Abstract
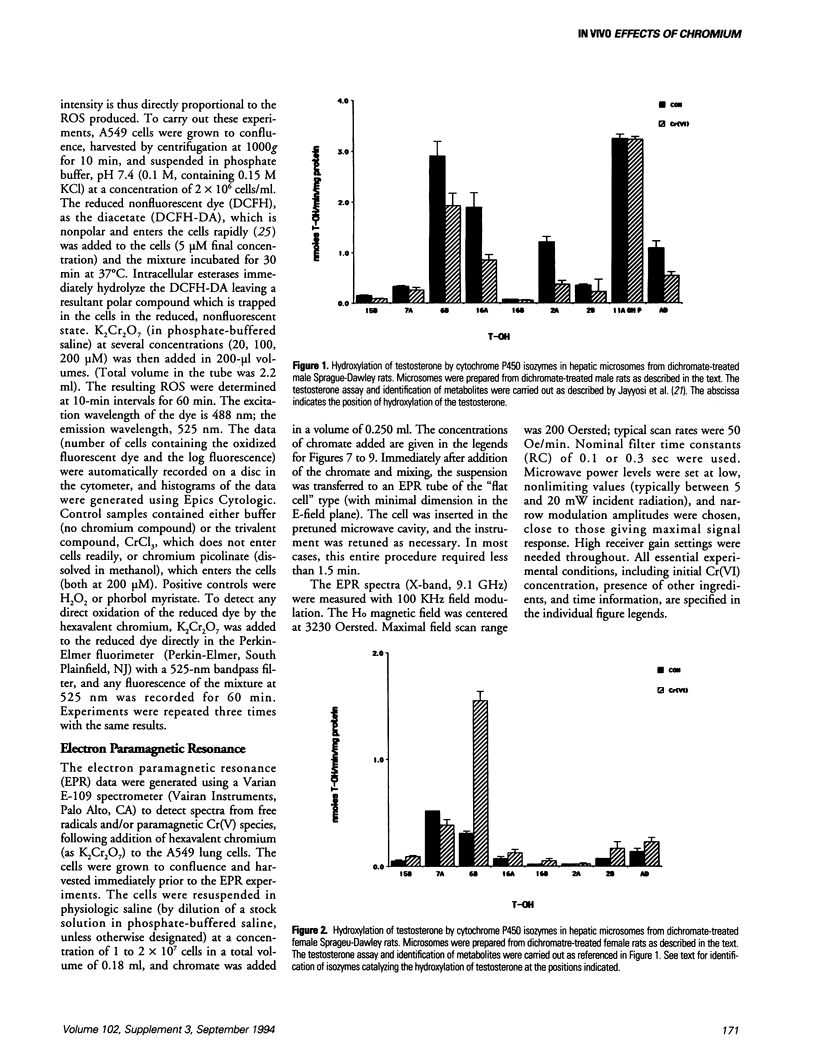
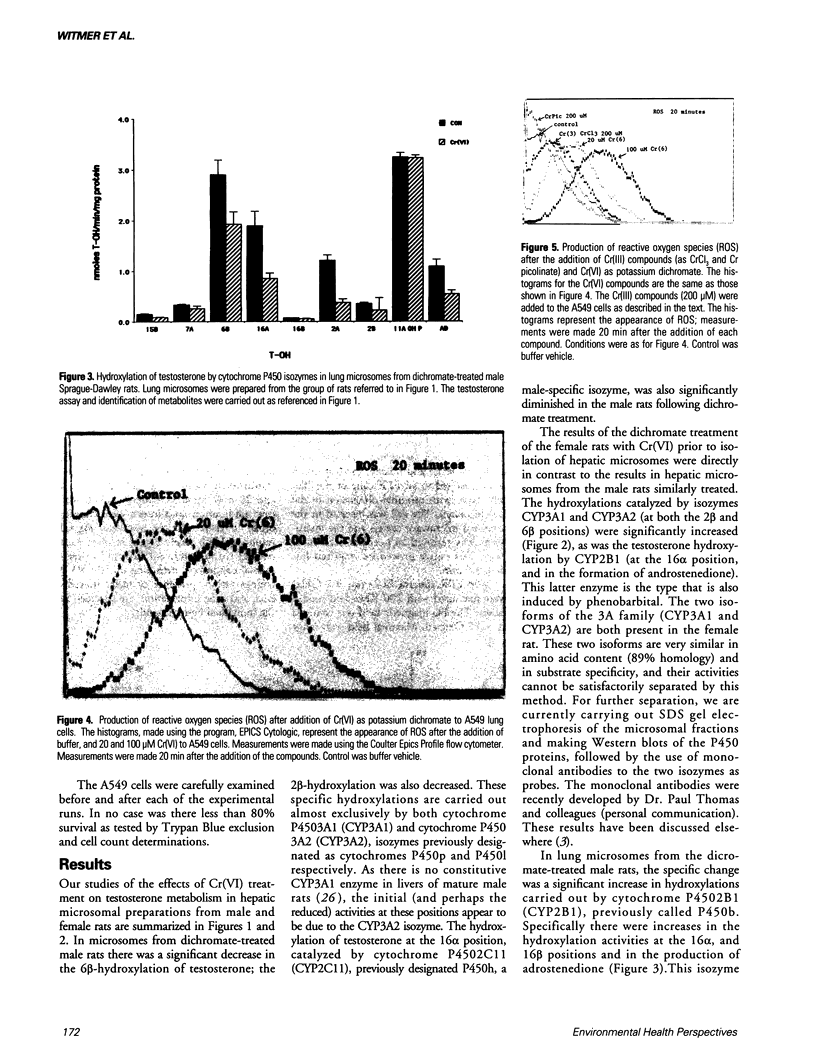
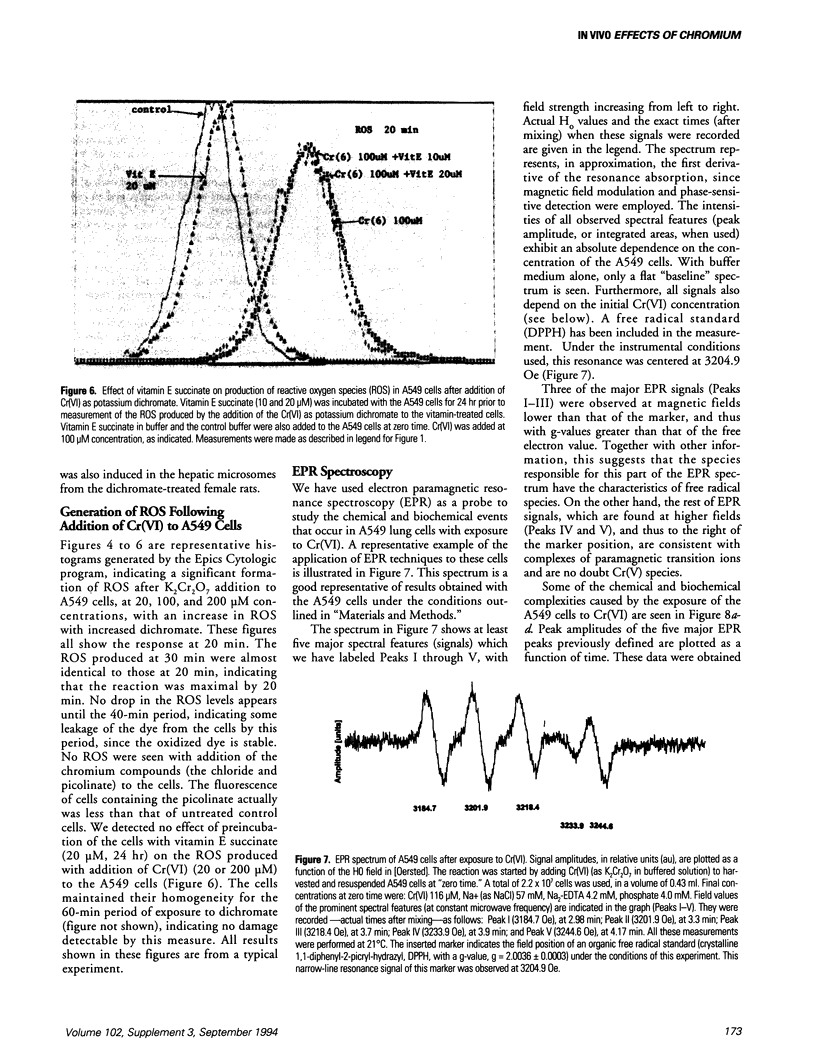
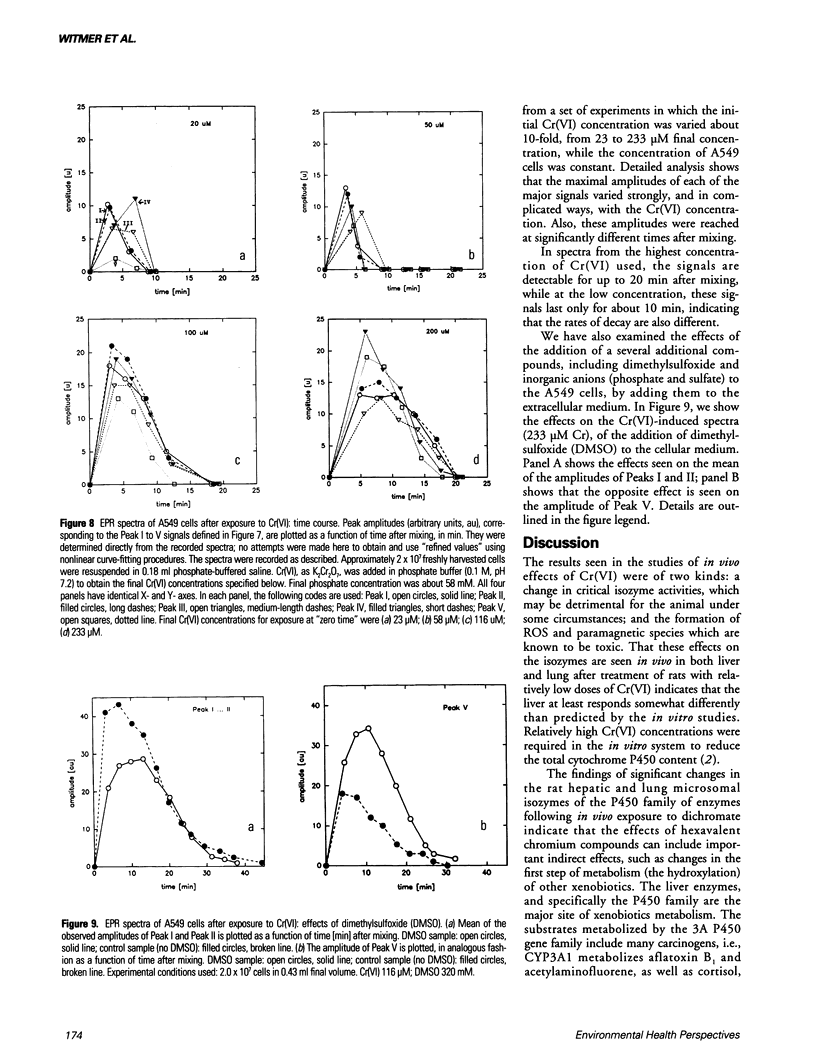
The production of reactive oxygen species on addition of hexavalent chromium (potassium dichromate, K2Cr2O7) to lung cells in culture was studied using flow cytometer analysis. A Coulter Epics Profile II flow cytometer was used to detect the formation of reactive oxygen species after K2Cr2O7 was added to A549 cells grown to confluence. The cells were loaded with the dye, 2',7'-dichlorofluorescein diacetate, after which cellular esterases removed the acetate groups and the dye was trapped intracellularly. Reactive oxygen species oxidized the dye, with resultant fluorescence. Increased doses of Cr(VI) caused increasing fluorescence (10-fold higher than background at 200 microM). Addition of Cr(III) compounds, as the picolinate or chloride, caused no increased fluorescence. Electron paramagnetic resonance (EPR) spectroscopic studies indicated that three (as yet unidentified) spectral "signals" of the free radical type were formed on addition of 20, 50, 100, and 200 microM Cr(VI) to the A549 cells in suspension. Two other EPR "signals" with the characteristics of Cr(V) entities were seen at field values lower than the standard free radical value. Liver microsomes from male Sprague-Dawley rats treated intraperitoneally with K2Cr2O7 (130 mumole/kg every 48 hr for six treatments) had decreased activity of cytochromes P4503A1 and/or 3A2, and 2C11. Hepatic microsomes from treated female Sprague-Dawley rats, in contrast, had increased activities of these isozymes. Lung microsomes from male Sprague-Dawley rats had increased activity of P4502C11.