Abstract
Carcinogenic arsenic, nickel, and chromium compounds induced morphological and neoplastic transformation but no mutation to ouabain resistance in 10T1/2 mouse embryo cells; lead chromate also did not induce mutation to ouabain or 6-thioguanine resistance in Chinese hamster ovary cells. The mechanism of metal-induced morphological transformation was likely not due to the specific base substitution mutations measured in ouabain resistance mutation assays, and for lead chromate, likely not due to this type of base substitution mutation or to frameshift mutations. Preliminary data indicate increases in steady-state levels of c-myc RNA in arsenic-, nickel-, and chromium-transformed cell lines. We also showed that carcinogenic nickel, chromium, and arsenic compounds and N-methyl-N-nitro-N-nitrosoguanidine (MNNG) induced stable anchorage independence (Al) in diploid human fibroblasts (DHF) but no focus formation or immortality. Nickel subsulfide and lead chromate induced Al but not mutation to 6-thioguanine resistance. The mechanism of induction of Al by metal salts in DHF was likely not by the type of base substitution or frameshift mutations measured in these assays. MNNG induced Al, mutation to 6-thioguanine resistance, and mutation to ouabain resistance, and might induce Al by base substitution or frameshift mutations. Dexamethasone, aspirin, and salicylic acid inhibited nickel subsulfide, MNNG, and 12-O-tetrade-canoylphorbol-13-acetate (TPA)-induced Al in DHF, suggesting that arachidonic acid metabolism and oxygen radical generation play a role in induction of Al. We propose that nickel compounds stimulate arachidonic acid metabolism, consequent oxygen radical generation, and oxygen radical attack upon DNA.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF
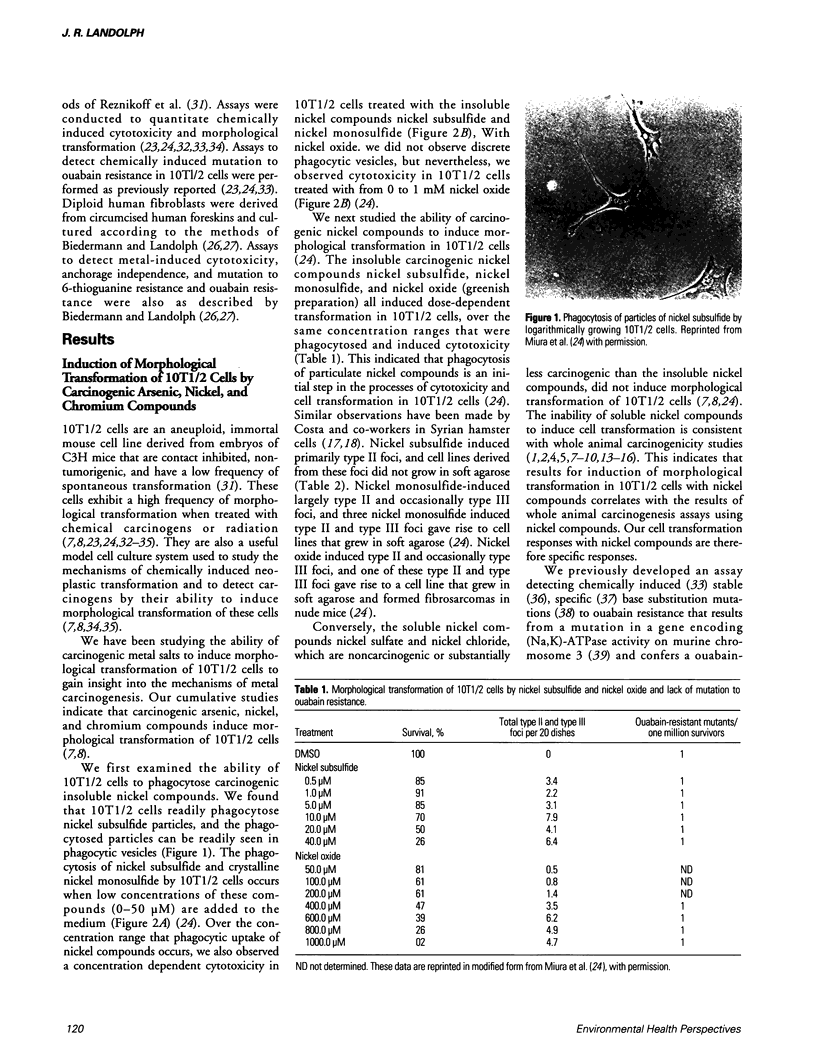
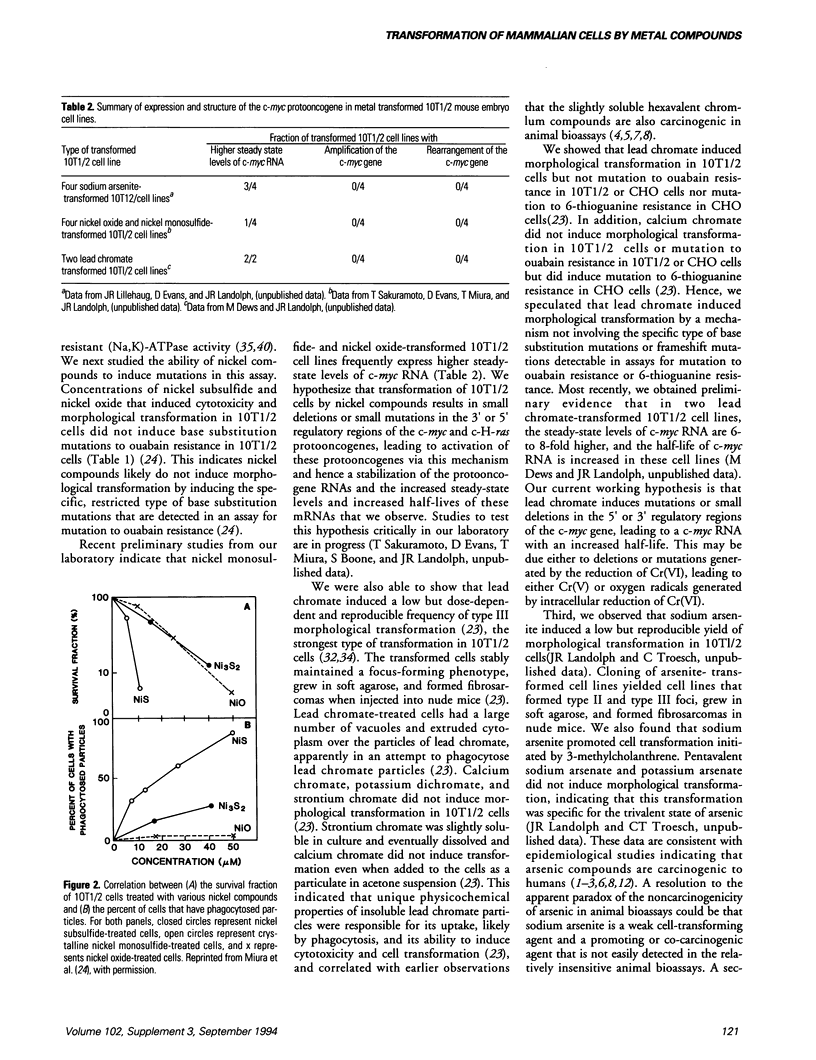
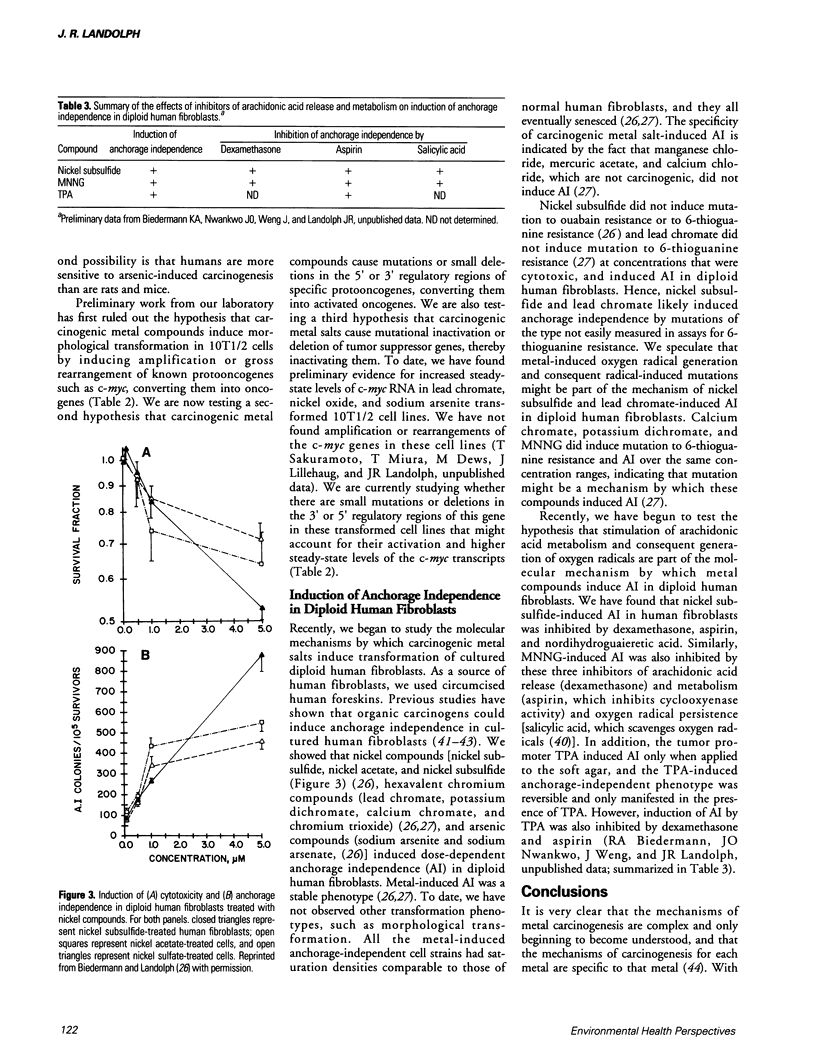



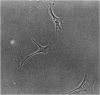
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biedermann K. A., Landolph J. R. Induction of anchorage independence in human diploid foreskin fibroblasts by carcinogenic metal salts. Cancer Res. 1987 Jul 15;47(14):3815–3823. [PubMed] [Google Scholar]
