Abstract
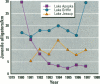
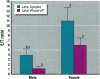
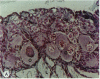
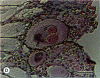
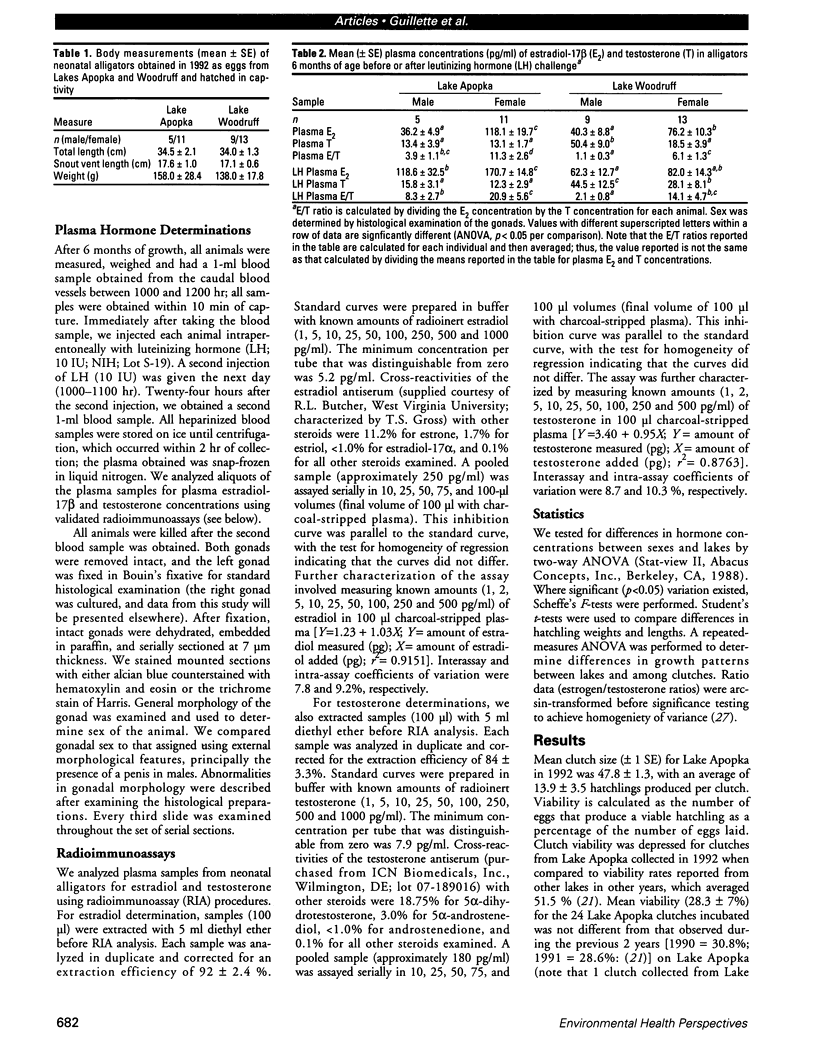
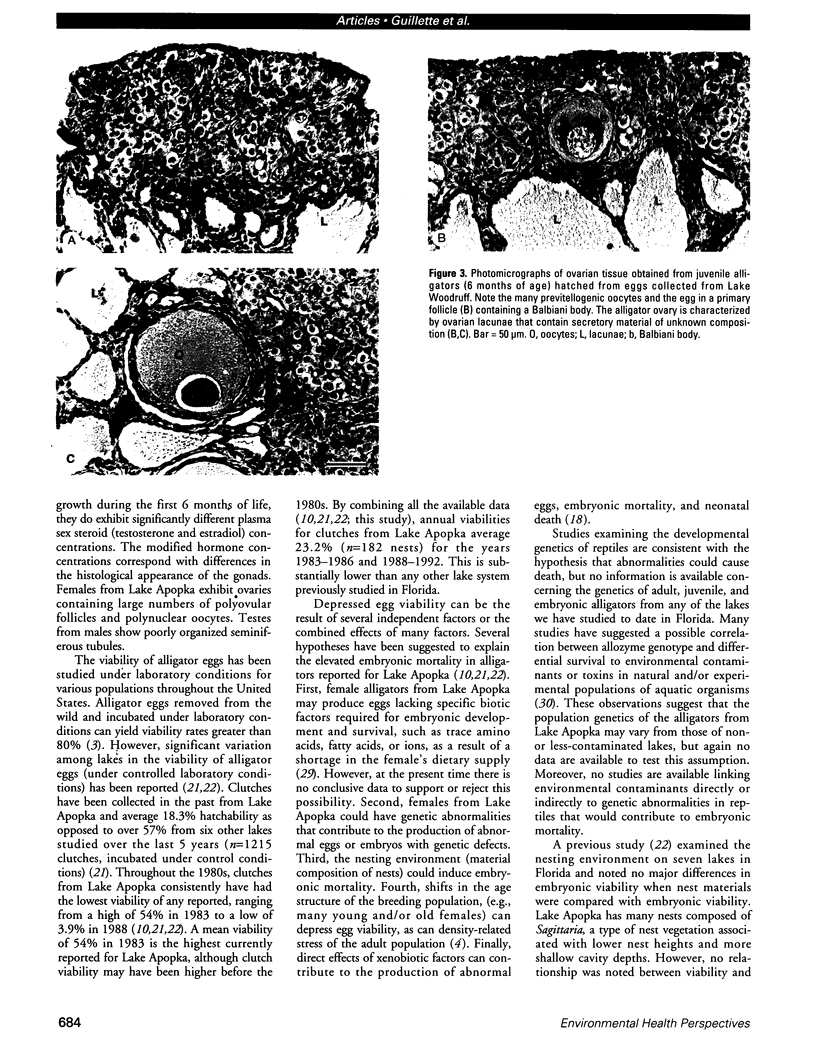
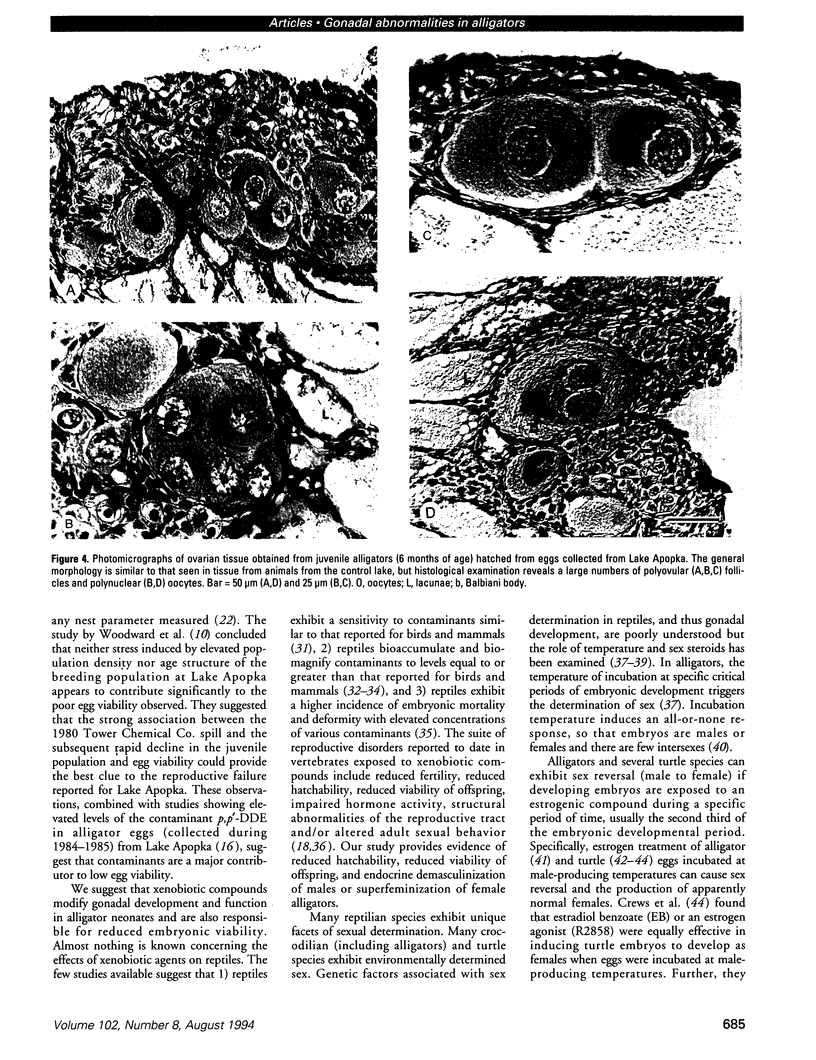
The reproductive development of alligators from a contaminated and a control lake in central Florida was examined. Lake Apopka is adjacent to an EPA Superfund site, listed due to an extensive spill of dicofol and DDT or its metabolites. These compounds can act as estrogens. Contaminants in the lake also have been derived from extensive agricultural activities around the lake that continue today and a sewage treatment facility associated with the city of Winter Garden, Florida. We examined the hypothesis that an estrogenic contaminant has caused the current failure in recruitment of alligators on Lake Apopka. Supporting data include the following: At 6 months of age, female alligators from Lake Apopka had plasma estradiol-17 beta concentrations almost two times greater than normal females from the control lake, Lake Woodruff. The Apopka females exhibited abnormal ovarian morphology with large numbers of polyovular follicles and polynuclear oocytes. Male juvenile alligators had significantly depressed plasma testosterone concentrations comparable to levels observed in normal Lake Woodruff females but more than three times lower than normal Lake Woodruff males. Additionally, males from Lake Apopka had poorly organized testes and abnormally small phalli. The differences between lakes and sexes in plasma hormone concentrations of juvenile alligators remain even after stimulation with luteinizing hormone. Our data suggest that the gonads of juveniles from Lake Apopka have been permanently modified in ovo, so that normal steroidogenesis is not possible, and thus normal sexual maturation is unlikely.