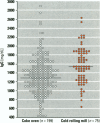
Abstract
We evaluated humoral immunity by measuring IgG, IgA, IgM, and IgE concentrations in 274 male workers in an iron foundry in Cracow, Poland. There were two groups: 199 coke oven workers and 76 cold-rolling mill workers. The groups were similar with respect to age, length of work (average 15 years), and smoking habits. Exposure to polycyclic aromatic hydrocarbons (PAHs), assessed by personal and area monitoring, ranged from 0.2 to 50 micrograms/m3 benzo[a]pyrene in coke plant workers and was of 3-5 magnitudes higher than in the cold-rolling mill employees. Comparison of the two groups revealed a marked depression of mean serum IgG and IgA in coke oven workers (p < 0.001, Student's unpaired t-test). In the same subjects, serum IgM had a tendency to decrease, whereas serum IgE showed a trend toward higher values. Thus, workers exposed chronically to complex mixtures of air pollutants, composed primarily of PAHs, develop immunosuppression. It remains to be established whether the immunosuppression described here is related to the frequent development of lung cancer reported in coke plant employees. Workers exposed chronically to PAHs should have serum immunoglobulins monitored regularly.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chau N., Bertrand J. P., Mur J. M., Figueredo A., Patris A., Moulin J. J., Pham Q. T. Mortality in retired coke oven plant workers. Br J Ind Med. 1993 Feb;50(2):127–135. doi: 10.1136/oem.50.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark G., Tritscher A., Bell D., Lucier G. Integrated approach for evaluating species and interindividual differences in responsiveness to dioxins and structural analogs. Environ Health Perspect. 1992 Nov;98:125–132. doi: 10.1289/ehp.9298125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermaszewski R. A., Webster A. D. Primary hypogammaglobulinaemia: a survey of clinical manifestations and complications. Q J Med. 1993 Jan;86(1):31–42. [PubMed] [Google Scholar]
- Holt P. G. Environmental pollutants as co-factors in IgE production. Curr Opin Immunol. 1989 Apr;1(4):643–646. doi: 10.1016/0952-7915(89)90034-4. [DOI] [PubMed] [Google Scholar]
- Lewtas J. Complex mixtures of air pollutants: characterizing the cancer risk of polycyclic organic matter. Environ Health Perspect. 1993 Apr;100:211–218. doi: 10.1289/ehp.93100211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstedt G., Sollenberg J. Polycyclic aromatic hydrocarbons in the occupational environment: with special reference to benzo[a]pyrene measurements in Swedish industry. Scand J Work Environ Health. 1982 Mar;8(1):1–19. doi: 10.5271/sjweh.2503. [DOI] [PubMed] [Google Scholar]
- Lucier G. W., Portier C. J., Gallo M. A. Receptor mechanisms and dose-response models for the effects of dioxins. Environ Health Perspect. 1993 Apr 22;101(1):36–44. doi: 10.1289/ehp.9310136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luster M. I., Rosenthal G. J. Chemical agents and the immune response. Environ Health Perspect. 1993 Apr;100:219–226. doi: 10.1289/ehp.93100219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera F. P., Hemminki K., Gryzbowska E., Motykiewicz G., Michalska J., Santella R. M., Young T. L., Dickey C., Brandt-Rauf P., De Vivo I. Molecular and genetic damage in humans from environmental pollution in Poland. Nature. 1992 Nov 19;360(6401):256–258. doi: 10.1038/360256a0. [DOI] [PubMed] [Google Scholar]
- Perera F. P., Tang D. L., O'Neill J. P., Bigbee W. L., Albertini R. J., Santella R., Ottman R., Tsai W. Y., Dickey C., Mooney L. A. HPRT and glycophorin A mutations in foundry workers: relationship to PAH exposure and to PAH-DNA adducts. Carcinogenesis. 1993 May;14(5):969–973. doi: 10.1093/carcin/14.5.969. [DOI] [PubMed] [Google Scholar]
- Perera F., Brenner D., Jeffrey A., Mayer J., Tang D., Warburton D., Young T. I., Wazneh L., Latriano L., Motykiewicz G. DNA adducts and related biomarkers in populations exposed to environmental carcinogens. Environ Health Perspect. 1992 Nov;98:133–137. doi: 10.1289/ehp.9298133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland A., Glover E. Comparison of 2,3,7,8-tetrachlorodibenzo-p-dioxin, a potent inducer of aryl hydrocarbon hydroxylase, with 3-methylcholanthrene. Mol Pharmacol. 1974 Mar;10(2):349–359. [PubMed] [Google Scholar]
- Redmond C. K. Cancer mortality among coke oven workers. Environ Health Perspect. 1983 Oct;52:67–73. doi: 10.1289/ehp.835267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond C. K., Strobino B. R., Cypess R. H. Cancer experience among coke by-product workers. Ann N Y Acad Sci. 1976;271:102–115. doi: 10.1111/j.1749-6632.1976.tb23099.x. [DOI] [PubMed] [Google Scholar]
- Sarto F., Zordan M., Tomanin R., Mazzotti D., Canova A., Cardin E. L., Bezze G., Levis A. G. Chromosomal alterations in peripheral blood lymphocytes, urinary mutagenicity and excretion of polycyclic aromatic hydrocarbons in six psoriatic patients undergoing coal tar therapy. Carcinogenesis. 1989 Feb;10(2):329–334. doi: 10.1093/carcin/10.2.329. [DOI] [PubMed] [Google Scholar]
- Szczeklik A., Sladek K., Szczerba A., Dropinski J. Serum immunoglobulin E response to myocardial infarction. Circulation. 1988 Jun;77(6):1245–1249. doi: 10.1161/01.cir.77.6.1245. [DOI] [PubMed] [Google Scholar]
- Ward E. C., Murray M. J., Lauer L. D., House R. V., Irons R., Dean J. H. Immunosuppression following 7,12-dimethylbenz[a]anthracene exposure in B6C3F1 mice. I. Effects on humoral immunity and host resistance. Toxicol Appl Pharmacol. 1984 Sep 15;75(2):299–308. doi: 10.1016/0041-008x(84)90212-6. [DOI] [PubMed] [Google Scholar]
- Watabe T., Ishizuka T., Isobe M., Ozawa N. A 7-hydroxymethyl sulfate ester as an active metabolite of 7,12-dimethylbenz[alpha]anthracene. Science. 1982 Jan 22;215(4531):403–405. doi: 10.1126/science.6800033. [DOI] [PubMed] [Google Scholar]