- Biedermann K. A., Landolph J. R. Role of valence state and solubility of chromium compounds on induction of cytotoxicity, mutagenesis, and anchorage independence in diploid human fibroblasts. Cancer Res. 1990 Dec 15;50(24):7835–7842. [PubMed] [Google Scholar]
- Bishop J. M. The molecular genetics of cancer. Science. 1987 Jan 16;235(4786):305–311. doi: 10.1126/science.3541204. [DOI] [PubMed] [Google Scholar]
- Ciccarelli R. B., Wetterhahn K. E. Nickel distribution and DNA lesions induced in rat tissues by the carcinogen nickel carbonate. Cancer Res. 1982 Sep;42(9):3544–3549. [PubMed] [Google Scholar]
- Ciccarelli R. B., Wetterhahn K. E. Nickel-bound chromatin, nucleic acids, and nuclear proteins from kidney and liver of rats treated with nickel carbonate in vivo. Cancer Res. 1984 Sep;44(9):3892–3897. [PubMed] [Google Scholar]
- Costa M., Mollenhauer H. H. Carcinogenic activity of particulate nickel compounds is proportional to their cellular uptake. Science. 1980 Jul 25;209(4455):515–517. doi: 10.1126/science.7394519. [DOI] [PubMed] [Google Scholar]
- Costa M., Nye J. S., Sunderman F. W., Jr, Allpass P. R., Gondos B. Induction of sarcomas in nude mice by implantation of Syrian hamster fetal cells exposed in vitro to nickel subsulfide. Cancer Res. 1979 Sep;39(9):3591–3597. [PubMed] [Google Scholar]
- Cupo D. Y., Wetterhahn K. E. Binding of chromium to chromatin and DNA from liver and kidney of rats treated with sodium dichromate and chromium(III) chloride in vivo. Cancer Res. 1985 Mar;45(3):1146–1151. [PubMed] [Google Scholar]
- DiPaolo J. A., Casto B. C. Quantitative studies of in vitro morphological transformation of Syrian hamster cells by inorganic metal salts. Cancer Res. 1979 Mar;39(3):1008–1013. [PubMed] [Google Scholar]
- Furst A., Schlauder M., Sasmore D. P. Tumorigenic activity of lead chromate. Cancer Res. 1976 May;36(5):1779–1783. [PubMed] [Google Scholar]
