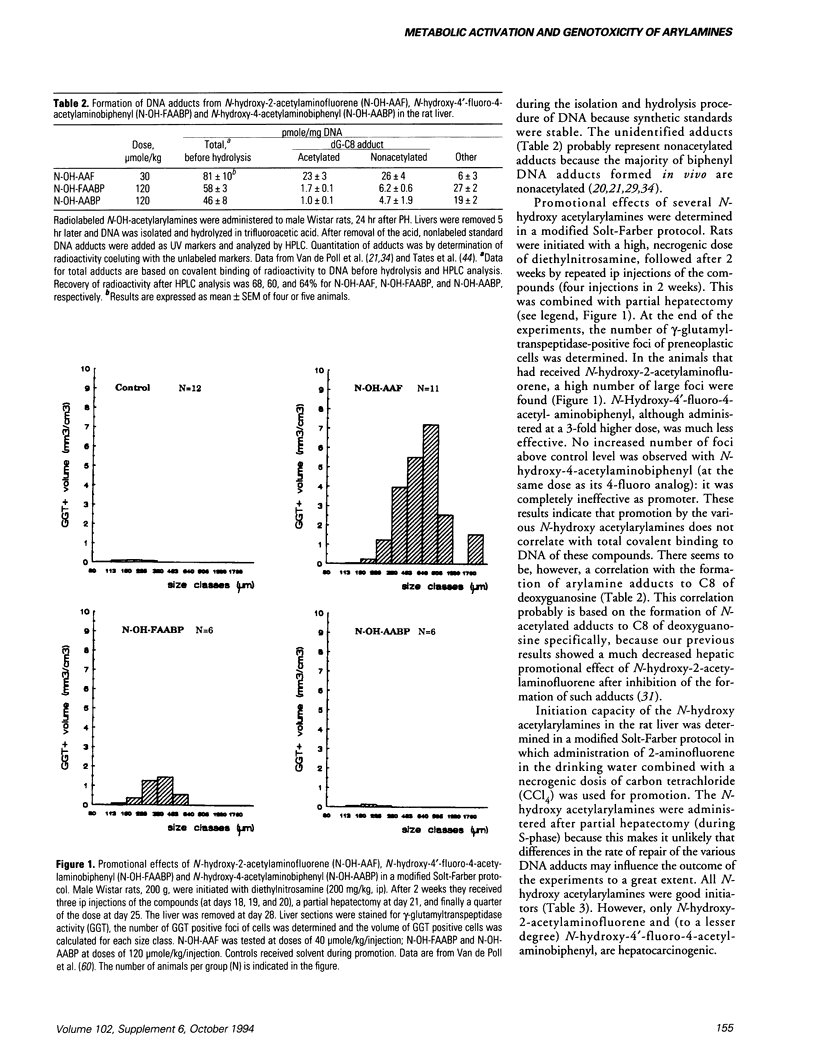
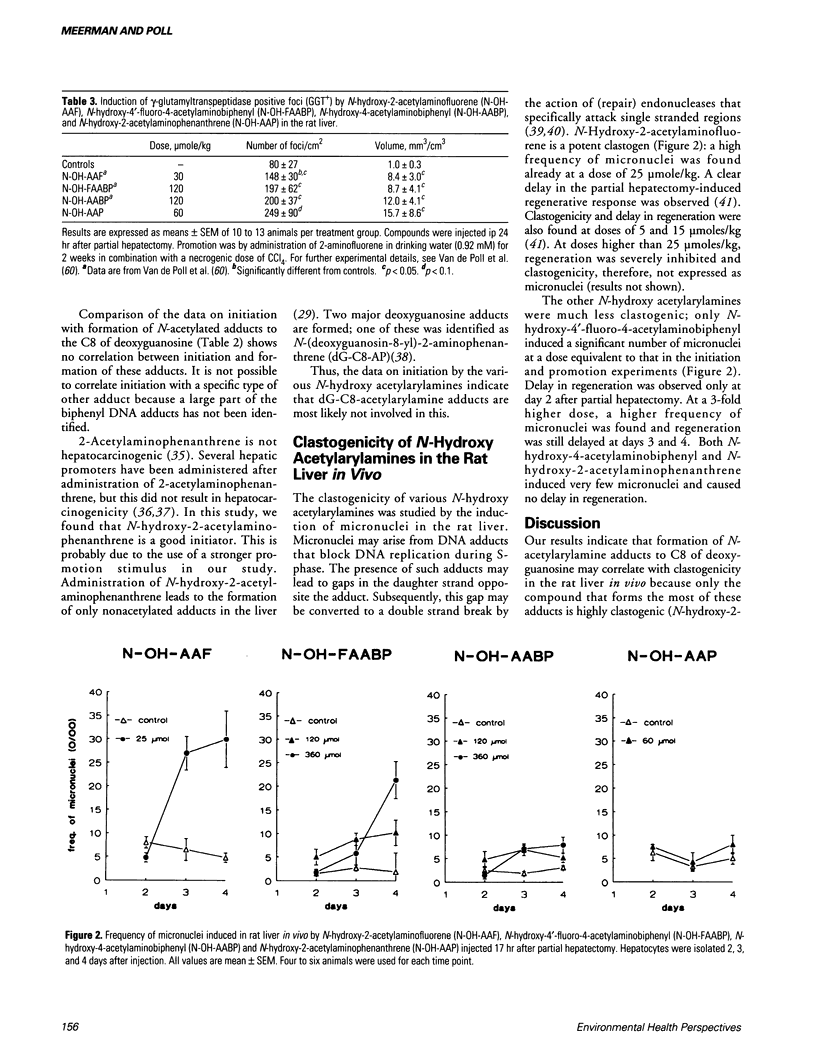
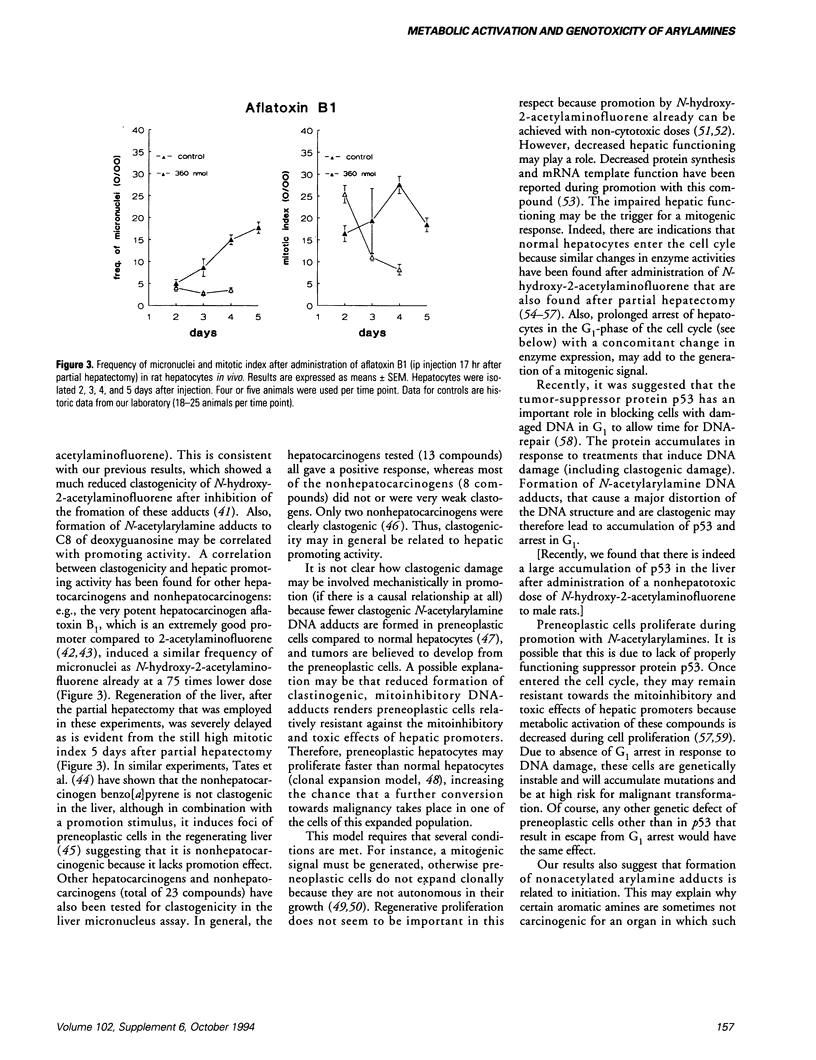
Two different types of DNA adducts are formed from many aromatic amines by bioactivation: N-acetylated and nonacetylated, arylamine DNA adducts. It has become clear from experiments using N-acetyl-2-aminofluorene and 2-aminofluorene adducts to C8 of deoxyguanosine that these two types of adducts may have different effects on DNA structure and DNA replication. We have determined blocking of DNA replication by various other N-acetylarylamine and arylamine deoxyguanosine adducts. It was found that the N-acetyl group in general is required for blocking of DNA replication; the nature of the aromatic moiety seems to be of minor importance. Little information is available on the genotoxic effects of these adducts in mammalian cells in vivo. We have tried to get more insight in this by investigating the clastogenicity, the initiation of preneoplastic cells, and the promotional effects of various aromatic amines from which different ratios of N-acetylarylamine DNA adducts to arylamine DNA adducts are formed in the rat liver. Our results show that formation of N-acetylarylamine adducts to C8 of deoxyguanosine in the liver is correlated with clastogenicity and hepatic promoting effect. Initiation capacities, however, seem to be correlated with formation of nonacetylated, arylamine adducts. Mechanisms by which formation of N-acetylarylamine DNA adducts may generate a promoting effect in the liver are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beland F. A., Beranek D. T., Dooley K. L., Heflich R. H., Kadlubar F. F. Arylamine-DNA adducts in vitro and in vivo: their role in bacterial mutagenesis and urinary bladder carcinogenesis. Environ Health Perspect. 1983 Mar;49:125–134. doi: 10.1289/ehp.8349125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beland F. A., Dooley K. L., Casciano D. A. Rapid isolation of carcinogen-bound DNA and RNA by hydroxyapatite chromatography. J Chromatogr. 1979 Jun 1;174(1):177–186. doi: 10.1016/s0021-9673(00)87048-x. [DOI] [PubMed] [Google Scholar]
