Abstract
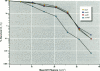
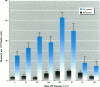
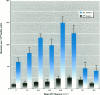
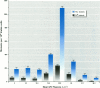
We compared mutagenic spectra induced by polychromatic near-ultraviolet radiation (near-UV; 300-400 nm) with superoxide anion (O2-) -dependent mutagenesis using a set of Escherichia coli tester strains. Near-UV radiation produced increased frequencies of G:C to A:T transitions, G:C to T:A and A:T to T:A transversions, and small increases in frameshift mutations in wild-type cells. Tester strains lacking superoxide dismutase (SOD) activity (sodAsodB double mutants) demonstrated high spontaneous mutation frequencies and increased near-UV sensitivity. The double mutants also showed increased mutations induced by near-UV compared to either isogenic wild type, sodA or sodB single mutants. Futhermore, these mutants had an unusual spontaneous mutation spectrum, with a predominance of A:T to T:A transversions, followed by G:C to T:A transversions and frameshifts generated in runs of adenines in both the +1 and -1 direction. Other frameshifts were detected to a lesser degree. The oxygen dependency and the type of mutations spontaneously induced in SOD-deficient cells indicated that this mutagenic spectrum was caused by oxidative DNA damage. However, no apparent synergistic action between near-UV radiation and an increased flux of O2- could be detected. From the frequency and types of mutations induced by the two agents, we speculate that near-UV-induced mutagenesis and O2--dependent mutagenesis involve, in part, different lesion(s) and/or mechanism(s). The nature and possible mutagenic pathways of each are discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science. 1983 Sep 23;221(4617):1256–1264. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- Ananthaswamy H. N., Eisenstark A. Near-UV-induced breaks in phage DNA: sensitization by hydrogen peroxide (a tryptophan photoproduct). Photochem Photobiol. 1976 Nov;24(5):439–442. doi: 10.1111/j.1751-1097.1976.tb06851.x. [DOI] [PubMed] [Google Scholar]
- Ananthaswamy H. N., Pierceall W. E. Molecular mechanisms of ultraviolet radiation carcinogenesis. Photochem Photobiol. 1990 Dec;52(6):1119–1136. doi: 10.1111/j.1751-1097.1990.tb08452.x. [DOI] [PubMed] [Google Scholar]
- Armstrong J. D., Kunz B. A. Site and strand specificity of UVB mutagenesis in the SUP4-o gene of yeast. Proc Natl Acad Sci U S A. 1990 Nov;87(22):9005–9009. doi: 10.1073/pnas.87.22.9005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au K. G., Cabrera M., Miller J. H., Modrich P. Escherichia coli mutY gene product is required for specific A-G----C.G mismatch correction. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9163–9166. doi: 10.1073/pnas.85.23.9163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Carlioz A., Touati D. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life? EMBO J. 1986 Mar;5(3):623–630. doi: 10.1002/j.1460-2075.1986.tb04256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham R. P., Saporito S. M., Spitzer S. G., Weiss B. Endonuclease IV (nfo) mutant of Escherichia coli. J Bacteriol. 1986 Dec;168(3):1120–1127. doi: 10.1128/jb.168.3.1120-1127.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupples C. G., Cabrera M., Cruz C., Miller J. H. A set of lacZ mutations in Escherichia coli that allow rapid detection of specific frameshift mutations. Genetics. 1990 Jun;125(2):275–280. doi: 10.1093/genetics/125.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupples C. G., Miller J. H. A set of lacZ mutations in Escherichia coli that allow rapid detection of each of the six base substitutions. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5345–5349. doi: 10.1073/pnas.86.14.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danpure H. J., Tyrrell R. M. Oxygen-dependence of near UV (365 NM) lethality and the interaction of near UV and X-rays in two mammalian cell lines. Photochem Photobiol. 1976 Mar;23(3):171–177. doi: 10.1111/j.1751-1097.1976.tb07238.x. [DOI] [PubMed] [Google Scholar]
- Demple B., Halbrook J., Linn S. Escherichia coli xth mutants are hypersensitive to hydrogen peroxide. J Bacteriol. 1983 Feb;153(2):1079–1082. doi: 10.1128/jb.153.2.1079-1082.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstark A. Bacterial genes involved in response to near-ultraviolet radiation. Adv Genet. 1989;26:99–147. doi: 10.1016/s0065-2660(08)60224-2. [DOI] [PubMed] [Google Scholar]
- Ewing D. Synergistic damage from H2O2 and OH radicals in irradiated cells. Radiat Res. 1983 Apr;94(1):171–189. [PubMed] [Google Scholar]