- Greiner J. W., Evans C. H., DiPaolo J. A. Carcinogen-induced anchorage-independent growth and in vivo lethality of human MRC-5 cells. Carcinogenesis. 1981;2(4):359–362. doi: 10.1093/carcin/2.4.359. [DOI] [PubMed] [Google Scholar]
- Heck J. D., Costa M. Influence of surface charge and dissolution on the selective phagocytosis of potentially carcinogenic particulate metal compounds. Cancer Res. 1983 Dec;43(12 Pt 1):5652–5656. [PubMed] [Google Scholar]
- Horowitz J. M., Yandell D. W., Park S. H., Canning S., Whyte P., Buchkovich K., Harlow E., Weinberg R. A., Dryja T. P. Point mutational inactivation of the retinoblastoma antioncogene. Science. 1989 Feb 17;243(4893):937–940. doi: 10.1126/science.2521957. [DOI] [PubMed] [Google Scholar]
- Klein C. B., Conway K., Wang X. W., Bhamra R. K., Lin X. H., Cohen M. D., Annab L., Barrett J. C., Costa M. Senescence of nickel-transformed cells by an X chromosome: possible epigenetic control. Science. 1991 Feb 15;251(4995):796–799. doi: 10.1126/science.1990442. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Cellular oncogenes and multistep carcinogenesis. Science. 1983 Nov 18;222(4625):771–778. doi: 10.1126/science.6356358. [DOI] [PubMed] [Google Scholar]
- Landolph J. R., Bhatt R. S., Telfer N., Heidelberger C. Comparison of adriamycin- and ouabain-induced cytotoxicity and inhibition of 86rubidium transport in wild-type and ouabain-resistant C3H/10T1/2 mouse fibroblasts. Cancer Res. 1980 Dec;40(12):4581–4588. [PubMed] [Google Scholar]
- Landolph J. R. Chemical transformation in C3H 10T1/2 Cl 8 mouse embryo fibroblasts: historical background, assessment of the transformation assay, and evolution and optimization of the transformation assay protocol. IARC Sci Publ. 1985;67:185–203. [PubMed] [Google Scholar]
- Landolph J. R., Fournier R. E. Microcell-mediated transfer of carcinogen-induced ouabain resistance from C3H/10T1/2 Cl 8 mouse fibroblasts to human cells. Mutat Res. 1983 Feb;107(2):447–463. doi: 10.1016/0027-5107(83)90183-5. [DOI] [PubMed] [Google Scholar]
