Abstract
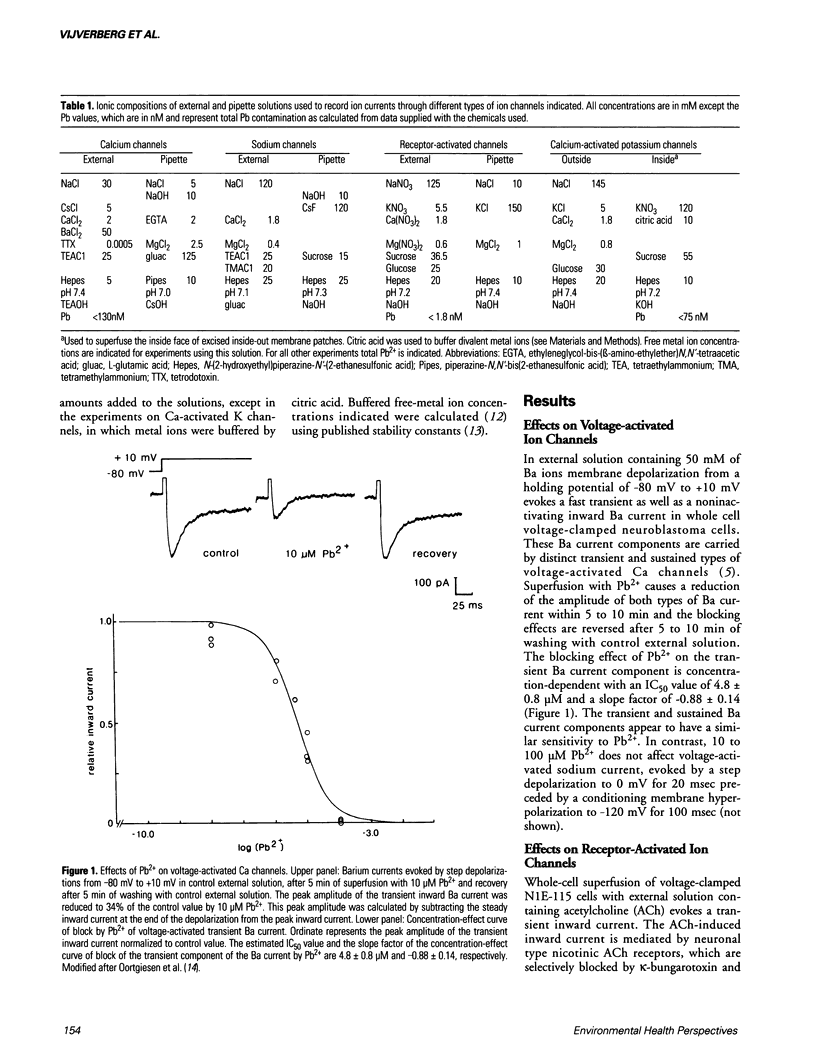
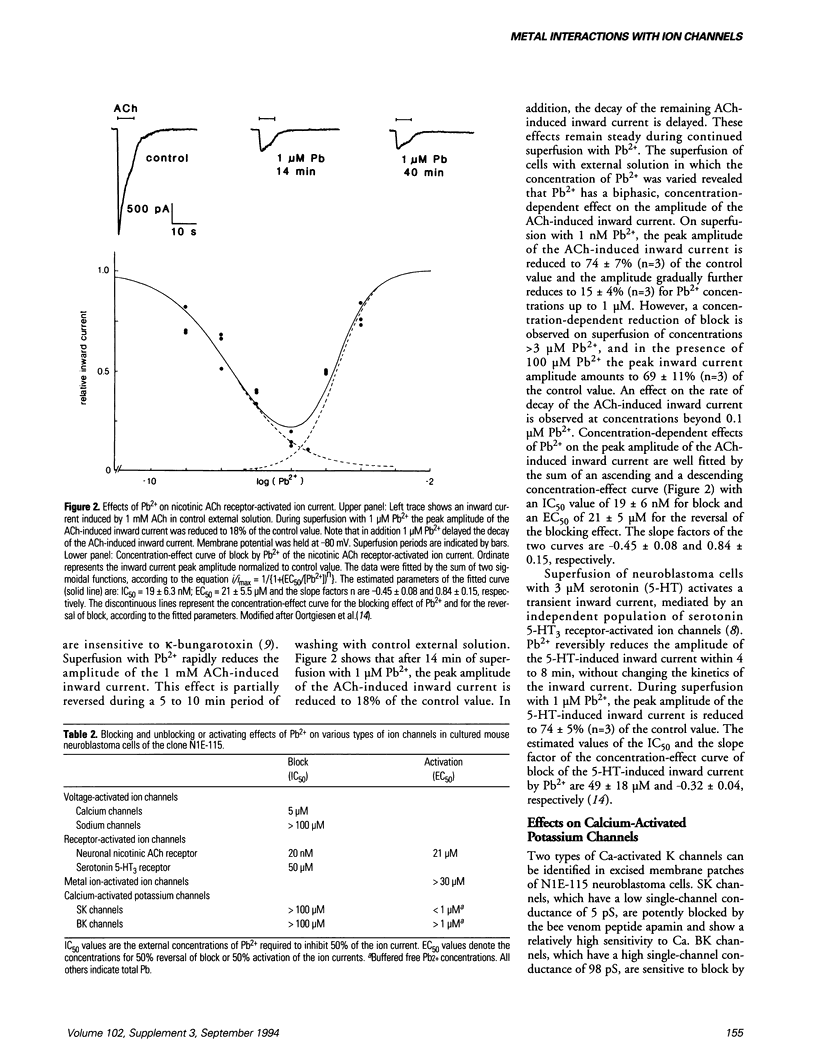
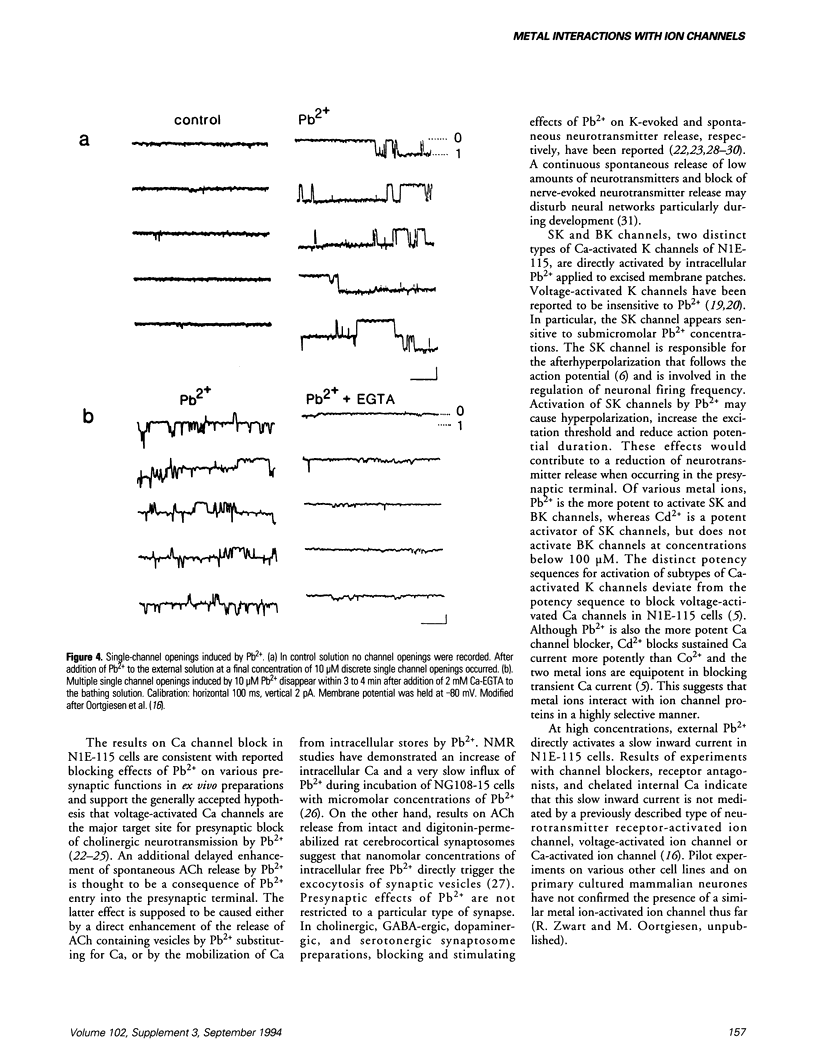
Effects of Pb and several other metal ions on various distinct types of voltage-, receptor- and Ca-activated ion channels have been investigated in cultured N1E-115 mouse neuroblastoma cells. Experiments were performed using the whole-cell voltage clamp and single-channel patch clamp techniques. External superfusion of nanomolar to submillimolar concentrations of Pb causes multiple effects on ion channels. Barium current through voltage-activated Ca channels is blocked by micromolar concentrations of Pb, whereas voltage-activated Na current appears insensitive. Neuronal type nicotinic acetylcholine receptor-activated ion current is blocked by nanomolar concentrations of Pb and this block is reversed at micromolar concentrations. Serotonin 5-HT3 receptor-activated ion current is much less sensitive to Pb. In addition, external superfusion with micromolar concentrations of Pb as well as of Cd and aluminum induces inward current, associated with the direct activation of nonselective cation channels by these metal ions. In excised inside-out membrane patches of neuroblastoma cells, micromolar concentrations of Ca activate small (SK) and big (BK) Ca-activated K channels. Internally applied Pb activates SK and BK channels more potently than Ca, whereas Cd is approximately equipotent to Pb with respect to SK channel activation, but fails to activate BK channels. The results show that metal ions cause distinct, selective effects on the various types of ion channels and that metal ion interaction sites of ion channels may be highly selective for particular metal ions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkondon M., Costa A. C., Radhakrishnan V., Aronstam R. S., Albuquerque E. X. Selective blockade of NMDA-activated channel currents may be implicated in learning deficits caused by lead. FEBS Lett. 1990 Feb 12;261(1):124–130. doi: 10.1016/0014-5793(90)80652-y. [DOI] [PubMed] [Google Scholar]
