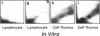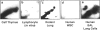Abstract
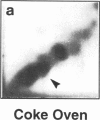
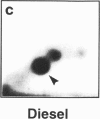
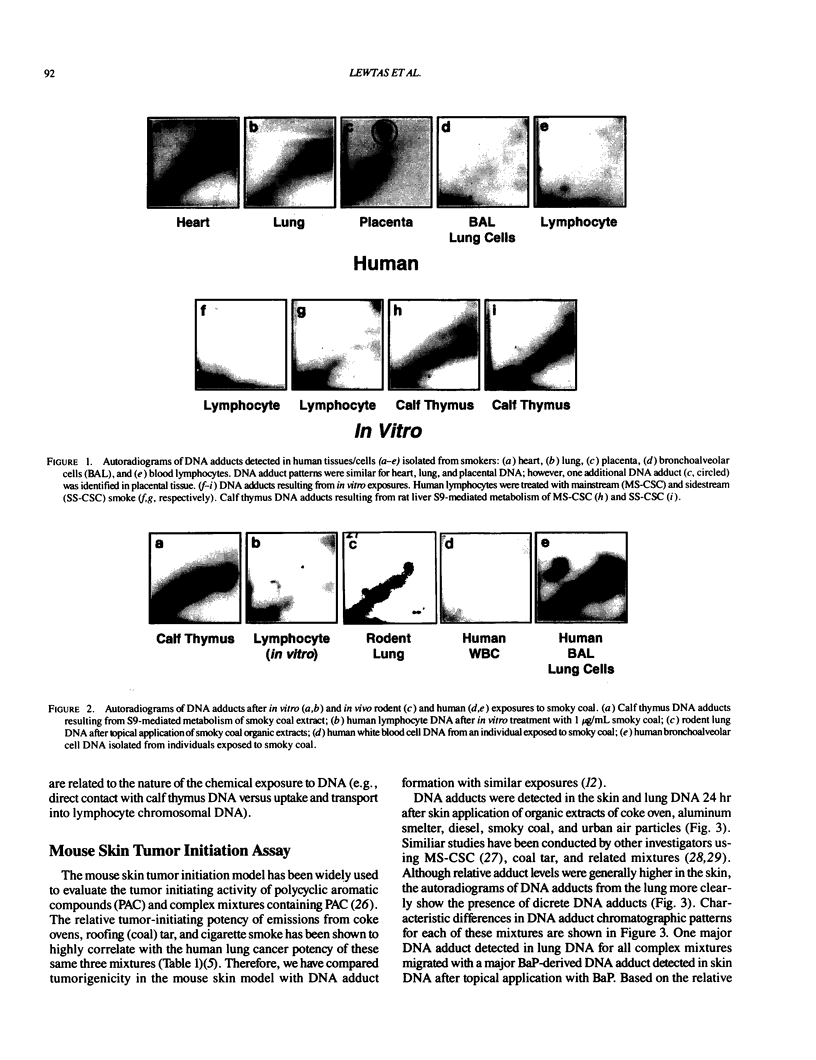
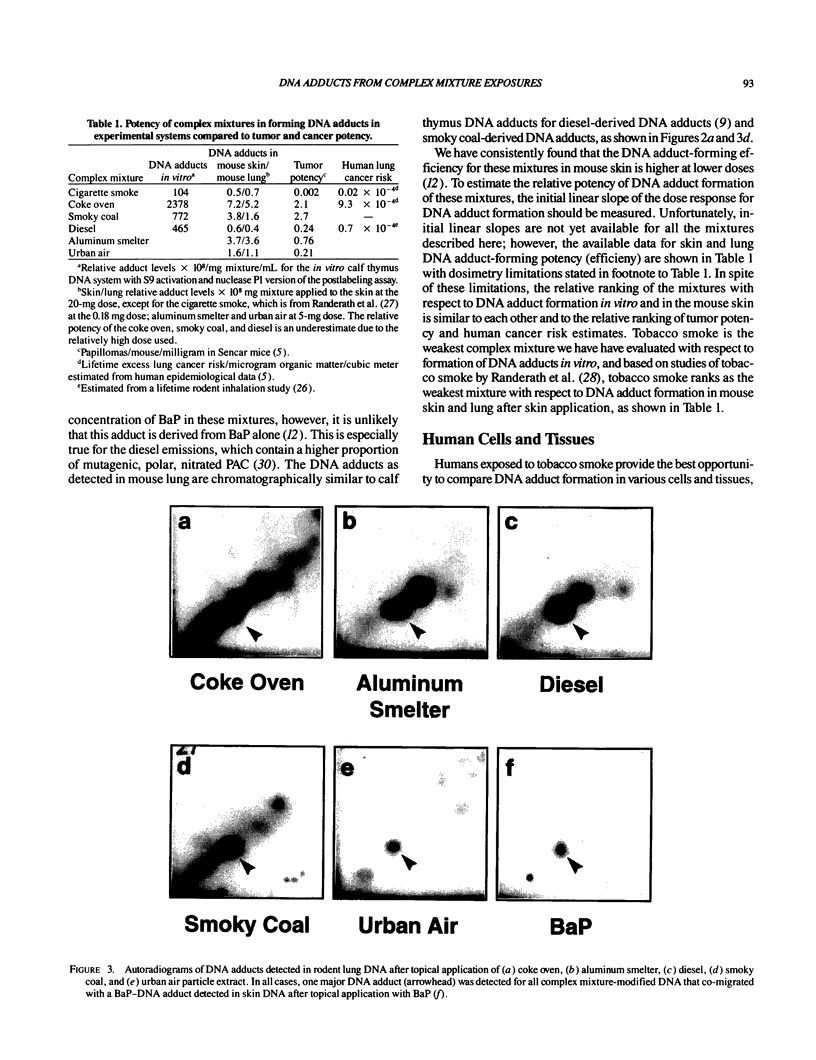
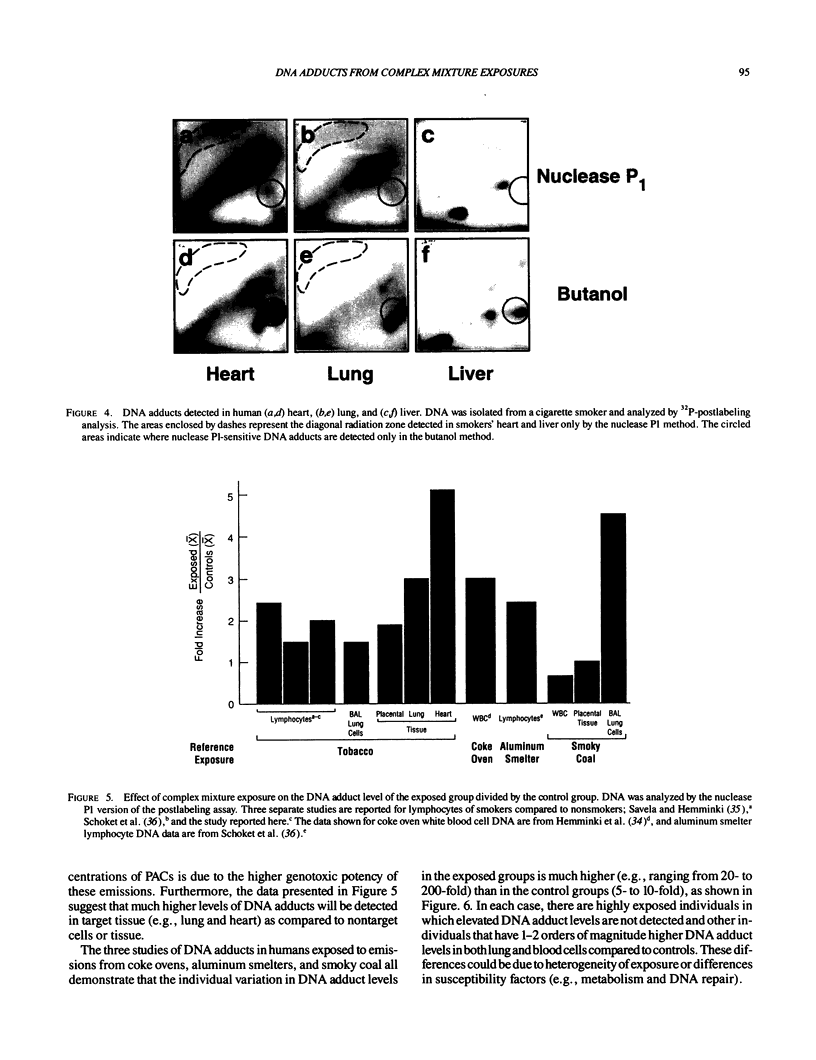
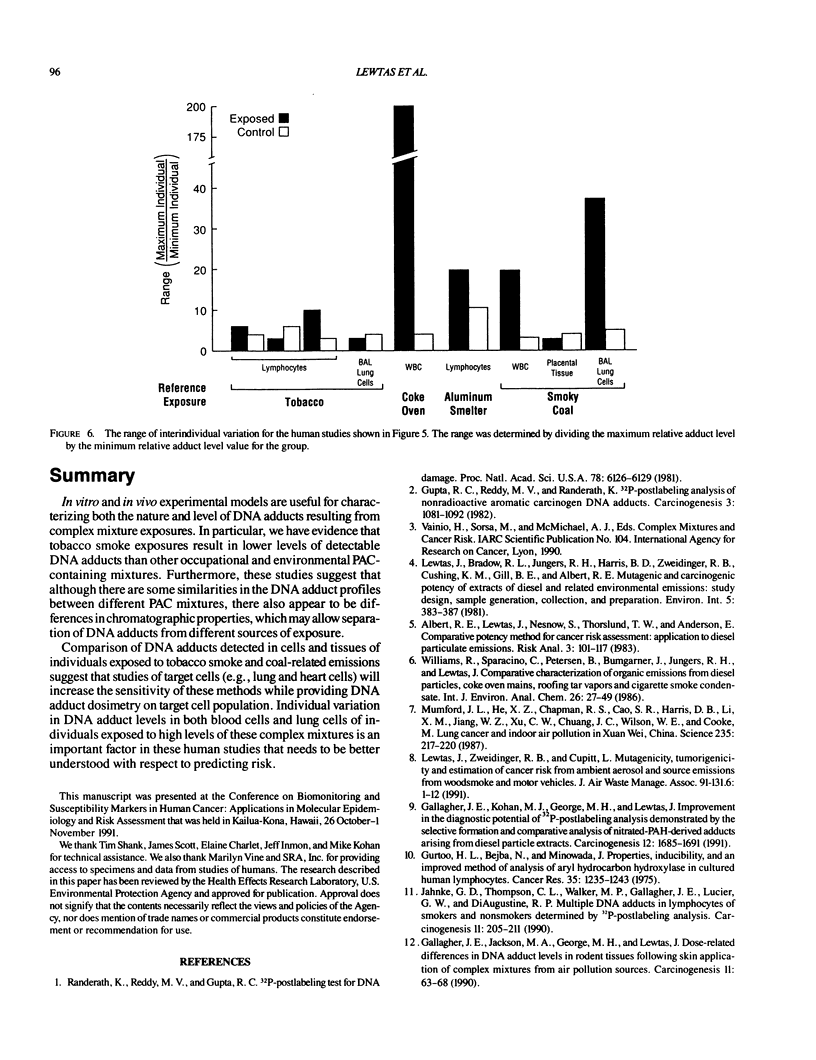
DNA adducts derived from complex mixtures of polycyclic aromatic compounds emitted from tobacco smoke are compared to industrial pollution sources (e.g., coke ovens and aluminum smelters), smoky coal burning, and urban air pollution. Exposures to coke oven emissions and smoky coal, both potent rodent skin tumor initiators and lung carcinogens in humans, result in high levels of DNA adducts compared to tobacco smoke in the in vitro calf thymus DNA model system, in cultured lymphocytes, and in the mouse skin assay. Using tobacco smoke as a model in human studies, we have compared relative DNA adduct levels detected in blood lymphocytes, placental tissue, bronchoalveolar lung lavage cells, sperm, and autopsy tissues of smokers and nonsmokers. Adduct levels in DNA isolated from smokers were highest in human heart and lung tissue with smaller but detectable differences in placental tissue and lung lavage cells. Comparison of the DNA adduct levels resulting from human exposure to different complex mixtures shows that emissions from coke ovens, aluminum smelters, and smoky coal result in higher DNA adduct levels than tobacco smoke exposure. These studies suggest that humans exposed to complex combustion mixtures will have higher DNA adduct levels in target cells (e.g., lung) as compared to nontarget cells (e.g., lymphocytes) and that the adduct levels will be dependent on the genotoxic and DNA adduct-forming potency of the mixture.