- Landolph J. R., Heidelberger C. Chemical carcinogens produce mutations to ouabain resistance in transformable C3H/10T1/2 Cl 8 mouse fibroblasts. Proc Natl Acad Sci U S A. 1979 Feb;76(2):930–934. doi: 10.1073/pnas.76.2.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landolph J. R., Jones P. A. Mutagenicity of 5-azacytidine and related nucleosides in C3H/10T 1/2 clone 8 and V79 cells. Cancer Res. 1982 Mar;42(3):817–823. [PubMed] [Google Scholar]
- Landolph J. R. Molecular and cellular mechanisms of transformation of C3H/10T1/2 Cl 8 and diploid human fibroblasts by unique carcinogenic, nonmutagenic metal compounds. A review. Biol Trace Elem Res. 1989 Jul-Sep;21:459–467. doi: 10.1007/BF02917289. [DOI] [PubMed] [Google Scholar]
- Landolph J. R., Telfer N., Heidelberger C. Further evidence that ouabain-resistant variants induced by chemical carcinogens in transformable C3H/10T1/2 Cl 8 mouse fibroblasts are mutants. Mutat Res. 1980 Sep;72(2):295–310. doi: 10.1016/0027-5107(80)90044-5. [DOI] [PubMed] [Google Scholar]
- Lee T. C., Oshimura M., Barrett J. C. Comparison of arsenic-induced cell transformation, cytotoxicity, mutation and cytogenetic effects in Syrian hamster embryo cells in culture. Carcinogenesis. 1985 Oct;6(10):1421–1426. doi: 10.1093/carcin/6.10.1421. [DOI] [PubMed] [Google Scholar]
- Lee T. C., Tanaka N., Lamb P. W., Gilmer T. M., Barrett J. C. Induction of gene amplification by arsenic. Science. 1988 Jul 1;241(4861):79–81. doi: 10.1126/science.3388020. [DOI] [PubMed] [Google Scholar]
- Milo G. E., Jr, DiPaolo J. A. Neoplastic transformation of human diploid cells in vitro after chemical carcinogen treatment. Nature. 1978 Sep 14;275(5676):130–132. doi: 10.1038/275130a0. [DOI] [PubMed] [Google Scholar]
- Miura T., Patierno S. R., Sakuramoto T., Landolph J. R. Morphological and neoplastic transformation of C3H/10T1/2 Cl 8 mouse embryo cells by insoluble carcinogenic nickel compounds. Environ Mol Mutagen. 1989;14(2):65–78. doi: 10.1002/em.2850140202. [DOI] [PubMed] [Google Scholar]
- Nishimura M., Umeda M. Inducation of chromosomal aberrations in cultured mammalian cells by nickel compounds. Mutat Res. 1979 Dec;68(4):337–349. doi: 10.1016/0165-1218(79)90166-6. [DOI] [PubMed] [Google Scholar]