- Amano T., Richelson E., Nirenberg M. Neurotransmitter synthesis by neuroblastoma clones (neuroblast differentiation-cell culture-choline acetyltransferase-acetylcholinesterase-tyrosine hydroxylase-axons-dendrites). Proc Natl Acad Sci U S A. 1972 Jan;69(1):258–263. doi: 10.1073/pnas.69.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audesirk G., Audesirk T. Effects of inorganic lead on voltage-sensitive calcium channels in N1E-115 neuroblastoma cells. Neurotoxicology. 1991 Fall;12(3):519–528. [PubMed] [Google Scholar]
- Bressler J. P., Goldstein G. W. Mechanisms of lead neurotoxicity. Biochem Pharmacol. 1991 Feb 15;41(4):479–484. doi: 10.1016/0006-2952(91)90617-e. [DOI] [PubMed] [Google Scholar]
- Evans M. L., Büsselberg D., Carpenter D. O. Pb2+ blocks calcium currents of cultured dorsal root ganglion cells. Neurosci Lett. 1991 Aug 5;129(1):103–106. doi: 10.1016/0304-3940(91)90730-h. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hugues M., Romey G., Duval D., Vincent J. P., Lazdunski M. Apamin as a selective blocker of the calcium-dependent potassium channel in neuroblastoma cells: voltage-clamp and biochemical characterization of the toxin receptor. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1308–1312. doi: 10.1073/pnas.79.4.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinders T., Vijverberg H. P. Ca2+ dependence of small Ca(2+)-activated K+ channels in cultured N1E-115 mouse neuroblastoma cells. Pflugers Arch. 1992 Dec;422(3):223–232. doi: 10.1007/BF00376206. [DOI] [PubMed] [Google Scholar]
- Leinders T., van Kleef R. G., Vijverberg H. P. Divalent cations activate small- (SK) and large-conductance (BK) channels in mouse neuroblastoma cells: selective activation of SK channels by cadmium. Pflugers Arch. 1992 Dec;422(3):217–222. doi: 10.1007/BF00376205. [DOI] [PubMed] [Google Scholar]
- Manalis R. S., Cooper G. P., Pomeroy S. L. Effects of lead on neuromuscular transmission in the frog. Brain Res. 1984 Feb 27;294(1):95–109. doi: 10.1016/0006-8993(84)91313-1. [DOI] [PubMed] [Google Scholar]
- Miller C. Genetic manipulation of ion channels: a new approach to structure and mechanism. Neuron. 1989 Mar;2(3):1195–1205. doi: 10.1016/0896-6273(89)90304-8. [DOI] [PubMed] [Google Scholar]