- Beland F. A., Dooley K. L., Jackson C. D. Persistence of DNA adducts in rat liver and kidney after multiple doses of the carcinogen N-hydroxy-2-acetylaminofluorene. Cancer Res. 1982 Apr;42(4):1348–1354. [PubMed] [Google Scholar]
- Daune M. P., Fuchs R. P., Leng M. Structural modification and protein recognition of DNA modified by N-2-fluorenylacetamide, its 7-iodo derivative, and by N-2-fluorenamine. Natl Cancer Inst Monogr. 1981 Dec;(58):201–210. [PubMed] [Google Scholar]
- DeBaun J. R., Miller E. C., Miller J. A. N-hydroxy-2-acetylaminofluorene sulfotransferase: its probable role in carcinogenesis and in protein-(methion-S-yl) binding in rat liver. Cancer Res. 1970 Mar;30(3):577–595. [PubMed] [Google Scholar]
- Dock L., Scheu G., Jernström B., Martinez M., Torndal U. B., Eriksson L. Benzo[a]pyrene metabolism and induction of enzyme-altered foci in regenerating rat liver. Chem Biol Interact. 1988;67(3-4):243–253. doi: 10.1016/0009-2797(88)90061-0. [DOI] [PubMed] [Google Scholar]
- Enomoto K., Farber E. Kinetics of phenotypic maturation of remodeling of hyperplastic nodules during liver carcinogenesis. Cancer Res. 1982 Jun;42(6):2330–2335. [PubMed] [Google Scholar]
- Farber E. Clonal adaptation during carcinogenesis. Biochem Pharmacol. 1990 Jun 15;39(12):1837–1846. doi: 10.1016/0006-2952(90)90599-g. [DOI] [PubMed] [Google Scholar]
- Gilissen R. A., Meerman J. H. Bioactivation of the hepatocarcinogen N-hydroxy-2-acetylaminofluorene by sulfation in the rat liver changes during the cell cycle. Life Sci. 1992;51(16):1255–1260. doi: 10.1016/0024-3205(92)90014-g. [DOI] [PubMed] [Google Scholar]
- Gupta P. K., Pandrangi R. G., Lee M. S., King C. M. Induction of mutations by N-acetoxy-N-acetyl-2-aminofluorene modified M13 viral DNA. Carcinogenesis. 1991 May;12(5):819–824. doi: 10.1093/carcin/12.5.819. [DOI] [PubMed] [Google Scholar]
- Gupta R. C., Dighe N. R. Formation and removal of DNA adducts in rat liver treated with N-hydroxy derivatives of 2-acetylaminofluorene, 4-acetylaminobiphenyl, and 2-acetylaminophenanthrene. Carcinogenesis. 1984 Mar;5(3):343–349. doi: 10.1093/carcin/5.3.343. [DOI] [PubMed] [Google Scholar]
- Gupta R. C., Earley K., Becker F. F. Analysis of DNA adducts in putative premalignant hepatic nodules and nontarget tissues of rats during 2-acetylaminofluorene carcinogenesis. Cancer Res. 1988 Sep 15;48(18):5270–5274. [PubMed] [Google Scholar]
