Abstract
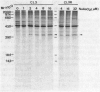
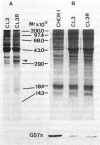
Human fibroblasts (HFW) were 10-fold more susceptible than Chinese hamster ovary (CHO-K1) cells to sodium arsenite. Comparison of cellular antioxidant enzyme activities showed that CHO-K1 cells contained 3- and 8-fold more glutathione-peroxidase and catalase activities, respectively, than HFW cells. Since vitamin E, methylamine, and benzyl alcohol could prevent, in part, the arsenite-induced killing of HFW cells, we suggest that arsenite can induce oxidative damage in HFW cells. We have also established arsenic-resistant cells, SA7 and CL3R, from CHO cells and from a human lung adenocarcinoma cell line (CL3), respectively. The arsenic resistance of SA7 cells was attributed mainly to elevation of glutathione S-transferase pi levels, and that of CL3R cells was possibly due to an increase in heme oxygenase activity. Since induction of heme oxygenase is a general response to oxidative stress, we suspect that the differential toxicity of arsenic to human and animal cells could be due to arsenic's more efficient induction of oxidative damage in human cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Akman S. A., Forrest G., Chu F. F., Esworthy R. S., Doroshow J. H. Antioxidant and xenobiotic-metabolizing enzyme gene expression in doxorubicin-resistant MCF-7 breast cancer cells. Cancer Res. 1990 Mar 1;50(5):1397–1402. [PubMed] [Google Scholar]
- Amstad P., Cerutti P. Genetic modulation of the cellular antioxidant defense capacity. Environ Health Perspect. 1990 Aug;88:77–82. doi: 10.1289/ehp.908877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applegate L. A., Luscher P., Tyrrell R. M. Induction of heme oxygenase: a general response to oxidant stress in cultured mammalian cells. Cancer Res. 1991 Feb 1;51(3):974–978. [PubMed] [Google Scholar]
- Blair P. C., Thompson M. B., Bechtold M., Wilson R. E., Moorman M. P., Fowler B. A. Evidence for oxidative damage to red blood cells in mice induced by arsine gas. Toxicology. 1990 Jul;63(1):25–34. doi: 10.1016/0300-483x(90)90065-o. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Breimer L. H. Molecular mechanisms of oxygen radical carcinogenesis and mutagenesis: the role of DNA base damage. Mol Carcinog. 1990;3(4):188–197. doi: 10.1002/mc.2940030405. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Burton G. W., Joyce A., Ingold K. U. Is vitamin E the only lipid-soluble, chain-breaking antioxidant in human blood plasma and erythrocyte membranes? Arch Biochem Biophys. 1983 Feb 15;221(1):281–290. doi: 10.1016/0003-9861(83)90145-5. [DOI] [PubMed] [Google Scholar]
- Cadenas E. Biochemistry of oxygen toxicity. Annu Rev Biochem. 1989;58:79–110. doi: 10.1146/annurev.bi.58.070189.000455. [DOI] [PubMed] [Google Scholar]
- Carlberg I., Mannervik B. Glutathione reductase. Methods Enzymol. 1985;113:484–490. doi: 10.1016/s0076-6879(85)13062-4. [DOI] [PubMed] [Google Scholar]
- Cerutti P. A. Prooxidant states and tumor promotion. Science. 1985 Jan 25;227(4685):375–381. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- Chen C. J., Chuang Y. C., Lin T. M., Wu H. Y. Malignant neoplasms among residents of a blackfoot disease-endemic area in Taiwan: high-arsenic artesian well water and cancers. Cancer Res. 1985 Nov;45(11 Pt 2):5895–5899. [PubMed] [Google Scholar]
- Cohn V. H., Lyle J. A fluorometric assay for glutathione. Anal Biochem. 1966 Mar;14(3):434–440. doi: 10.1016/0003-2697(66)90286-7. [DOI] [PubMed] [Google Scholar]
- Goldstein B. D., Czerniecki B., Witz G. The role of free radicals in tumor promotion. Environ Health Perspect. 1989 May;81:55–57. doi: 10.1289/ehp.898155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinde B. Inhibition of autophagy by benzyl alcohol. Biochim Biophys Acta. 1984 Jul 30;800(2):140–144. doi: 10.1016/0304-4165(84)90052-7. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Jakoby W. B. Assays for differentiation of glutathione S-transferases. Methods Enzymol. 1981;77:398–405. doi: 10.1016/s0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Jakoby W. B. The glutathione S-transferases: a group of multifunctional detoxification proteins. Adv Enzymol Relat Areas Mol Biol. 1978;46:383–414. doi: 10.1002/9780470122914.ch6. [DOI] [PubMed] [Google Scholar]