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiyar J., Berkovits H. J., Floyd R. A., Wetterhahn K. E. Reaction of chromium(VI) with glutathione or with hydrogen peroxide: identification of reactive intermediates and their role in chromium(VI)-induced DNA damage. Environ Health Perspect. 1991 May;92:53–62. doi: 10.1289/ehp.919253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIDSTRUP P. L., CASE R. A. Carcinoma of the lung in workmen in the bichromates-producing industry in Great Britain. Br J Ind Med. 1956 Oct;13(4):260–264. doi: 10.1136/oem.13.4.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass D. A., Parce J. W., Dechatelet L. R., Szejda P., Seeds M. C., Thomas M. Flow cytometric studies of oxidative product formation by neutrophils: a graded response to membrane stimulation. J Immunol. 1983 Apr;130(4):1910–1917. [PubMed] [Google Scholar]
- Cooper K. O., Reik L. M., Jayyosi Z., Bandiera S., Kelley M., Ryan D. E., Daniel R., McCluskey S. A., Levin W., Thomas P. E. Regulation of two members of the steroid-inducible cytochrome P450 subfamily (3A) in rats. Arch Biochem Biophys. 1993 Mar;301(2):345–354. doi: 10.1006/abbi.1993.1154. [DOI] [PubMed] [Google Scholar]
- Costa M., Cantoni O., de Mars M., Swartzendruber D. E. Toxic metals produce an S-phase-specific cell cycle block. Res Commun Chem Pathol Pharmacol. 1982 Dec;38(3):405–419. [PubMed] [Google Scholar]
- Davies D. M., Holdsworth E. S., Sherriff J. L. The isolation of glucose tolerance factors from brewer's yeast and their relationship to chromium. Biochem Med. 1985 Jun;33(3):297–311. doi: 10.1016/0006-2944(85)90004-3. [DOI] [PubMed] [Google Scholar]