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Everson R. B., Randerath E., Santella R. M., Avitts T. A., Weinstein I. B., Randerath K. Quantitative associations between DNA damage in human placenta and maternal smoking and birth weight. J Natl Cancer Inst. 1988 Jun 15;80(8):567–576. doi: 10.1093/jnci/80.8.567. [DOI] [PubMed] [Google Scholar]
- Gallagher J. E., Jackson M. A., George M. H., Lewtas J. Dose-related differences in DNA adduct levels in rodent tissues following skin application of complex mixtures from air pollution sources. Carcinogenesis. 1990 Jan;11(1):63–68. doi: 10.1093/carcin/11.1.63. [DOI] [PubMed] [Google Scholar]
- Gallagher J. E., Jackson M. A., George M. H., Lewtas J., Robertson I. G. Differences in detection of DNA adducts in the 32P-postlabelling assay after either 1-butanol extraction or nuclease P1 treatment. Cancer Lett. 1989 Apr;45(1):7–12. doi: 10.1016/0304-3835(89)90029-3. [DOI] [PubMed] [Google Scholar]
- Gallagher J. E., Kohan M. J., George M. H., Lewtas J. Improvement in the diagnostic potential of 32P-postlabeling analysis demonstrated by the selective formation and comparative analysis of nitrated-PAH-derived adducts arising from diesel particle extracts. Carcinogenesis. 1991 Sep;12(9):1685–1691. doi: 10.1093/carcin/12.9.1685. [DOI] [PubMed] [Google Scholar]
- Gupta R. C., Earley K. 32P-adduct assay: comparative recoveries of structurally diverse DNA adducts in the various enhancement procedures. Carcinogenesis. 1988 Sep;9(9):1687–1693. doi: 10.1093/carcin/9.9.1687. [DOI] [PubMed] [Google Scholar]
- Gupta R. C. Enhanced sensitivity of 32P-postlabeling analysis of aromatic carcinogen:DNA adducts. Cancer Res. 1985 Nov;45(11 Pt 2):5656–5662. [PubMed] [Google Scholar]