- Ito N., Tatematsu M., Nakanishi K., Hasegawa R., Takano T., Imaida K., Ogiso T. The effects of various chemicals on the development of hyperplastic liver nodules in hepatectomized rats treated with N-nitrosodiethylamine or N-2-fluorenylacetamide. Gan. 1980 Dec;71(6):832–842. [PubMed] [Google Scholar]
- Ito N., Tsuda H., Tatematsu M., Inoue T., Tagawa Y., Aoki T., Uwagawa S., Kagawa M., Ogiso T., Masui T. Enhancing effect of various hepatocarcinogens on induction of preneoplastic glutathione S-transferase placental form positive foci in rats--an approach for a new medium-term bioassay system. Carcinogenesis. 1988 Mar;9(3):387–394. doi: 10.1093/carcin/9.3.387. [DOI] [PubMed] [Google Scholar]
- Kaderbhai M. A., Bradshaw T. K., Freedman R. B. Alterations in the enzyme activity and polypeptide composition of rat hepatic endoplasmic reticulum during acute exposure to 2-acetylaminofluorene. Chem Biol Interact. 1982 Apr;39(3):279–299. doi: 10.1016/0009-2797(82)90046-1. [DOI] [PubMed] [Google Scholar]
- Koffel-Schwartz N., Maenhaut-Michel G., Fuchs R. P. Specific strand loss in N-2-acetylaminofluorene-modified DNA. J Mol Biol. 1987 Feb 20;193(4):651–659. doi: 10.1016/0022-2836(87)90348-2. [DOI] [PubMed] [Google Scholar]
- Kondo S., Carr B. I., Takagi K., Huang T. H., Chou Y. M., Yokoyama K., Itakura K. Expression of rat microsomal epoxide hydrolase gene during liver chemical carcinogenesis. Cancer Res. 1990 Oct 1;50(19):6222–6228. [PubMed] [Google Scholar]
- Kriek E., Hengeveld G. M. Reaction products of the carcinogen N-hydroxy-4-acetylamino-4'-fluorobiphenyl with DNA in liver and kidney of the rat. Chem Biol Interact. 1978 Jun;21(2-3):179–201. doi: 10.1016/0009-2797(78)90018-2. [DOI] [PubMed] [Google Scholar]
- Kriek E. Persistent binding of a new reaction product of the carcinogen N-hydroxy-N-2-acetylaminofluorene with guanine in rat liver DNA in vivo. Cancer Res. 1972 Oct;32(10):2042–2048. [PubMed] [Google Scholar]
- Kroese E. D., van de Poll M. L., Mulder G. J., Meerman J. H. The role of N-sulfation in the N-hydroxy-2-acetylaminofluorene-mediated outgrowth of diethylnitrosamine-initiated hepatocytes to gamma-glutamyltranspeptidase-positive foci in male rat liver. Carcinogenesis. 1988 Nov;9(11):1953–1958. doi: 10.1093/carcin/9.11.1953. [DOI] [PubMed] [Google Scholar]