- De Flora S., Morelli A., Basso C., Romano M., Serra D., De Flora A. Prominent role of DT-diaphorase as a cellular mechanism reducing chromium(VI) and reverting its mutagenicity. Cancer Res. 1985 Jul;45(7):3188–3196. [PubMed] [Google Scholar]
- Goodgame D. M., Joy A. M. Relatively long-lived chromium(V) species are produced by the action of glutathione on carcinogenic chromium(VI). J Inorg Biochem. 1986 Mar;26(3):219–224. doi: 10.1016/0162-0134(86)80044-7. [DOI] [PubMed] [Google Scholar]
- Jayyosi Z., Cooper K. O., Thomas P. E. Brain cytochrome P450 and testosterone metabolism by rat brain subcellular fractions: presence of cytochrome P450 3A immunoreactive protein in rat brain mitochondria. Arch Biochem Biophys. 1992 Oct;298(1):265–270. doi: 10.1016/0003-9861(92)90122-d. [DOI] [PubMed] [Google Scholar]
- Kawanishi S., Inoue S., Sano S. Mechanism of DNA cleavage induced by sodium chromate(VI) in the presence of hydrogen peroxide. J Biol Chem. 1986 May 5;261(13):5952–5958. [PubMed] [Google Scholar]
- MERTZ W., SCHWARZ K. Relation of glucose tolerance factor to impaired intravenous glucose tolerance of rats on stock diets. Am J Physiol. 1959 Mar;196(3):614–618. doi: 10.1152/ajplegacy.1959.196.3.614. [DOI] [PubMed] [Google Scholar]
- Petrilli F. L., De Flora S. Metabolic deactivation of hexavalent chromium mutagenicity. Mutat Res. 1978 Oct;54(2):139–147. doi: 10.1016/0165-1161(78)90034-1. [DOI] [PubMed] [Google Scholar]
- Petrilli F. L., Rossi G. A., Camoirano A., Romano M., Serra D., Bennicelli C., De Flora A., De Flora S. Metabolic reduction of chromium by alveolar macrophages and its relationships to cigarette smoke. J Clin Invest. 1986 Jun;77(6):1917–1924. doi: 10.1172/JCI112520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder R. D. Role of active oxygen species in metal-induced DNA strand breakage in human diploid fibroblasts. Mutat Res. 1988 May;193(3):237–246. doi: 10.1016/0167-8817(88)90034-x. [DOI] [PubMed] [Google Scholar]
