Abstract
Mutations arising in vivo in recorder genes of human blood cells provide biomarkers for molecular epidemiology by serving as surrogates for cancer-causing genetic changes. Current markers include mutations of the glycophorin-A (GPA) or hemoglobin (Hb) genes, measured in red blood cells, or mutations of the hypoxanthine-guanine phosphoribosyltransferase (hprt) or HLA genes, measured in T-lymphocytes. Mean mutant frequencies (variant frequencies) for normal young adults are approximately: Hb (4 x 10(-8)) < hprt (5 x 10(-6)) = GPA (10 x 10(-6)) < HLA (30 x 10(-6)). Mutagen-exposed individuals show decided elevations. Molecular mutational spectra are also being defined. For the hprt marker system, about 15% of background mutations are gross structural alterations of the hprt gene (e.g., deletions); the remainder are point mutations (e.g., base substitutions or frameshifts). Ionizing radiations result in dose-related increases in total gene deletions. Large deletions may encompass several megabases as shown by co-deletions of linked markers. Possible hprt spectra for defining radiation and chemical exposures are being sought. In addition to their responsiveness to environmental mutagens/carcinogens, three additional findings suggest that the in vivo recorder mutations are relevant in vivo surrogates for cancer mutations. First, a large fraction of GPA and HLA mutations show exchanges due to homologous recombination, an important mutational event in cancer. Second, hprt mutations arise preferentially in dividing T-cells, which can accumulate additional mutations in the same clone, reminiscent of the multiple hits required in the evolution of malignancy.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertini R. J., Castle K. L., Borcherding W. R. T-cell cloning to detect the mutant 6-thioguanine-resistant lymphocytes present in human peripheral blood. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6617–6621. doi: 10.1073/pnas.79.21.6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertini R. J., Nicklas J. A., O'Neill J. P., Robison S. H. In vivo somatic mutations in humans: measurement and analysis. Annu Rev Genet. 1990;24:305–326. doi: 10.1146/annurev.ge.24.120190.001513. [DOI] [PubMed] [Google Scholar]
- Albertini R. J. Somatic gene mutations in vivo as indicated by the 6-thioguanine-resistant T-lymphocytes in human blood. Mutat Res. 1985 Jun-Jul;150(1-2):411–422. doi: 10.1016/0027-5107(85)90138-1. [DOI] [PubMed] [Google Scholar]
- Albertini R. J., Sullivan L. M., Berman J. K., Greene C. J., Stewart J. A., Silveira J. M., O'Neill J. P. Mutagenicity monitoring in humans by autoradiographic assay for mutant T lymphocytes. Mutat Res. 1988 Mar;204(3):481–492. doi: 10.1016/0165-1218(88)90043-2. [DOI] [PubMed] [Google Scholar]
- Ammenheuser M. M., Au W. W., Whorton E. B., Jr, Belli J. A., Ward J. B., Jr Comparison of hprt variant frequencies and chromosome aberration frequencies in lymphocytes from radiotherapy and chemotherapy patients: a prospective study. Environ Mol Mutagen. 1991;18(2):126–135. doi: 10.1002/em.2850180208. [DOI] [PubMed] [Google Scholar]