- Lane D. P. Cancer. p53, guardian of the genome. Nature. 1992 Jul 2;358(6381):15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- Lutgerink J. T., Retèl J., Loman H. Effects of adduct formation on the biological activity of single- and double-stranded øX174 DNA, modified by N-acetoxy-N-acetyl-2-aminofluorene. Biochim Biophys Acta. 1984 Feb 24;781(1-2):81–91. doi: 10.1016/0167-4781(84)90126-x. [DOI] [PubMed] [Google Scholar]
- Lutgerink J. T., Retèl J., Westra J. G., Welling M. C., Loman H., Kriek E. By-pass of the major aminofluorene-DNA adduct during in vivo replication of single- and double-stranded phi X174 DNA treated with N-hydroxy-2-aminofluorene. Carcinogenesis. 1985 Oct;6(10):1501–1506. doi: 10.1093/carcin/6.10.1501. [DOI] [PubMed] [Google Scholar]
- MILLER E. C., MILLER J. A., HARTMANN H. A. N-Hydroxy-2-acetylaminofluorene: a metabolite of 2-acetylaminofluorene with increased carcinogenic activity in the rat. Cancer Res. 1961 Jul;21:815–824. [PubMed] [Google Scholar]
- MILLER J. A., SANDIN R. B., MILLER E. C., RUSCH H. P. The carcinogenicity of compounds related to 2-acetylaminofluorene. II. Variations in the bridges and the 2-substituent. Cancer Res. 1955 Mar;15(3):188–199. [PubMed] [Google Scholar]
- Meerman J. H., Mulder G. J. Prevention of the hepatotoxic action of N-hydroxy-2-acetylaminofluorene in the rat by inhibition of N-O-sulfation by pentachlorophenol. Life Sci. 1981 May 21;28(21):2361–2365. doi: 10.1016/0024-3205(81)90501-4. [DOI] [PubMed] [Google Scholar]
- Meerman J. H. The initiation of gamma-glutamyltranspeptidase positive foci in the rat liver by N-hydroxy-2-acetylaminofluorene. The effect of the sulfation inhibitor pentachlorophenol. Carcinogenesis. 1985 Jun;6(6):893–897. doi: 10.1093/carcin/6.6.893. [DOI] [PubMed] [Google Scholar]
