Abstract
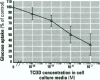
This study examined the changes in cellular glucose uptake induced by 2,3,7,8 tetrachlorodibenzo-p-dioxin (TCDD) as measured by quantification of intracellular radioactivity in the NIH 3T3 L1 preadipocyte cell line after a 30-minute incubation with the non-metabolizable radioactive analogue of glucose, 3-O-methyl-D-[1-3H] glucose. Treatment of differentiated NIH 3T3 L1 cells with TCDD produced a time- and dose-dependent decrease in the cellular uptake of glucose. Treatment of cells for 3 hr with 10(-8) M TCDD significantly reduced glucose uptake to about 10% of control values (p </= 0.05). Furthermore, cytochalasin B, a specific inhibitor of facilitative glucose transporter proteins totally abolished the portion of glucose transport activity that is sensitive to TCDD. The role of the Ah receptor in TCDD-mediated reduction in glucose uptake was investigated. Pretreatment of 3T3 L1 cells with the Ah receptor blocker 4,7-phenanthroline antagonized the effect of TCDD on glucose uptake. Structure-activity relationship studies with TCDD and two polychlorinated biphenyl (PCB) congeners revealed a rank order for their potency in the inhibition of glucose transport as follows: TCDD <<3,3',4,4' tetrachlorobiphenyl <2,2',5,5' tetrachlorobiphenyl (TCB). Such a rank order correlates both with previously determined biological activity of TCDD and the more active 3,3',4,4'- and less active 2,2',5,5'-TCB and with affinity for binding to the Ah receptor. The thyroid hormone T4, like TCDD, reduced glucose uptake and blocked the action of TCDD to further reduce glucose uptake. Experimental evidence is consistent with a proposed mechanism for TCDD to reduce the titer of functional glucose transporter proteins through its interaction with the Ah receptor.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell G. I., Kayano T., Buse J. B., Burant C. F., Takeda J., Lin D., Fukumoto H., Seino S. Molecular biology of mammalian glucose transporters. Diabetes Care. 1990 Mar;13(3):198–208. doi: 10.2337/diacare.13.3.198. [DOI] [PubMed] [Google Scholar]
- Burbach K. M., Poland A., Bradfield C. A. Cloning of the Ah-receptor cDNA reveals a distinctive ligand-activated transcription factor. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8185–8189. doi: 10.1073/pnas.89.17.8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casla A., Rovira A., Wells J. A., Dohm G. L. Increased glucose transporter (GLUT4) protein expression in hyperthyroidism. Biochem Biophys Res Commun. 1990 Aug 31;171(1):182–188. doi: 10.1016/0006-291x(90)91374-2. [DOI] [PubMed] [Google Scholar]
- Clancy B. M., Czech M. P. Hexose transport stimulation and membrane redistribution of glucose transporter isoforms in response to cholera toxin, dibutyryl cyclic AMP, and insulin in 3T3-L1 adipocytes. J Biol Chem. 1990 Jul 25;265(21):12434–12443. [PubMed] [Google Scholar]
- Enan E., Liu P. C., Matsumura F. 2,3,7,8-Tetrachlorodibenzo-p-dioxin causes reduction of glucose transporting activities in the plasma membranes of adipose tissue and pancreas from the guinea pig. J Biol Chem. 1992 Oct 5;267(28):19785–19791. [PubMed] [Google Scholar]
- Foley J. E., Cushman S. W., Salans L. B. Intracellular glucose concentration in small and large rat adipose cells. Am J Physiol. 1980 Feb;238(2):E180–E185. doi: 10.1152/ajpendo.1980.238.2.E180. [DOI] [PubMed] [Google Scholar]
- Gasiewicz T. A., Neal R. A. 2,3,7,8-Tetrachlorodibenzo-p-dioxin tissue distribution, excretion, and effects on clinical chemical parameters in guinea pigs. Toxicol Appl Pharmacol. 1979 Nov;51(2):329–339. doi: 10.1016/0041-008x(79)90475-7. [DOI] [PubMed] [Google Scholar]
- Horuk R., Rodbell M., Cushman S. W., Wardzala L. J. Proposed mechanism of insulin-resistant glucose transport in the isolated guinea pig adipocyte. Small intracellular pool of glucose transporters. J Biol Chem. 1983 Jun 25;258(12):7425–7429. [PubMed] [Google Scholar]