- Ammenheuser M. M., Ward J. B., Jr, Whorton E. B., Jr, Killian J. M., Legator M. S. Elevated frequencies of 6-thioguanine-resistant lymphocytes in multiple sclerosis patients treated with cyclophosphamide: a prospective study. Mutat Res. 1988 Mar;204(3):509–520. doi: 10.1016/0165-1218(88)90045-6. [DOI] [PubMed] [Google Scholar]
- Antonarakis S. E., Kazazian H. H., Jr, Orkin S. H. DNA polymorphism and molecular pathology of the human globin gene clusters. Hum Genet. 1985;69(1):1–14. doi: 10.1007/BF00295521. [DOI] [PubMed] [Google Scholar]
- Aplan P. D., Lombardi D. P., Ginsberg A. M., Cossman J., Bertness V. L., Kirsch I. R. Disruption of the human SCL locus by "illegitimate" V-(D)-J recombinase activity. Science. 1990 Dec 7;250(4986):1426–1429. doi: 10.1126/science.2255914. [DOI] [PubMed] [Google Scholar]
- Bernini L. F., Natarajan A. T., Schreuder-Rotteveel A. H., Giordano P. C., Ploem J. S., Tates A. Assay for somatic mutation of human hemoglobins. Prog Clin Biol Res. 1990;340C:57–67. [PubMed] [Google Scholar]
- Bigbee W. L., Wyrobek A. J., Langlois R. G., Jensen R. H., Everson R. B. The effect of chemotherapy on the in vivo frequency of glycophorin A 'null' variant erythrocytes. Mutat Res. 1990 Mar;240(3):165–175. doi: 10.1016/0165-1218(90)90056-8. [DOI] [PubMed] [Google Scholar]
- Bradley W. E., Gareau J. L., Seifert A. M., Messing K. Molecular characterization of 15 rearrangements among 90 human in vivo somatic mutants shows that deletions predominate. Mol Cell Biol. 1987 Feb;7(2):956–960. doi: 10.1128/mcb.7.2.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branda R. F., O'Neill J. P., Jacobson-Kram D., Albertini R. J. Factors influencing mutation at the hprt locus in T-lymphocytes: studies in normal women and women with benign and malignant breast masses. Environ Mol Mutagen. 1992;19(4):274–281. doi: 10.1002/em.2850190403. [DOI] [PubMed] [Google Scholar]
- Bridges B. A., Cole J., Arlett C. F., Green M. H., Waugh A. P., Beare D., Henshaw D. L., Last R. D. Possible association between mutant frequency in peripheral lymphocytes and domestic radon concentrations. Lancet. 1991 May 18;337(8751):1187–1189. doi: 10.1016/0140-6736(91)92859-z. [DOI] [PubMed] [Google Scholar]
- Brown L., Cheng J. T., Chen Q., Siciliano M. J., Crist W., Buchanan G., Baer R. Site-specific recombination of the tal-1 gene is a common occurrence in human T cell leukemia. EMBO J. 1990 Oct;9(10):3343–3351. doi: 10.1002/j.1460-2075.1990.tb07535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrysostomou A., Seshadri R., Morley A. A. Mutation frequency in nurses and pharmacists working with cytotoxic drugs. Aust N Z J Med. 1984 Dec;14(6):831–834. doi: 10.1111/j.1445-5994.1984.tb03782.x. [DOI] [PubMed] [Google Scholar]
- Cole J., Green M. H., James S. E., Henderson L., Cole H. A further assessment of factors influencing measurements of thioguanine-resistant mutant frequency in circulating T-lymphocytes. Mutat Res. 1988 Mar;204(3):493–507. doi: 10.1016/0165-1218(88)90044-4. [DOI] [PubMed] [Google Scholar]