- Moore P. D., Rabkin S. D., Strauss B. S. Termination of vitro DNA synthesis at AAF adducts in the DNA. Nucleic Acids Res. 1980 Oct 10;8(19):4473–4484. doi: 10.1093/nar/8.19.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriya M., Takeshita M., Johnson F., Peden K., Will S., Grollman A. P. Targeted mutations induced by a single acetylaminofluorene DNA adduct in mammalian cells and bacteria. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1586–1589. doi: 10.1073/pnas.85.5.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan A. T., Obe G., van Zeeland A. A., Palitti F., Meijers M., Verdegaal-Immerzeel E. A. Molecular mechanisms involved in the production of chromosomal aberrations. II. Utilization of Neurospora endonuclease for the study of aberration production by X-rays in G1 and G2 stages of the cell cycle. Mutat Res. 1980 Feb;69(2):293–305. doi: 10.1016/0027-5107(80)90094-9. [DOI] [PubMed] [Google Scholar]
- Poirier M. C., Fullerton N. F., Patterson E. D., Beland F. A. DNA adduct formation and removal in hepatic chromatin fractions from rats chronically fed 2-acetylaminofluorene. Carcinogenesis. 1990 Aug;11(8):1343–1347. doi: 10.1093/carcin/11.8.1343. [DOI] [PubMed] [Google Scholar]
- Poirier M. C., True B., Laishes B. A. Formation and removal of (guan-8-yl)-DNA-2-acetylaminofluorene adducts in liver and kidney of male rats given dietary 2-acetylaminofluorene. Cancer Res. 1982 Apr;42(4):1317–1321. [PubMed] [Google Scholar]
- Reid T. M., Lee M. S., King C. M. Mutagenesis by site-specific arylamine adducts in plasmid DNA: enhancing replication of the adducted strand alters mutation frequency. Biochemistry. 1990 Jul 3;29(26):6153–6161. doi: 10.1021/bi00478a007. [DOI] [PubMed] [Google Scholar]
- Ringer D. P., Kampschmidt K., King R. L., Jr, Jackson S., Kizer D. E. Rapid decrease in N-hydroxy-2-acetylaminofluorene sulfotransferase activity of liver cytosols from rats fed carcinogen. Biochem Pharmacol. 1983 Jan 15;32(2):315–319. doi: 10.1016/0006-2952(83)90561-0. [DOI] [PubMed] [Google Scholar]
- Roberts E., Ahluwalia M. B., Lee G., Chan C., Sarma D. S., Farber E. Resistance to hepatotoxins acquired by hepatocytes during liver regeneration. Cancer Res. 1983 Jan;43(1):28–34. [PubMed] [Google Scholar]
- Saeter G., Schwarze P. E., Nesland J. M., Seglen P. O. 2-Acetylaminofluorene promotion of liver carcinogenesis by a non-cytotoxic mechanism. Carcinogenesis. 1988 Apr;9(4):581–587. doi: 10.1093/carcin/9.4.581. [DOI] [PubMed] [Google Scholar]