- Minnema D. J., Greenland R. D., Michaelson I. A. Effect of in vitro inorganic lead on dopamine release from superfused rat striatal synaptosomes. Toxicol Appl Pharmacol. 1986 Jun 30;84(2):400–411. doi: 10.1016/0041-008x(86)90148-1. [DOI] [PubMed] [Google Scholar]
- Minnema D. J., Michaelson I. A., Cooper G. P. Calcium efflux and neurotransmitter release from rat hippocampal synaptosomes exposed to lead. Toxicol Appl Pharmacol. 1988 Mar 15;92(3):351–357. doi: 10.1016/0041-008x(88)90175-5. [DOI] [PubMed] [Google Scholar]
- Minnema D. J., Michaelson I. A. Differential effects of inorganic lead and delta-aminolevulinic acid in vitro on synaptosomal gamma-aminobutyric acid release. Toxicol Appl Pharmacol. 1986 Dec;86(3):437–447. doi: 10.1016/0041-008x(86)90371-6. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Spector I. Ionic currents in cultured mouse neuroblastoma cells under voltage-clamp conditions. J Physiol. 1978 May;278:265–286. doi: 10.1113/jphysiol.1978.sp012303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T., Tsunoo A., Yoshii M. Characterization of two types of calcium channels in mouse neuroblastoma cells. J Physiol. 1987 Feb;383:231–249. doi: 10.1113/jphysiol.1987.sp016406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neijt H. C., Plomp J. J., Vijverberg H. P. Kinetics of the membrane current mediated by serotonin 5-HT3 receptors in cultured mouse neuroblastoma cells. J Physiol. 1989 Apr;411:257–269. doi: 10.1113/jphysiol.1989.sp017572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oortgiesen M., Vijverberg H. P. Properties of neuronal type acetylcholine receptors in voltage clamped mouse neuroblastoma cells. Neuroscience. 1989;31(1):169–179. doi: 10.1016/0306-4522(89)90038-9. [DOI] [PubMed] [Google Scholar]
- Oortgiesen M., van Kleef R. G., Bajnath R. B., Vijverberg H. P. Nanomolar concentrations of lead selectively block neuronal nicotinic acetylcholine responses in mouse neuroblastoma cells. Toxicol Appl Pharmacol. 1990 Mar 15;103(1):165–174. doi: 10.1016/0041-008x(90)90272-v. [DOI] [PubMed] [Google Scholar]
