Abstract
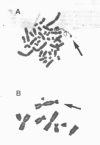
Carcinogenic, water-insoluble Ni compounds are phagocytized by cells; and the particles undergo dissolution inside the cell, releasing Ni ions that interact with chromatin. Ni produces highly selective damage to heterochromatin. The longest contiguous region of heterochromatin in the Chinese hamster genome is found on the q arm of the X chromosome, and this region is selectively damaged by Ni. More than half of the male mice in which there were Ni-induced transformations of Chinese hamster cells exhibited complete deletion of the long arm of the X chromosome. The introduction of a normal X chromosome into these cells resulted in cellular senescence, suggesting that the Ni interacted with Chinese hamster genome to inactivate a senescence gene. Investigations were conducted into the mechanisms by which Ni produced damage to chromatin. Ni ions have a much higher affinity for proteins and amino acids than for DNA (by five to seven orders of magnitude). Therefore, Ni interacted with chromatin because of the protein present, not because of its reactivity for DNA. Studies have shown that Ni produced an increase in oxidative products in cells as indicated by oxidation of the fluorescent dye dichlorofluorescein; Ni has also been shown to produce oxidation of proteins in cells, as measured by carbonyl formation. Ni cross-linked certain amino acids and proteins to DNA. These covalent cross-links were not dissociated by EDTA and are inconsistent with direct Ni involvement, but they are consistent with Ni acting catalytically. Using subtractive hybridization, we have isolated a number of clones that are expressed in normal but not in Ni-transformed cells.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Castle V. P., Ou X., O'Rourke K., Dixit V. M. High level thrombospondin 1 expression in two NIH 3T3 cloned lines confers serum- and anchorage-independent growth. J Biol Chem. 1993 Feb 5;268(4):2899–2903. [PubMed] [Google Scholar]
- Conway K., Costa M. Nonrandom chromosomal alterations in nickel-transformed Chinese hamster embryo cells. Cancer Res. 1989 Nov 1;49(21):6032–6038. [PubMed] [Google Scholar]
- Costa M. Molecular mechanisms of nickel carcinogenesis. Annu Rev Pharmacol Toxicol. 1991;31:321–337. doi: 10.1146/annurev.pa.31.040191.001541. [DOI] [PubMed] [Google Scholar]
- Huang X., Frenkel K., Klein C. B., Costa M. Nickel induces increased oxidants in intact cultured mammalian cells as detected by dichlorofluorescein fluorescence. Toxicol Appl Pharmacol. 1993 May;120(1):29–36. doi: 10.1006/taap.1993.1083. [DOI] [PubMed] [Google Scholar]
- Kasprzak K. S. The role of oxidative damage in metal carcinogenicity. Chem Res Toxicol. 1991 Nov-Dec;4(6):604–615. doi: 10.1021/tx00024a002. [DOI] [PubMed] [Google Scholar]
- Klein C. B., Conway K., Wang X. W., Bhamra R. K., Lin X. H., Cohen M. D., Annab L., Barrett J. C., Costa M. Senescence of nickel-transformed cells by an X chromosome: possible epigenetic control. Science. 1991 Feb 15;251(4995):796–799. doi: 10.1126/science.1990442. [DOI] [PubMed] [Google Scholar]
- Klein C. B., Frenkel K., Costa M. The role of oxidative processes in metal carcinogenesis. Chem Res Toxicol. 1991 Nov-Dec;4(6):592–604. doi: 10.1021/tx00024a001. [DOI] [PubMed] [Google Scholar]
- Levine R. L., Garland D., Oliver C. N., Amici A., Climent I., Lenz A. G., Ahn B. W., Shaltiel S., Stadtman E. R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- Lin X., Zhuang Z., Costa M. Analysis of residual amino acid--DNA crosslinks induced in intact cells by nickel and chromium compounds. Carcinogenesis. 1992 Oct;13(10):1763–1768. doi: 10.1093/carcin/13.10.1763. [DOI] [PubMed] [Google Scholar]
- Mosher D. F. Physiology of thrombospondin. Annu Rev Med. 1990;41:85–97. doi: 10.1146/annurev.me.41.020190.000505. [DOI] [PubMed] [Google Scholar]
- Patierno S. R., Costa M. Effects of nickel(II) on nuclear protein binding to DNA in intact mammalian cells. Cancer Biochem Biophys. 1987 May;9(2):113–126. [PubMed] [Google Scholar]
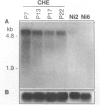
- Salnikow K., Cosentino S., Klein C., Costa M. Loss of thrombospondin transcriptional activity in nickel-transformed cells. Mol Cell Biol. 1994 Jan;14(1):851–858. doi: 10.1128/mcb.14.1.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. W., Lin X., Klein C. B., Bhamra R. K., Lee Y. W., Costa M. A conserved region in human and Chinese hamster X chromosomes can induce cellular senescence of nickel-transformed Chinese hamster cell lines. Carcinogenesis. 1992 Apr;13(4):555–561. doi: 10.1093/carcin/13.4.555. [DOI] [PubMed] [Google Scholar]