- Gupta R. C. Nonrandom binding of the carcinogen N-hydroxy-2-acetylaminofluorene to repetitive sequences of rat liver DNA in vivo. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6943–6947. doi: 10.1073/pnas.81.22.6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. C., Reddy M. V., Randerath K. 32P-postlabeling analysis of non-radioactive aromatic carcinogen--DNA adducts. Carcinogenesis. 1982;3(9):1081–1092. doi: 10.1093/carcin/3.9.1081. [DOI] [PubMed] [Google Scholar]
- Gurtoo H. L., Bejba N., Minowada J. Properties, inducibility, and an improved method of analysis of aryl hydrocarbon hydroxylase in cultured human lymphocytes. Cancer Res. 1975 May;35(5):1235–1243. [PubMed] [Google Scholar]
- Hemminki K., Grzybowska E., Chorazy M., Twardowska-Saucha K., Sroczynski J. W., Putman K. L., Randerath K., Phillips D. H., Hewer A., Santella R. M. DNA adducts in human environmentally exposed to aromatic compounds in an industrial area of Poland. Carcinogenesis. 1990 Jul;11(7):1229–1231. doi: 10.1093/carcin/11.7.1229. [DOI] [PubMed] [Google Scholar]
- Jahnke G. D., Thompson C. L., Walker M. P., Gallagher J. E., Lucier G. W., DiAugustine R. P. Multiple DNA adducts in lymphocytes of smokers and nonsmokers determined by 32P-postlabeling analysis. Carcinogenesis. 1990 Feb;11(2):205–211. doi: 10.1093/carcin/11.2.205. [DOI] [PubMed] [Google Scholar]
- Koren H. S., Devlin R. B., Graham D. E., Mann R., McGee M. P., Horstman D. H., Kozumbo W. J., Becker S., House D. E., McDonnell W. F. Ozone-induced inflammation in the lower airways of human subjects. Am Rev Respir Dis. 1989 Feb;139(2):407–415. doi: 10.1164/ajrccm/139.2.407. [DOI] [PubMed] [Google Scholar]
- Lu L. J., Disher R. M., Reddy M. V., Randerath K. 32P-postlabeling assay in mice of transplacental DNA damage induced by the environmental carcinogens safrole, 4-aminobiphenyl, and benzo(a)pyrene. Cancer Res. 1986 Jun;46(6):3046–3054. [PubMed] [Google Scholar]