- Kaestner K. H., Christy R. J., McLenithan J. C., Braiterman L. T., Cornelius P., Pekala P. H., Lane M. D. Sequence, tissue distribution, and differential expression of mRNA for a putative insulin-responsive glucose transporter in mouse 3T3-L1 adipocytes. Proc Natl Acad Sci U S A. 1989 May;86(9):3150–3154. doi: 10.1073/pnas.86.9.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasanicki M. A., Pilch P. F. Regulation of glucose-transporter function. Diabetes Care. 1990 Mar;13(3):219–227. doi: 10.2337/diacare.13.3.219. [DOI] [PubMed] [Google Scholar]
- King A. P., Tai P. K., Carter-Su C. Cytochalasin B interferes with conformational changes of the human erythrocyte glucose transporter induced by internal and external sugar binding. Biochemistry. 1991 Dec 10;30(49):11546–11553. doi: 10.1021/bi00113a009. [DOI] [PubMed] [Google Scholar]
- Kozka I. J., Clark A. E., Holman G. D. Chronic treatment with insulin selectively down-regulates cell-surface GLUT4 glucose transporters in 3T3-L1 adipocytes. J Biol Chem. 1991 Jun 25;266(18):11726–11731. [PubMed] [Google Scholar]
- Kuruvilla A. K., Perez C., Ismail-Beigi F., Loeb J. N. Regulation of glucose transport in Clone 9 cells by thyroid hormone. Biochim Biophys Acta. 1991 Sep 24;1094(3):300–308. doi: 10.1016/0167-4889(91)90090-k. [DOI] [PubMed] [Google Scholar]
- Mahon M. J., Gasiewicz T. A. Chelatable metal ions are not required for aryl hydrocarbon receptor transformation to a DNA binding form: phenanthrolines are possible competitive antagonists of 2,3,7,8-tetrachlorodibenzo-p-dioxin. Arch Biochem Biophys. 1992 Aug 15;297(1):1–8. doi: 10.1016/0003-9861(92)90633-8. [DOI] [PubMed] [Google Scholar]
- Oka Y., Asano T., Shibasaki Y., Kasuga M., Kanazawa Y., Takaku F. Studies with antipeptide antibody suggest the presence of at least two types of glucose transporter in rat brain and adipocyte. J Biol Chem. 1988 Sep 15;263(26):13432–13439. [PubMed] [Google Scholar]
- Poellinger L., Göttlicher M., Gustafsson J. A. The dioxin and peroxisome proliferator-activated receptors: nuclear receptors in search of endogenous ligands. Trends Pharmacol Sci. 1992 Jun;13(6):241–245. doi: 10.1016/0165-6147(92)90076-i. [DOI] [PubMed] [Google Scholar]
- Poland A., Knutson J. C. 2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu Rev Pharmacol Toxicol. 1982;22:517–554. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- Rozman K., Rozman T., Greim H. Effect of thyroidectomy and thyroxine on 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) induced toxicity. Toxicol Appl Pharmacol. 1984 Feb;72(2):372–376. doi: 10.1016/0041-008x(84)90322-3. [DOI] [PubMed] [Google Scholar]
- Safe S., Bandiera S., Sawyer T., Robertson L., Safe L., Parkinson A., Thomas P. E., Ryan D. E., Reik L. M., Levin W. PCBs: structure-function relationships and mechanism of action. Environ Health Perspect. 1985 May;60:47–56. doi: 10.1289/ehp.856047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwetz B. A., Norris J. M., Sparschu G. L., Rowe U. K., Gehring P. J., Emerson J. L., Gerbig C. G. Toxicology of chlorinated dibenzo-p-dioxins. Environ Health Perspect. 1973 Sep;5:87–99. doi: 10.1289/ehp.730587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seefeld M. D., Corbett S. W., Keesey R. E., Peterson R. E. Characterization of the wasting syndrome in rats treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol. 1984 Apr;73(2):311–322. doi: 10.1016/0041-008x(84)90337-5. [DOI] [PubMed] [Google Scholar]
- Student A. K., Hsu R. Y., Lane M. D. Induction of fatty acid synthetase synthesis in differentiating 3T3-L1 preadipocytes. J Biol Chem. 1980 May 25;255(10):4745–4750. [PubMed] [Google Scholar]
- Unger R. H. Diabetic hyperglycemia: link to impaired glucose transport in pancreatic beta cells. Science. 1991 Mar 8;251(4998):1200–1205. doi: 10.1126/science.2006409. [DOI] [PubMed] [Google Scholar]
- Yang J., Holman G. D. Comparison of GLUT4 and GLUT1 subcellular trafficking in basal and insulin-stimulated 3T3-L1 cells. J Biol Chem. 1993 Mar 5;268(7):4600–4603. [PubMed] [Google Scholar]