- Ottolenghi A. D., Haseman J. K., Payne W. W., Falk H. L., MacFarland H. N. Inhalation studies of nickel sulfide in pulmonary carcinogenesis of rats. J Natl Cancer Inst. 1975 May;54(5):1165–1172. doi: 10.1093/jnci/54.5.1165. [DOI] [PubMed] [Google Scholar]
- Patierno S. R., Banh D., Landolph J. R. Transformation of C3H/10T1/2 mouse embryo cells to focus formation and anchorage independence by insoluble lead chromate but not soluble calcium chromate: relationship to mutagenesis and internalization of lead chromate particles. Cancer Res. 1988 Sep 15;48(18):5280–5288. [PubMed] [Google Scholar]
- Peters J. M., Thomas D., Falk H., Oberdörster G., Smith T. J. Contribution of metals to respiratory cancer. Environ Health Perspect. 1986 Dec;70:71–83. doi: 10.1289/ehp.867071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J. F., Weller R. A., Schudlich R. R. Wind-driven ocean currents and ekman transport. Science. 1987 Dec 11;238(4833):1534–1538. doi: 10.1126/science.238.4833.1534. [DOI] [PubMed] [Google Scholar]
- Reznikoff C. A., Bertram J. S., Brankow D. W., Heidelberger C. Quantitative and qualitative studies of chemical transformation of cloned C3H mouse embryo cells sensitive to postconfluence inhibition of cell division. Cancer Res. 1973 Dec;33(12):3239–3249. [PubMed] [Google Scholar]
- Reznikoff C. A., Brankow D. W., Heidelberger C. Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res. 1973 Dec;33(12):3231–3238. [PubMed] [Google Scholar]
- Rivedal E., Sanner T. Metal salts as promoters of in vitro morphological transformation of hamster embryo cells initiated by benzo(a)pyrene. Cancer Res. 1981 Jul;41(7):2950–2953. [PubMed] [Google Scholar]
- Saxholm H. J., Reith A., Brøgger A. Oncogenic transformation and cell lysis in C3H/10T 1/2 cells and increased sister chromatid exchange in human lymphocytes by nickel subsulfide. Cancer Res. 1981 Oct;41(10):4136–4139. [PubMed] [Google Scholar]
- Shibuya M. L., Miura T., Lillehaug J. R., Farley R. A., Landolph J. R. Ouabain-resistant (Na+,K+)-ATPase enzyme activity in chemically induced ouabain-resistant C3H/10T1/2 cells. Mol Toxicol. 1989 Apr-Jun;2(2):75–98. [PubMed] [Google Scholar]