- Dempsey J. L., Seshadri R. S., Morley A. A. Increased mutation frequency following treatment with cancer chemotherapy. Cancer Res. 1985 Jun;45(6):2873–2877. [PubMed] [Google Scholar]
- Fuscoe J. C., Zimmerman L. J., Lippert M. J., Nicklas J. A., O'Neill J. P., Albertini R. J. V(D)J recombinase-like activity mediates hprt gene deletion in human fetal T-lymphocytes. Cancer Res. 1991 Nov 1;51(21):6001–6005. [PubMed] [Google Scholar]
- Hakoda M., Akiyama M., Kyoizumi S., Awa A. A., Yamakido M., Otake M. Increased somatic cell mutant frequency in atomic bomb survivors. Mutat Res. 1988 Sep;201(1):39–48. doi: 10.1016/0027-5107(88)90109-1. [DOI] [PubMed] [Google Scholar]
- Hakoda M., Hirai Y., Kyoizumi S., Akiyama M. Molecular analyses of in vivo hprt mutant T cells from atomic bomb survivors. Environ Mol Mutagen. 1989;13(1):25–33. doi: 10.1002/em.2850130103. [DOI] [PubMed] [Google Scholar]
- Hüttner E., Mergner U., Braun R., Schöneich J. Increased frequency of 6-thioguanine-resistant lymphocytes in peripheral blood of workers employed in cyclophosphamide production. Mutat Res. 1990 Feb;243(2):101–107. doi: 10.1016/0165-7992(90)90030-n. [DOI] [PubMed] [Google Scholar]
- Janatipour M., Trainor K. J., Kutlaca R., Bennett G., Hay J., Turner D. R., Morley A. A. Mutations in human lymphocytes studied by an HLA selection system. Mutat Res. 1988 Mar;198(1):221–226. doi: 10.1016/0027-5107(88)90058-9. [DOI] [PubMed] [Google Scholar]
- Kudo S., Fukuda M. Structural organization of glycophorin A and B genes: glycophorin B gene evolved by homologous recombination at Alu repeat sequences. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4619–4623. doi: 10.1073/pnas.86.12.4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyoizumi S., Nakamura N., Hakoda M., Awa A. A., Bean M. A., Jensen R. H., Akiyama M. Detection of somatic mutations at the glycophorin A locus in erythrocytes of atomic bomb survivors using a single beam flow sorter. Cancer Res. 1989 Feb 1;49(3):581–588. [PubMed] [Google Scholar]
- Langlois R. G., Bigbee W. L., Jensen R. H. Measurements of the frequency of human erythrocytes with gene expression loss phenotypes at the glycophorin A locus. Hum Genet. 1986 Dec;74(4):353–362. doi: 10.1007/BF00280485. [DOI] [PubMed] [Google Scholar]
- Langlois R. G., Bigbee W. L., Jensen R. H. The glycophorin A assay for somatic cell mutations in humans. Prog Clin Biol Res. 1990;340C:47–56. [PubMed] [Google Scholar]
- Langlois R. G., Bigbee W. L., Kyoizumi S., Nakamura N., Bean M. A., Akiyama M., Jensen R. H. Evidence for increased somatic cell mutations at the glycophorin A locus in atomic bomb survivors. Science. 1987 Apr 24;236(4800):445–448. doi: 10.1126/science.3563520. [DOI] [PubMed] [Google Scholar]