- Mumford J. L., He X. Z., Chapman R. S., Cao S. R., Harris D. B., Li X. M., Xian Y. L., Jiang W. Z., Xu C. W., Chuang J. C. Lung cancer and indoor air pollution in Xuan Wei, China. Science. 1987 Jan 9;235(4785):217–220. doi: 10.1126/science.3798109. [DOI] [PubMed] [Google Scholar]
- Mumford J. L., Lee X., Lewtas J., Young T. L., Santella R. M. DNA adducts as biomarkers for assessing exposure to polycyclic aromatic hydrocarbons in tissues from Xuan Wei women with high exposure to coal combustion emissions and high lung cancer mortality. Environ Health Perspect. 1993 Mar;99:83–87. doi: 10.1289/ehp.939983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera F. P., Hemminki K., Young T. L., Brenner D., Kelly G., Santella R. M. Detection of polycyclic aromatic hydrocarbon-DNA adducts in white blood cells of foundry workers. Cancer Res. 1988 Apr 15;48(8):2288–2291. [PubMed] [Google Scholar]
- Phillips D. H., Hemminki K., Alhonen A., Hewer A., Grover P. L. Monitoring occupational exposure to carcinogens: detection by 32P-postlabelling of aromatic DNA adducts in white blood cells from iron foundry workers. Mutat Res. 1988 Mar;204(3):531–541. doi: 10.1016/0165-1218(88)90047-x. [DOI] [PubMed] [Google Scholar]
- Phillips D. H., Schoket B., Hewer A., Bailey E., Kostic S., Vincze I. Influence of cigarette smoking on the levels of DNA adducts in human bronchial epithelium and white blood cells. Int J Cancer. 1990 Oct 15;46(4):569–575. doi: 10.1002/ijc.2910460403. [DOI] [PubMed] [Google Scholar]
- Randerath E., Mittal D., Randerath K. Tissue distribution of covalent DNA damage in mice treated dermally with cigarette 'tar': preference for lung and heart DNA. Carcinogenesis. 1988 Jan;9(1):75–80. doi: 10.1093/carcin/9.1.75. [DOI] [PubMed] [Google Scholar]