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin H. B. The effects of estradiol and testosterone on mullerian-duct regression in the American alligator (Alligator mississippiensis). Gen Comp Endocrinol. 1989 Dec;76(3):461–472. doi: 10.1016/0016-6480(89)90143-3. [DOI] [PubMed] [Google Scholar]
- Bishop C. A., Brooks R. J., Carey J. H., Ng P., Norstrom R. J., Lean D. R. The case for a cause-effect linkage between environmental contamination and development in eggs of the common snapping turtle (Chelydra S.serpentina) from Ontario, Canada. J Toxicol Environ Health. 1991 Aug;33(4):521–547. doi: 10.1080/15287399109531539. [DOI] [PubMed] [Google Scholar]
- Bryan A. M., Stone W. B., Olafsson P. G. Disposition of toxic PCB congeners in snapping turtle eggs: expressed as toxic equivalents of TCDD. Bull Environ Contam Toxicol. 1987 Nov;39(5):791–796. doi: 10.1007/BF01855856. [DOI] [PubMed] [Google Scholar]
- Bull J. J., Gutzke W. H., Crews D. Sex reversal by estradiol in three reptilian orders. Gen Comp Endocrinol. 1988 Jun;70(3):425–428. doi: 10.1016/0016-6480(88)90117-7. [DOI] [PubMed] [Google Scholar]
- Crews D., Wibbels T., Gutzke W. H. Action of sex steroid hormones on temperature-induced sex determination in the snapping turtle (Chelydra serpentina). Gen Comp Endocrinol. 1989 Oct;76(1):159–166. doi: 10.1016/0016-6480(89)90042-7. [DOI] [PubMed] [Google Scholar]
- Deeming D. C., Ferguson M. W. Environmental regulation of sex determination in reptiles. Philos Trans R Soc Lond B Biol Sci. 1988 Dec 1;322(1208):19–39. doi: 10.1098/rstb.1988.0111. [DOI] [PubMed] [Google Scholar]