- Malissen M., Malissen B., Jordan B. R. Exon/intron organization and complete nucleotide sequence of an HLA gene. Proc Natl Acad Sci U S A. 1982 Feb;79(3):893–897. doi: 10.1073/pnas.79.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinniss M. J., Falta M. T., Sullivan L. M., Albertini R. J. In vivo hprt mutant frequencies in T-cells of normal human newborns. Mutat Res. 1990 Feb;240(2):117–126. doi: 10.1016/0165-1218(90)90015-t. [DOI] [PubMed] [Google Scholar]
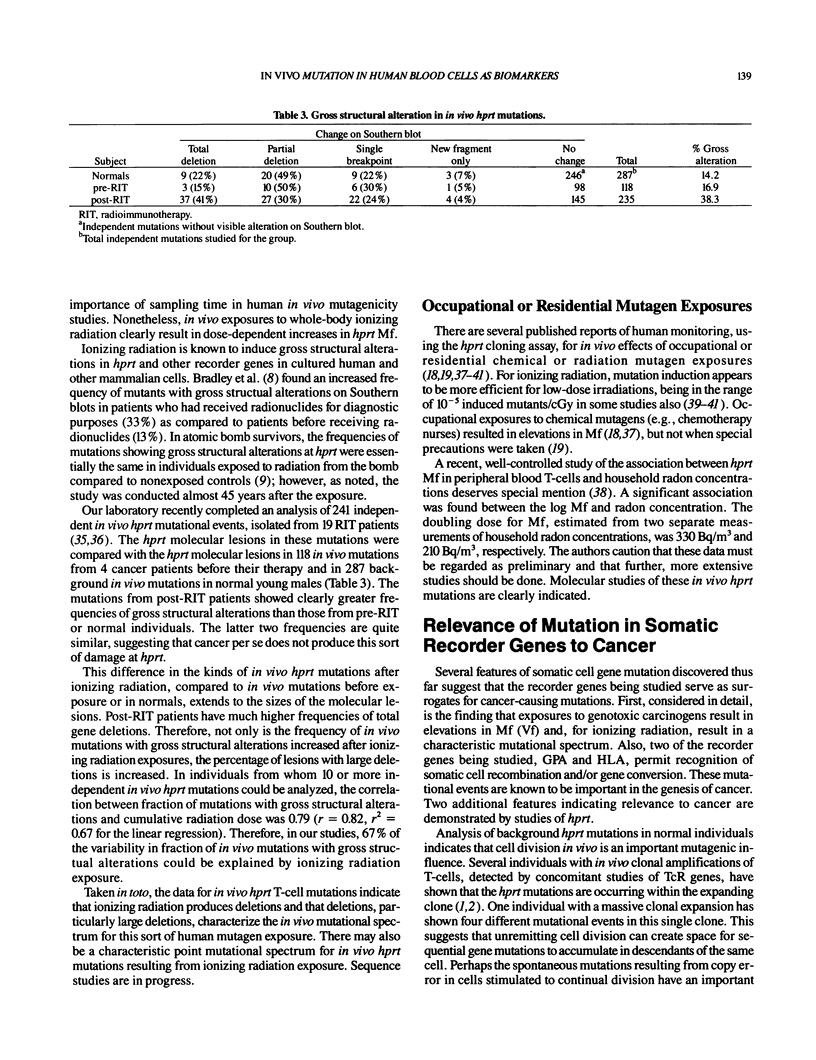
- McGinniss M. J., Nicklas J. A., Albertini R. J. Molecular analyses of in vivo hprt mutations in human T-lymphocytes: IV. Studies in newborns. Environ Mol Mutagen. 1989;14(4):229–237. doi: 10.1002/em.2850140404. [DOI] [PubMed] [Google Scholar]
- Messing K., Bradley W. E. In vivo mutant frequency rises among breast cancer patients after exposure to high doses of gamma-radiation. Mutat Res. 1985 Oct;152(1):107–112. doi: 10.1016/0027-5107(85)90051-x. [DOI] [PubMed] [Google Scholar]
- Messing K., Ferraris J., Bradley W. E., Swartz J., Seifert A. M. Mutant frequency of radiotherapy technicians appears to be associated with recent dose of ionizing radiation. Health Phys. 1989 Oct;57(4):537–544. doi: 10.1097/00004032-198910000-00003. [DOI] [PubMed] [Google Scholar]
- Messing K., Seifert A. M., Bradley W. E. In vivo mutant frequency of technicians professionally exposed to ionizing radiation. Prog Clin Biol Res. 1986;207:87–97. [PubMed] [Google Scholar]
- Morley A. A., Grist S. A., Turner D. R., Kutlaca A., Bennett G. Molecular nature of in vivo mutations in human cells at the autosomal HLA-A locus. Cancer Res. 1990 Aug 1;50(15):4584–4587. [PubMed] [Google Scholar]
- Morley A. A., Trainor K. J., Seshadri R., Ryall R. G. Measurement of in vivo mutations in human lymphocytes. Nature. 1983 Mar 10;302(5904):155–156. doi: 10.1038/302155a0. [DOI] [PubMed] [Google Scholar]
- Nicklas J. A., Falta M. T., Hunter T. C., O'Neill J. P., Jacobson-Kram D., Williams J. R., Albertini R. J. Molecular analysis of in vivo hprt mutations in human T lymphocytes. V. Effects of total body irradiation secondary to radioimmunoglobulin therapy (RIT) Mutagenesis. 1990 Sep;5(5):461–468. doi: 10.1093/mutage/5.5.461. [DOI] [PubMed] [Google Scholar]
- Nicklas J. A., Hunter T. C., O'Neill J. P., Albertini R. J. Fine structure mapping of the hypoxanthine-guanine phosphoribosyltransferase (HPRT) gene region of the human X chromosome (Xq26). Am J Hum Genet. 1991 Aug;49(2):267–278. [PMC free article] [PubMed] [Google Scholar]
- Nicklas J. A., Hunter T. C., O'Neill J. P., Albertini R. J. Molecular analyses of in vivo hprt mutations in human T-lymphocytes. III. Longitudinal study of hprt gene structural alterations and T-cell clonal origins. Mutat Res. 1989 Dec;215(2):147–160. doi: 10.1016/0027-5107(89)90178-4. [DOI] [PubMed] [Google Scholar]
- Nicklas J. A., Hunter T. C., Sullivan L. M., Berman J. K., O'Neill J. P., Albertini R. J. Molecular analyses of in vivo hprt mutations in human T-lymphocytes. I. Studies of low frequency 'spontaneous' mutants by Southern blots. Mutagenesis. 1987 Sep;2(5):341–347. doi: 10.1093/mutage/2.5.341. [DOI] [PubMed] [Google Scholar]