- Schaaper R. M., Koffel-Schwartz N., Fuchs R. P. N-acetoxy-N-acetyl-2-aminofluorene-induced mutagenesis in the lacI gene of Escherichia coli. Carcinogenesis. 1990 Jul;11(7):1087–1095. doi: 10.1093/carcin/11.7.1087. [DOI] [PubMed] [Google Scholar]
- Scribner J. D., Mottet N. K. DDT acceleration of mammary gland tumors induced in the male Sprague-Dawley rat by 2-acetamidophenanthrene. Carcinogenesis. 1981;2(12):1235–1239. doi: 10.1093/carcin/2.12.1235. [DOI] [PubMed] [Google Scholar]
- Scribner J. D., Woodworth B., Koponen G., Holmes E. H. Use of 2-acetamidophenanthrene and 2-acetamidofluorene in investigations of mechanisms of hepatocarcinogenesis. Environ Health Perspect. 1983 Mar;49:81–86. doi: 10.1289/ehp.834981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura N., King C. M. Comparative survival of aminobiphenyl- and aminofluorene-substituted plasmid DNA in Escherichia coli Uvr endonuclease deficient strains. Carcinogenesis. 1990 Apr;11(4):535–540. doi: 10.1093/carcin/11.4.535. [DOI] [PubMed] [Google Scholar]
- Tang M., Lieberman M. W., King C. M. uvr Genes function differently in repair of acetylaminofluorene and aminofluorene DNA adducts. Nature. 1982 Oct 14;299(5884):646–648. doi: 10.1038/299646a0. [DOI] [PubMed] [Google Scholar]
- Tatematsu M., Takano T., Hasegawa R., Imaida K., Nakanowatari J., Ito N. A sequential quantitative study of the reversibility or irreversibility of liver hyperplastic nodules in rats exposed to hepatocarcinogens. Gan. 1980 Dec;71(6):843–855. [PubMed] [Google Scholar]
- Tiwawech D., Hasegawa R., Kurata Y., Tatematsu M., Shibata M. A., Thamavit W., Ito N. Dose-dependent effects of 2-acetylaminofluorene on hepatic foci development and cell proliferation in rats. Carcinogenesis. 1991 Jun;12(6):985–990. doi: 10.1093/carcin/12.6.985. [DOI] [PubMed] [Google Scholar]
- Westra J. G., Flammang T. J., Fullerton N. F., Beland F. A., Weis C. C., Kadlubar F. F. Formation of DNA adducts in vivo in rat liver and intestinal epithelium after administration of the carcinogen 3,2'-dimethyl-4-aminobiphenyl and its hydroxamic acid. Carcinogenesis. 1985 Jan;6(1):37–44. doi: 10.1093/carcin/6.1.37. [DOI] [PubMed] [Google Scholar]