- DiBartolomeis M. J., Moore R. W., Peterson R. E., Christian B. J., Jefcoate C. R. Altered regulation of adrenal steroidogenesis in 2,3,7,8-tetrachlorodibenzo-p-dioxin-treated rats. Biochem Pharmacol. 1987 Jan 1;36(1):59–67. doi: 10.1016/0006-2952(87)90382-0. [DOI] [PubMed] [Google Scholar]
- DiBartolomeis M. J., Moore R. W., Peterson R. E., Jefcoate C. R. Hypercholesterolemia and the regulation of adrenal steroidogenesis in 2,3,7,8-tetrachlorodibenzo-p-dioxin-treated rats. Toxicol Appl Pharmacol. 1986 Sep 30;85(3):313–323. doi: 10.1016/0041-008x(86)90338-8. [DOI] [PubMed] [Google Scholar]
- Faber K. A., Basham K., Hughes C. L., Jr The effect of neonatal exposure to DES and o,p'-DDT on pituitary responsiveness to GnRH in adult castrated rats. Reprod Toxicol. 1991;5(4):363–369. doi: 10.1016/0890-6238(91)90095-w. [DOI] [PubMed] [Google Scholar]
- Faber K. A., Hughes C. L., Jr The effect of neonatal exposure to diethylstilbestrol, genistein, and zearalenone on pituitary responsiveness and sexually dimorphic nucleus volume in the castrated adult rat. Biol Reprod. 1991 Oct;45(4):649–653. doi: 10.1095/biolreprod45.4.649. [DOI] [PubMed] [Google Scholar]
- Ferguson M. W., Joanen T. Temperature of egg incubation determines sex in Alligator mississippiensis. Nature. 1982 Apr 29;296(5860):850–853. doi: 10.1038/296850a0. [DOI] [PubMed] [Google Scholar]
- Gutzke W. H., Bull J. J. Steroid hormones reverse sex in turtles. Gen Comp Endocrinol. 1986 Dec;64(3):368–372. doi: 10.1016/0016-6480(86)90070-5. [DOI] [PubMed] [Google Scholar]
- Halling A., Forsberg J. G. Effects of neonatal exposure to diethylstilbestrol on early mouse embryo development in vivo and in vitro. Biol Reprod. 1991 Jul;45(1):157–162. doi: 10.1095/biolreprod45.1.157. [DOI] [PubMed] [Google Scholar]
- Iguchi T. Cellular effects of early exposure to sex hormones and antihormones. Int Rev Cytol. 1992;139:1–57. doi: 10.1016/s0074-7696(08)61409-6. [DOI] [PubMed] [Google Scholar]