- Silinskas K. C., Kateley S. A., Tower J. E., Maher V. M., McCormick J. J. Induction of anchorage-independent growth in human fibroblasts by propane sultone. Cancer Res. 1981 May;41(5):1620–1627. [PubMed] [Google Scholar]
- Smith A. H., Hopenhayn-Rich C., Bates M. N., Goeden H. M., Hertz-Picciotto I., Duggan H. M., Wood R., Kosnett M. J., Smith M. T. Cancer risks from arsenic in drinking water. Environ Health Perspect. 1992 Jul;97:259–267. doi: 10.1289/ehp.9297259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderman F. W., Jr Carcinogenic effects of metals. Fed Proc. 1978 Jan;37(1):40–46. [PubMed] [Google Scholar]
- Sunderman F. W., Jr, Maenza R. M. Comparisons of carcinogenicities of nickel compounds in rats. Res Commun Chem Pathol Pharmacol. 1976 Jun;14(2):319–330. [PubMed] [Google Scholar]
- Sunderman F. W., Jr Mechanisms of nickel carcinogenesis. Scand J Work Environ Health. 1989 Feb;15(1):1–12. doi: 10.5271/sjweh.1888. [DOI] [PubMed] [Google Scholar]
- Tsapakos M. J., Hampton T. H., Jennette K. W. The carcinogen chromate induces DNA cross-links in rat liver and kidney. J Biol Chem. 1981 Apr 25;256(8):3623–3626. [PubMed] [Google Scholar]
- Weinberg R. A. The action of oncogenes in the cytoplasm and nucleus. Science. 1985 Nov 15;230(4727):770–776. doi: 10.1126/science.2997917. [DOI] [PubMed] [Google Scholar]
- Weissman B. E., Saxon P. J., Pasquale S. R., Jones G. R., Geiser A. G., Stanbridge E. J. Introduction of a normal human chromosome 11 into a Wilms' tumor cell line controls its tumorigenic expression. Science. 1987 Apr 10;236(4798):175–180. doi: 10.1126/science.3031816. [DOI] [PubMed] [Google Scholar]
- Wetterhahn K. E., Demple B., Kulesz-Martin M., Copeland E. S. Workshop report from the Division of Research Grants, National Institutes of Health. Metal carcinogenesis--a Chemical Pathology Study Section Workshop. Cancer Res. 1992 Jul 15;52(14):4058–4063. [PubMed] [Google Scholar]
- Yu M. C., Garabrant D. H., Peters J. M., Mack T. M. Tobacco, alcohol, diet, occupation, and carcinoma of the esophagus. Cancer Res. 1988 Jul 1;48(13):3843–3848. [PubMed] [Google Scholar]