- Nicklas J. A., O'Neill J. P., Hunter T. C., Falta M. T., Lippert M. J., Jacobson-Kram D., Williams J. R., Albertini R. J. In vivo ionizing irradiations produce deletions in the hprt gene of human T-lymphocytes. Mutat Res. 1991 Sep-Oct;250(1-2):383–396. doi: 10.1016/0027-5107(91)90195-t. [DOI] [PubMed] [Google Scholar]
- Ostrosky-Wegman P., Montero R., Palao A., Cortinas de Nava C., Hurtado F., Albertini R. J. 6-Thioguanine-resistant T-lymphocyte autoradiographic assay. Determination of variation frequencies in individuals suspected of radiation exposure. Mutat Res. 1990 Sep;232(1):49–56. doi: 10.1016/0027-5107(90)90109-h. [DOI] [PubMed] [Google Scholar]
- Patel P. I., Framson P. E., Caskey C. T., Chinault A. C. Fine structure of the human hypoxanthine phosphoribosyltransferase gene. Mol Cell Biol. 1986 Feb;6(2):393–403. doi: 10.1128/mcb.6.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala-Trepat M., Cole J., Green M. H., Rigaud O., Vilcoq J. R., Moustacchi E. Genotoxic effects of radiotherapy and chemotherapy on the circulating lymphocytes of breast cancer patients. III: Measurement of mutant frequency to 6-thioguanine resistance. Mutagenesis. 1990 Nov;5(6):593–598. doi: 10.1093/mutage/5.6.593. [DOI] [PubMed] [Google Scholar]
- Seifert A. M., Bradley W. E., Messing K. Exposure of nuclear medicine patients to ionizing radiation is associated with rises in HPRT- mutant frequency in peripheral T-lymphocytes. Mutat Res. 1987 May;191(1):57–63. doi: 10.1016/0165-7992(87)90171-0. [DOI] [PubMed] [Google Scholar]
- Tates A. D., Bernini L. F., Natarajan A. T., Ploem J. S., Verwoerd N. P., Cole J., Green M. H., Arlett C. F., Norris P. N. Detection of somatic mutants in man: HPRT mutations in lymphocytes and hemoglobin mutations in erythrocytes. Mutat Res. 1989 Jul;213(1):73–82. doi: 10.1016/0027-5107(89)90033-x. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Trainor K. J., Wigmore D. J., Chrysostomou A., Dempsey J. L., Seshadri R., Morley A. A. Mutation frequency in human lymphocytes increases with age. Mech Ageing Dev. 1984 Sep;27(1):83–86. doi: 10.1016/0047-6374(84)90084-8. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Jaffe E., Cossman J., Gorham J., Nowell P. C., Croce C. M. Clustering of breakpoints on chromosome 11 in human B-cell neoplasms with the t(11;14) chromosome translocation. Nature. 1985 May 23;315(6017):340–343. doi: 10.1038/315340a0. [DOI] [PubMed] [Google Scholar]
- Turner D. R., Grist S. A., Janatipour M., Morley A. A. Mutations in human lymphocytes commonly involve gene duplication and resemble those seen in cancer cels. Proc Natl Acad Sci U S A. 1988 May;85(9):3189–3192. doi: 10.1073/pnas.85.9.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner D. R., Morley A. A., Haliandros M., Kutlaca R., Sanderson B. J. In vivo somatic mutations in human lymphocytes frequently result from major gene alterations. Nature. 1985 May 23;315(6017):343–345. doi: 10.1038/315343a0. [DOI] [PubMed] [Google Scholar]
- Turner D. R., Morley A. A. Human somatic mutation at the autosomal HLA-A locus. Prog Clin Biol Res. 1990;340C:37–46. [PubMed] [Google Scholar]