- Farr S. B., D'Ari R., Touati D. Oxygen-dependent mutagenesis in Escherichia coli lacking superoxide dismutase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8268–8272. doi: 10.1073/pnas.83.21.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr S. B., Kogoma T. Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol Rev. 1991 Dec;55(4):561–585. doi: 10.1128/mr.55.4.561-585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr S. B., Natvig D. O., Kogoma T. Toxicity and mutagenicity of plumbagin and the induction of a possible new DNA repair pathway in Escherichia coli. J Bacteriol. 1985 Dec;164(3):1309–1316. doi: 10.1128/jb.164.3.1309-1316.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner P. R., Fridovich I. Superoxide sensitivity of the Escherichia coli 6-phosphogluconate dehydratase. J Biol Chem. 1991 Jan 25;266(3):1478–1483. [PubMed] [Google Scholar]
- Gralla E. B., Valentine J. S. Null mutants of Saccharomyces cerevisiae Cu,Zn superoxide dismutase: characterization and spontaneous mutation rates. J Bacteriol. 1991 Sep;173(18):5918–5920. doi: 10.1128/jb.173.18.5918-5920.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg J. T., Demple B. Overproduction of peroxide-scavenging enzymes in Escherichia coli suppresses spontaneous mutagenesis and sensitivity to redox-cycling agents in oxyR-mutants. EMBO J. 1988 Aug;7(8):2611–2617. doi: 10.1002/j.1460-2075.1988.tb03111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. Oxidants and human disease: some new concepts. FASEB J. 1987 Nov;1(5):358–364. [PubMed] [Google Scholar]
- Hoerter J., Eisenstark A., Touati D. Mutations by near-ultraviolet radiation in Escherichia coli strains lacking superoxide dismutase. Mutat Res. 1989 Dec;215(2):161–165. doi: 10.1016/0027-5107(89)90179-6. [DOI] [PubMed] [Google Scholar]
- Imlay J. A., Linn S. Mutagenesis and stress responses induced in Escherichia coli by hydrogen peroxide. J Bacteriol. 1987 Jul;169(7):2967–2976. doi: 10.1128/jb.169.7.2967-2976.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li I. C., Chang C. C., Trosko J. E. Thymidylate synthetase gene as a quantitative mutation marker in Chinese hamster cells. Mutat Res. 1990 Mar;243(3):233–239. doi: 10.1016/0165-7992(90)90096-3. [DOI] [PubMed] [Google Scholar]
- McBride T. J., Preston B. D., Loeb L. A. Mutagenic spectrum resulting from DNA damage by oxygen radicals. Biochemistry. 1991 Jan 8;30(1):207–213. doi: 10.1021/bi00215a030. [DOI] [PubMed] [Google Scholar]
- Michaels M. L., Pham L., Nghiem Y., Cruz C., Miller J. H. MutY, an adenine glycosylase active on G-A mispairs, has homology to endonuclease III. Nucleic Acids Res. 1990 Jul 11;18(13):3841–3845. doi: 10.1093/nar/18.13.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. H., Low K. B. Specificity of mutagenesis resulting from the induction of the SOS system in the absence of mutagenic treatment. Cell. 1984 Jun;37(2):675–682. doi: 10.1016/0092-8674(84)90400-8. [DOI] [PubMed] [Google Scholar]
- Moody C. S., Hassan H. M. Mutagenicity of oxygen free radicals. Proc Natl Acad Sci U S A. 1982 May;79(9):2855–2859. doi: 10.1073/pnas.79.9.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nghiem Y., Cabrera M., Cupples C. G., Miller J. H. The mutY gene: a mutator locus in Escherichia coli that generates G.C----T.A transversions. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2709–2713. doi: 10.1073/pnas.85.8.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peak M. J., Jones C. A., Sedita B. A., Dudek E. J., Spitz D. R., Peak J. G. Evidence that hydrogen peroxide generated by 365-nm UVA radiation is not important in mammalian cell killing. Radiat Res. 1990 Aug;123(2):220–223. [PubMed] [Google Scholar]
- Peak M. J., Peak J. G. Solar-ultraviolet-induced damage to DNA. Photodermatol. 1989 Feb;6(1):1–15. [PubMed] [Google Scholar]
- Prieto-Alamo M. J., Abril N., Pueyo C. Mutagenesis in Escherichia coli K-12 mutants defective in superoxide dismutase or catalase. Carcinogenesis. 1993 Feb;14(2):237–244. doi: 10.1093/carcin/14.2.237. [DOI] [PubMed] [Google Scholar]
- Smyk-Randall E., Brown O. R., Wilke A., Eisenstark A., Flint D. H. Near ultraviolet light inactivation of dihydroxyacid dehydratase in Escherichia coli. Free Radic Biol Med. 1993 Jun;14(6):609–613. doi: 10.1016/0891-5849(93)90142-h. [DOI] [PubMed] [Google Scholar]
- Storz G., Christman M. F., Sies H., Ames B. N. Spontaneous mutagenesis and oxidative damage to DNA in Salmonella typhimurium. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8917–8921. doi: 10.1073/pnas.84.24.8917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touati D. Molecular genetics of superoxide dismutases. Free Radic Biol Med. 1988;5(5-6):393–402. doi: 10.1016/0891-5849(88)90113-x. [DOI] [PubMed] [Google Scholar]
- Turner M. A., Webb R. B. Comparative mutagenesis and interaction between near-ultraviolet (313- to 405-nm) and far-ultraviolet (254-nm) radiation in Escherichia coli strains with differing repair capabilities. J Bacteriol. 1981 Aug;147(2):410–417. doi: 10.1128/jb.147.2.410-417.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]