- Iguchi T., Fukazawa Y., Uesugi Y., Takasugi N. Polyovular follicles in mouse ovaries exposed neonatally to diethylstilbestrol in vivo and in vitro. Biol Reprod. 1990 Sep;43(3):478–484. doi: 10.1095/biolreprod43.3.478. [DOI] [PubMed] [Google Scholar]
- Iguchi T., Kamiya K., Uesugi Y., Sayama K., Takasugi N. In vitro fertilization of oocytes from polyovular follicles in mouse ovaries exposed neonatally to diethylstilbestrol. In Vivo. 1991 Jul-Aug;5(4):359–363. [PubMed] [Google Scholar]
- Janzen F. J., Paukstis G. L. Environmental sex determination in reptiles: ecology, evolution, and experimental design. Q Rev Biol. 1991 Jun;66(2):149–179. doi: 10.1086/417143. [DOI] [PubMed] [Google Scholar]
- Moore R. W., Jefcoate C. R., Peterson R. E. 2,3,7,8-Tetrachlorodibenzo-p-dioxin inhibits steroidogenesis in the rat testis by inhibiting the mobilization of cholesterol to cytochrome P450scc. Toxicol Appl Pharmacol. 1991 Jun 1;109(1):85–97. doi: 10.1016/0041-008x(91)90193-i. [DOI] [PubMed] [Google Scholar]
- Moore R. W., Parsons J. A., Bookstaff R. C., Peterson R. E. Plasma concentrations of pituitary hormones in 2,3,7,8-tetrachlorodibenzo-p-dioxin-treated male rats. J Biochem Toxicol. 1989 Fall;4(3):165–172. doi: 10.1002/jbt.2570040305. [DOI] [PubMed] [Google Scholar]
- Moore R. W., Potter C. L., Theobald H. M., Robinson J. A., Peterson R. E. Androgenic deficiency in male rats treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol. 1985 Jun 15;79(1):99–111. doi: 10.1016/0041-008x(85)90372-2. [DOI] [PubMed] [Google Scholar]
- Sharpe R. M., Skakkebaek N. E. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet. 1993 May 29;341(8857):1392–1395. doi: 10.1016/0140-6736(93)90953-e. [DOI] [PubMed] [Google Scholar]