- Westra J. G., Kriek E., Hittenhausen H. Identification of the persistently bound form of the carcinogen N-acetyl-2-aminofluorene to rat liver DNA in vivo. Chem Biol Interact. 1976 Oct 2;15(2):149–164. doi: 10.1016/0009-2797(76)90160-5. [DOI] [PubMed] [Google Scholar]
- Yerokun T., Norton T. R., Howell B., Ringer D. P. Modulation of hepatic mRNA translation activity and specific expression of arylsulfotransferase IV during acetylaminofluorene-induced rat hepatocarcinogenesis. Cancer Res. 1991 Jan 15;51(2):504–509. [PubMed] [Google Scholar]
- van de Poll M. L., Lafleur M. V., van Gog F., Vrieling H., Meerman J. H. N-acetylated and deacetylated 4'-fluoro-4-aminobiphenyl and 4-aminobiphenyl adducts differ in their ability to inhibit DNA replication of single-stranded M13 in vitro and of single-stranded phi X174 in Escherichia coli. Carcinogenesis. 1992 May;13(5):751–758. doi: 10.1093/carcin/13.5.751. [DOI] [PubMed] [Google Scholar]
- van de Poll M. L., Tijdens R. B., Vondrácek P., Bruins A. P., Meijer D. K., Meerman J. H. The role of sulfation in the metabolic activation of N-hydroxy-4'-fluoro-4-acetylaminobiphenyl. Carcinogenesis. 1989 Dec;10(12):2285–2291. doi: 10.1093/carcin/10.12.2285. [DOI] [PubMed] [Google Scholar]
- van de Poll M. L., Venizelos V., Meerman J. H. Sulfation-dependent formation of N-acetylated and deacetylated DNA adducts of N-hydroxy-4-acetylaminobiphenyl in male rat liver in vivo and in isolated hepatocytes. Carcinogenesis. 1990 Oct;11(10):1775–1781. doi: 10.1093/carcin/11.10.1775. [DOI] [PubMed] [Google Scholar]
- van de Poll M. L., van der Hulst D. A., Tates A. D., Meerman J. H. Correlation between clastogenicity and promotion activity in liver carcinogenesis by N-hydroxy-2-acetylaminofluorene, N-hydroxy-4'-fluoro-4-acetylaminobiphenyl and N-hydroxy-4-acetylaminobiphenyl. Carcinogenesis. 1990 Feb;11(2):333–339. doi: 10.1093/carcin/11.2.333. [DOI] [PubMed] [Google Scholar]
- van de Poll M. L., van der Hulst D. A., Tates A. D., Mulder G. J., Meerman J. H. The role of specific DNA adducts in the induction of micronuclei by N-hydroxy-2-acetylaminofluorene in rat liver in vivo. Carcinogenesis. 1989 Apr;10(4):717–722. doi: 10.1093/carcin/10.4.717. [DOI] [PubMed] [Google Scholar]