- Srivastava S. K., Beutler E. The transport of oxidized glutathione from the erythrocytes of various species in the presence of chromate. Biochem J. 1969 Oct;114(4):833–837. doi: 10.1042/bj1140833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama M. Effects of vitamins on chromium(VI)-induced damage. Environ Health Perspect. 1991 May;92:63–70. doi: 10.1289/ehp.919263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama M. Role of physiological antioxidants in chromium(VI)-induced cellular injury. Free Radic Biol Med. 1992;12(5):397–407. doi: 10.1016/0891-5849(92)90089-y. [DOI] [PubMed] [Google Scholar]
- Suzuki Y. Reduction of hexavalent chromium by ascorbic acid in rat lung lavage fluid. Arch Toxicol. 1988;62(2-3):116–122. doi: 10.1007/BF00570129. [DOI] [PubMed] [Google Scholar]
- Thomas P. E., Reik L. M., Ryan D. E., Levin W. Induction of two immunochemically related rat liver cytochrome P-450 isozymes, cytochromes P-450c and P-450d, by structurally diverse xenobiotics. J Biol Chem. 1983 Apr 10;258(7):4590–4598. [PubMed] [Google Scholar]
- Witmer C. M., Harris R., Shupack S. I. Oral bioavailability of chromium from a specific site. Environ Health Perspect. 1991 May;92:105–110. doi: 10.1289/ehp.92-1519374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witmer C. M., Park H. S., Shupack S. I. Mutagenicity and disposition of chromium. Sci Total Environ. 1989 Oct 1;86(1-2):131–148. doi: 10.1016/0048-9697(89)90200-3. [DOI] [PubMed] [Google Scholar]




