Background and epidemiology: The identification of the SARS-associated coronavirus (SARS-CoV)1,2 has led to the development of serologic and virologic tests for the disease. As of Apr. 30, 2003, the US Centers for Disease Control and Prevention (CDC) has revised their surveillance case definitions for SARS to include laboratory criteria for evidence of infection3 (see Box [on next page] and Figure).
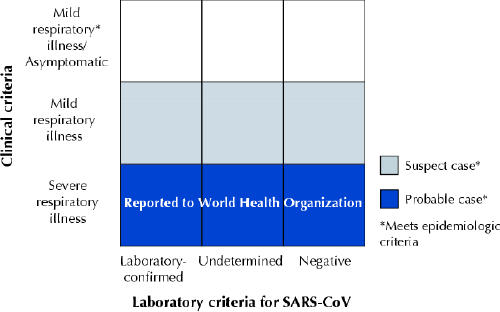
Figure: Clinical and laboratory criteria for probable and suspect severe acute respiratory syndrome (SARS) cases and SARS-associated coronavirus (SARS-CoV) infection. [Source: US Centers for Disease Control and Prevention (www.cdc.gov/ncidod/sars/casedefinition.htm [released 2003 Apr. 30]).
Clinical implications: Serologic testing can be done using indirect fluorescent antibody or enzyme-linked immunosorbent assays specific for SARS-CoV antibody. Because some patients do not have detectable coronavirus antibodies during the acute phase of their illness, a definitive diagnosis of SARS should be made with antibody testing more than 21 days after the onset of initial symptoms.
Detection of the SARS-CoV itself has been done using clinical specimens of serum, nasal secretions and stool. This was done through viral isolation and electron microscopy, viral culture, or reverse transcription polymerase chain reaction (RT-PCR) to test for viral RNA.1,2 Although PCR results have been positive during the acute phase of SARS illness in some patients, the natural course of the viremia and viral shedding is unknown. Thus, a negative PCR test or viral culture result does not exclude coronavirus infection. Similarly, a negative serum antibody test result obtained less than 21 days after symptom onset is not reliable enough to rule out infection.
Prevention: Fundamental public health measures of respiratory isolation of patients and quarantine of close contacts should remain in effect. Suspect and probable SARS cases should be reported to public health authorities. In addition, clinicians should report the results of serologic tests if available. It is important, both for patients and for public health authorities, to obtain convalescent serum samples to make a definitive determination of SARS.
Although no cases of SARS-CoV infection (determined by serologic tests) have been found among exposed asymptomatic people, it remains possible that SARS-CoV infection might be asymptomatic in some, or cause nonrespiratory symptoms in others. This possibility has not been fully explored; however, testing of close contacts of infected cases who have not manifested symptoms will provide a better answer. Until then, the CDC cautions that there is insufficient evidence to exclude the possibility that asymptomatic, or atypical, infected people can transmit the disease.
John Hoey CMAJ
Footnotes
Published at www.cmaj.ca on May 1, 2003.
References
- 1.Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med 2003;348(20):1947-58. Available: http://content.nejm.org/cgi/reprint/NEJMoa030781v3.pdf (accessed 2003 Apr 30). [DOI] [PubMed]
- 2.Drosten C, Günther S, Preiser W, van der Werf S, Brodt HR, Becker S, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med [online early release 2003 Apr 10]. Available: http://content.nejm.org/cgi/reprint/NEJMoa030747v2.pdf (accessed 2003 Apr 30). [DOI] [PubMed]
- 3.US Centers for Disease Control and Prevention (CDC). Updated interim U.S. case definition of severe acute respiratory syndrome (SARS). Atlanta: The CDC; 2003 Apr 30. Available: www.cdc.gov/ncidod/sars/casedefinition.htm (accessed 2003 Apr 30).