- Randerath K., Reddy M. V., Gupta R. C. 32P-labeling test for DNA damage. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6126–6129. doi: 10.1073/pnas.78.10.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy M. V., Randerath K. Nuclease P1-mediated enhancement of sensitivity of 32P-postlabeling test for structurally diverse DNA adducts. Carcinogenesis. 1986 Sep;7(9):1543–1551. doi: 10.1093/carcin/7.9.1543. [DOI] [PubMed] [Google Scholar]
- Savela K., Hemminki K. DNA adducts in lymphocytes and granulocytes of smokers and nonsmokers detected by the 32P-postlabelling assay. Carcinogenesis. 1991 Mar;12(3):503–508. doi: 10.1093/carcin/12.3.503. [DOI] [PubMed] [Google Scholar]
- Schoket B., Hewer A., Grover P. L., Phillips D. H. 32P-postlabelling analysis of DNA adducts in the skin of mice treated with petrol and diesel engine lubricating oils and exhaust condensates. Carcinogenesis. 1989 Aug;10(8):1485–1490. doi: 10.1093/carcin/10.8.1485. [DOI] [PubMed] [Google Scholar]
- Schoket B., Hewer A., Grover P. L., Phillips D. H. Covalent binding of components of coal-tar, creosote and bitumen to the DNA of the skin and lungs of mice following topical application. Carcinogenesis. 1988 Jul;9(7):1253–1258. doi: 10.1093/carcin/9.7.1253. [DOI] [PubMed] [Google Scholar]
- Schoket B., Phillips D. H., Hewer A., Vincze I. 32P-postlabelling detection of aromatic DNA adducts in peripheral blood lymphocytes from aluminium production plant workers. Mutat Res. 1991 May;260(1):89–98. doi: 10.1016/0165-1218(91)90084-y. [DOI] [PubMed] [Google Scholar]
- Williams R., Sparacino C., Petersen B., Bumgarner J., Jungers R. H., Lewtas J. Comparative characterization of organic emissions from diesel particles, coke oven mains, roofing tar vapors and cigarette smoke condensate. Int J Environ Anal Chem. 1986;26(1):27–49. doi: 10.1080/03067318608077102. [DOI] [PubMed] [Google Scholar]