- Oortgiesen M., van Kleef R. G., Vijverberg H. P. Novel type of ion channel activated by Pb2+, Cd2+, and Al3+ in cultured mouse neuroblastoma cells. J Membr Biol. 1990 Feb;113(3):261–268. doi: 10.1007/BF01870077. [DOI] [PubMed] [Google Scholar]
- Oudar P., Caillard L., Fillion G. The effects of inorganic lead on the spontaneous and potassium-evoked release of 3H-5-HT from rat cortical synaptosome interaction with calcium. Pharmacol Toxicol. 1989 May;64(5):459–463. doi: 10.1111/j.1600-0773.1989.tb00687.x. [DOI] [PubMed] [Google Scholar]
- Pickett J. B., Bornstein J. C. Some effects of lead at mammalian neuromuscular junction. Am J Physiol. 1984 Mar;246(3 Pt 1):C271–C276. doi: 10.1152/ajpcell.1984.246.3.C271. [DOI] [PubMed] [Google Scholar]
- Reuveny E., Narahashi T. Potent blocking action of lead on voltage-activated calcium channels in human neuroblastoma cells SH-SY5Y. Brain Res. 1991 Apr 5;545(1-2):312–314. doi: 10.1016/0006-8993(91)91304-j. [DOI] [PubMed] [Google Scholar]
- Schanne F. A., Kane A. B., Young E. E., Farber J. L. Calcium dependence of toxic cell death: a final common pathway. Science. 1979 Nov 9;206(4419):700–702. doi: 10.1126/science.386513. [DOI] [PubMed] [Google Scholar]
- Schanne F. A., Moskal J. R., Gupta R. K. Effect of lead on intracellular free calcium ion concentration in a presynaptic neuronal model: 19F-NMR study of NG108-15 cells. Brain Res. 1989 Dec 4;503(2):308–311. doi: 10.1016/0006-8993(89)91680-6. [DOI] [PubMed] [Google Scholar]
- Shao Z., Suszkiw J. B. Ca2(+)-surrogate action of Pb2+ on acetylcholine release from rat brain synaptosomes. J Neurochem. 1991 Feb;56(2):568–574. doi: 10.1111/j.1471-4159.1991.tb08187.x. [DOI] [PubMed] [Google Scholar]
- Suszkiw J., Toth G., Murawsky M., Cooper G. P. Effects of Pb2+ and Cd2+ on acetylcholine release and Ca2+ movements in synaptosomes and subcellular fractions from rat brain and Torpedo electric organ. Brain Res. 1984 Dec 3;323(1):31–46. doi: 10.1016/0006-8993(84)90262-2. [DOI] [PubMed] [Google Scholar]
- van Heeswijk M. P., Geertsen J. A., van Os C. H. Kinetic properties of the ATP-dependent Ca2+ pump and the Na+/Ca2+ exchange system in basolateral membranes from rat kidney cortex. J Membr Biol. 1984;79(1):19–31. doi: 10.1007/BF01868523. [DOI] [PubMed] [Google Scholar]