- Keyse S. M., Tyrrell R. M. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc Natl Acad Sci U S A. 1989 Jan;86(1):99–103. doi: 10.1073/pnas.86.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawrence R. A., Burk R. F. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun. 1976 Aug 23;71(4):952–958. doi: 10.1016/0006-291x(76)90747-6. [DOI] [PubMed] [Google Scholar]
- Lee T. C., Ko J. L., Jan K. Y. Differential cytotoxicity of sodium arsenite in human fibroblasts and Chinese hamster ovary cells. Toxicology. 1989 Jun 16;56(3):289–299. doi: 10.1016/0300-483x(89)90092-9. [DOI] [PubMed] [Google Scholar]
- Lee T. C., Wei M. L., Chang W. J., Ho I. C., Lo J. F., Jan K. Y., Huang H. Elevation of glutathione levels and glutathione S-transferase activity in arsenic-resistant Chinese hamster ovary cells. In Vitro Cell Dev Biol. 1989 May;25(5):442–448. doi: 10.1007/BF02624629. [DOI] [PubMed] [Google Scholar]
- Lo J. F., Wang H. F., Tam M. F., Lee T. C. Glutathione S-transferase pi in an arsenic-resistant Chinese hamster ovary cell line. Biochem J. 1992 Dec 15;288(Pt 3):977–982. doi: 10.1042/bj2880977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léonard A., Lauwerys R. R. Carcinogenicity, teratogenicity and mutagenicity of arsenic. Mutat Res. 1980 Jan;75(1):49–62. doi: 10.1016/0165-1110(80)90027-5. [DOI] [PubMed] [Google Scholar]
- Mabuchi K., Lilienfeld A. M., Snell L. M. Lung cancer among pesticide workers exposed to inorganic arsenicals. Arch Environ Health. 1979 Sep-Oct;34(5):312–320. doi: 10.1080/00039896.1979.10667423. [DOI] [PubMed] [Google Scholar]
- Mimnaugh E. G., Dusre L., Atwell J., Myers C. E. Differential oxygen radical susceptibility of adriamycin-sensitive and -resistant MCF-7 human breast tumor cells. Cancer Res. 1989 Jan 1;49(1):8–15. [PubMed] [Google Scholar]
- Sakaida I., Kyle M. E., Farber J. L. Autophagic degradation of protein generates a pool of ferric iron required for the killing of cultured hepatocytes by an oxidative stress. Mol Pharmacol. 1990 Mar;37(3):435–442. [PubMed] [Google Scholar]
- Seglen P. O., Grinde B., Solheim A. E. Inhibition of the lysosomal pathway of protein degradation in isolated rat hepatocytes by ammonia, methylamine, chloroquine and leupeptin. Eur J Biochem. 1979 Apr 2;95(2):215–225. doi: 10.1111/j.1432-1033.1979.tb12956.x. [DOI] [PubMed] [Google Scholar]
- Slaga T. J., Klein-Szanto A. J., Triplett L. L., Yotti L. P., Trosko K. E. Skin tumor-promoting activity of benzoyl peroxide, a widely used free radical-generating compound. Science. 1981 Aug 28;213(4511):1023–1025. doi: 10.1126/science.6791284. [DOI] [PubMed] [Google Scholar]
- Spitz D. R., Oberley L. W. An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal Biochem. 1989 May 15;179(1):8–18. doi: 10.1016/0003-2697(89)90192-9. [DOI] [PubMed] [Google Scholar]
- Starke P. E., Gilbertson J. D., Farber J. L. Lysosomal origin of the ferric iron required for cell killing by hydrogen peroxide. Biochem Biophys Res Commun. 1985 Dec 17;133(2):371–379. doi: 10.1016/0006-291x(85)90916-7. [DOI] [PubMed] [Google Scholar]
- Tam G. K., Charbonneau S. M., Bryce F., Pomroy C., Sandi E. Metabolism of inorganic arsenic (74As) in humans following oral ingestion. Toxicol Appl Pharmacol. 1979 Sep 15;50(2):319–322. doi: 10.1016/0041-008x(79)90157-1. [DOI] [PubMed] [Google Scholar]
- Tseng W. P. Effects and dose--response relationships of skin cancer and blackfoot disease with arsenic. Environ Health Perspect. 1977 Aug;19:109–119. doi: 10.1289/ehp.7719109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahter M. Biotransformation of trivalent and pentavalent inorganic arsenic in mice and rats. Environ Res. 1981 Aug;25(2):286–293. doi: 10.1016/0013-9351(81)90030-x. [DOI] [PubMed] [Google Scholar]
- Yamanaka K., Hasegawa A., Sawamura R., Okada S. Dimethylated arsenics induce DNA strand breaks in lung via the production of active oxygen in mice. Biochem Biophys Res Commun. 1989 Nov 30;165(1):43–50. doi: 10.1016/0006-291x(89)91031-0. [DOI] [PubMed] [Google Scholar]