Abstract
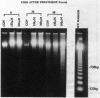
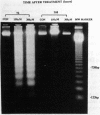
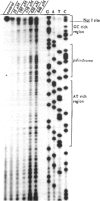
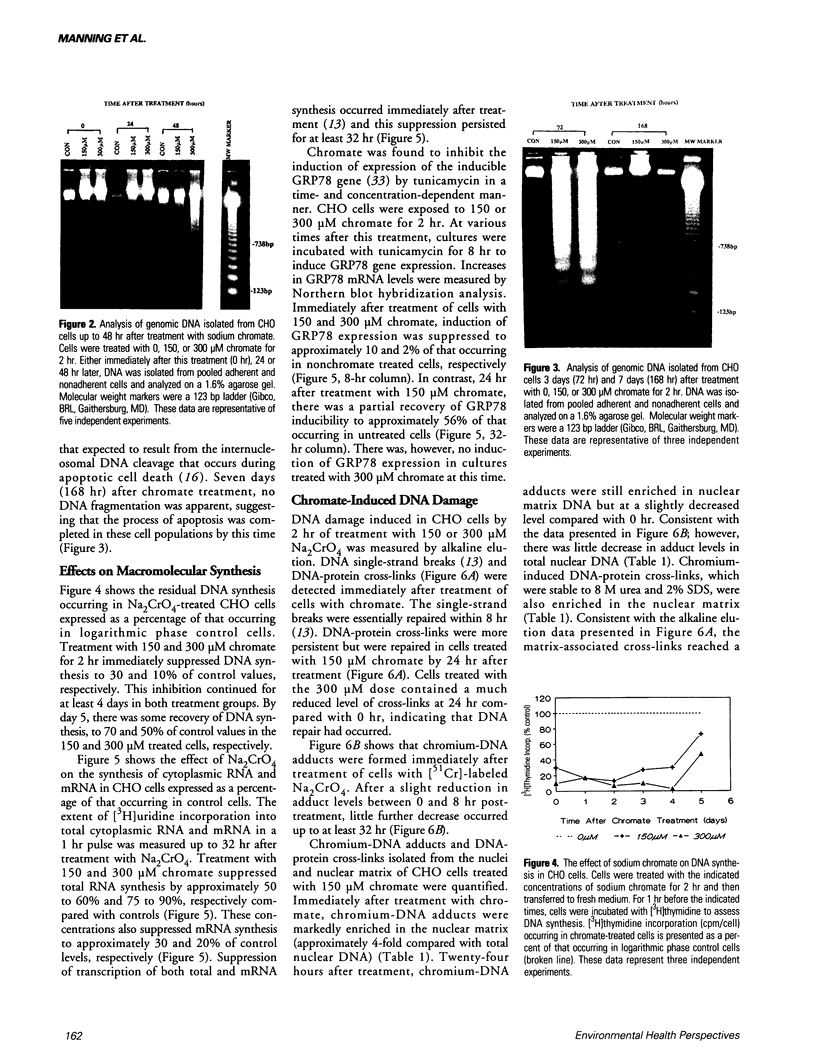
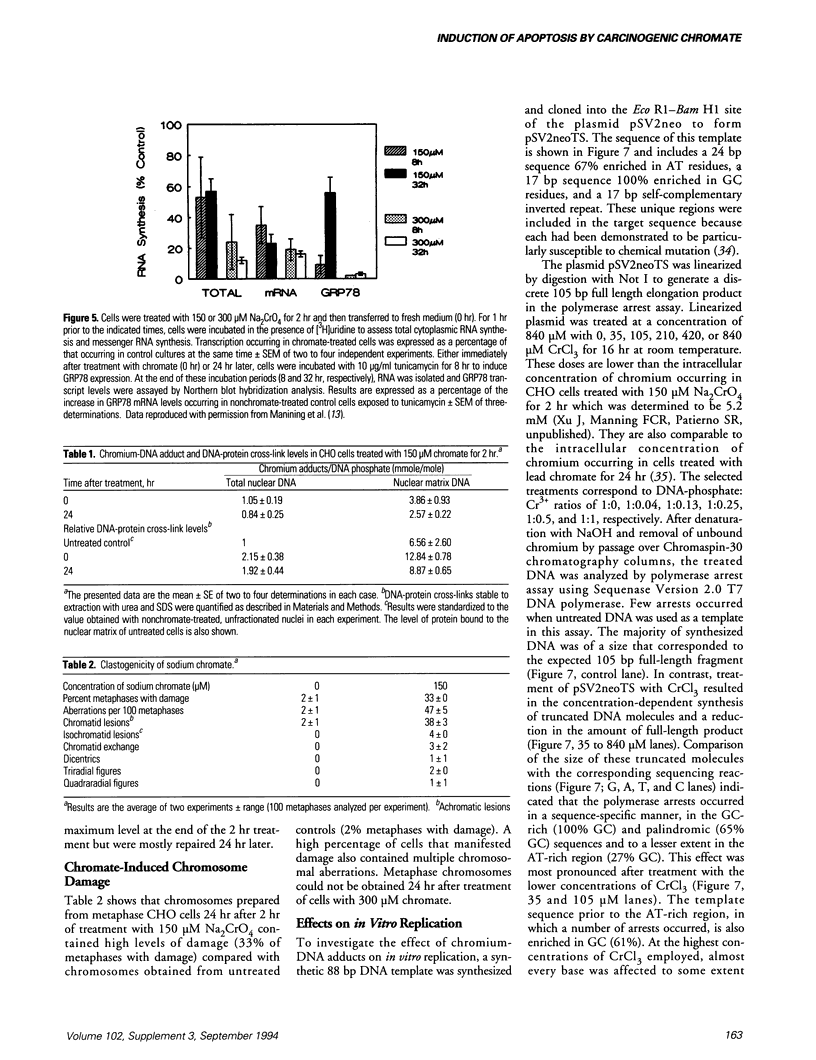
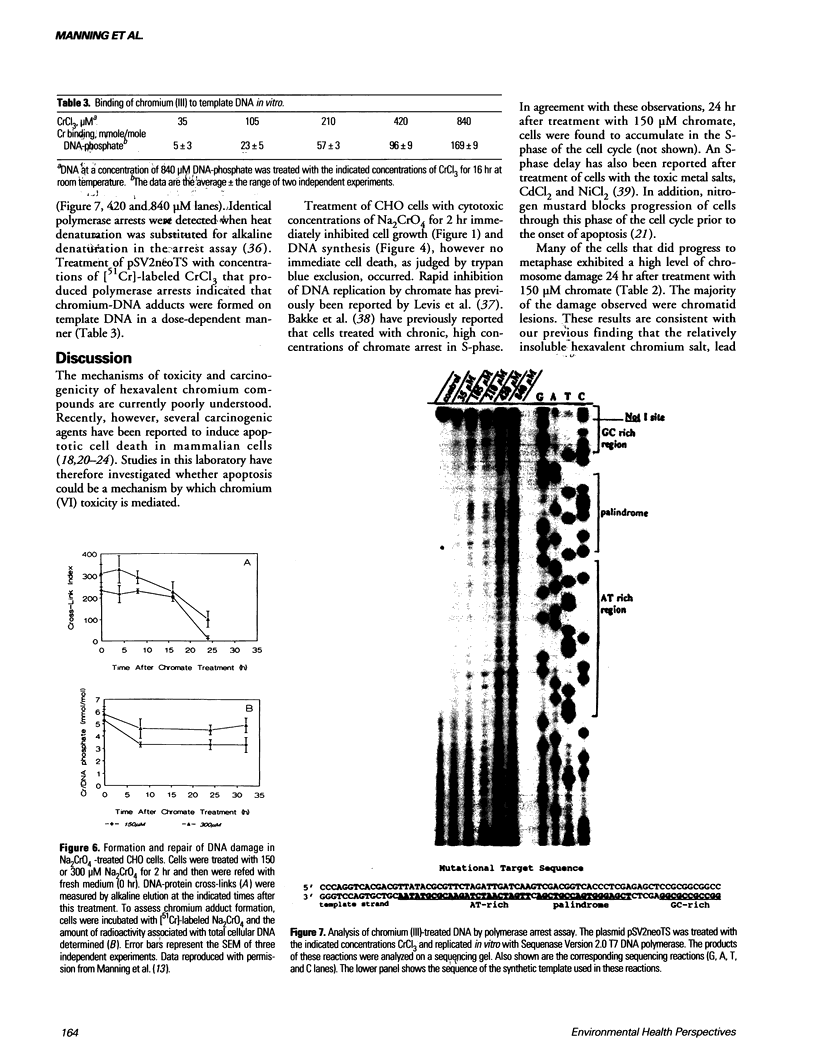
Hexavalent chromium (Cr) compounds are respiratory carcinogens in humans and animals. Treatment of Chinese hamster ovary cells with 150 and 300 microM sodium chromate (Na2CrO4) for 2 hr decreased colony-forming efficiency by 46 and 92%, respectively. These treatments induced dose-dependent internucleosomal fragmentation of cellular DNA beyond 24 hr after chromate treatment. This fragmentation pattern is characteristic of apoptosis as a mechanism of cell death. These treatments also induced an immediate inhibition of macromolecular synthesis and delayed progression of cells through S-phase of the cell cycle. Cell growth (as evidenced by DNA synthesis) was inhibited for at least 4 days and transcription remained suppressed for at least 32 hr. Many of the cells that did progress to metaphase exhibited chromosome damage. Chromate caused the dose-dependent formation of DNA single-strand breaks and DNA-protein cross-links, but these were repaired 8 and 24 hr after removal of the treatment, respectively. In contrast, Cr-DNA adducts (up to 1/100 base-pairs) were extremely resistant to repair and were still detectable even 5 days after treatment. Compared with other regions of the genome, DNA-protein cross-links and Cr adducts were preferentially associated with the nuclear matrix DNA of treated cells, which was 4.5-fold enriched in actively transcribed genes. Chromium adducts, formed on DNA in vitro at a similar level to that detected in nuclear matrix DNA, arrested the progression of a DNA polymerase in a sequence-specific manner, possibly through the formation of DNA-DNA cross-links.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakke O., Jakobsen K., Eik-Nes K. B. Concentration-dependent effects of potassium dichromate on the cell cycle. Cytometry. 1984 Sep;5(5):482–486. doi: 10.1002/cyto.990050508. [DOI] [PubMed] [Google Scholar]
- Barry M. A., Behnke C. A., Eastman A. Activation of programmed cell death (apoptosis) by cisplatin, other anticancer drugs, toxins and hyperthermia. Biochem Pharmacol. 1990 Nov 15;40(10):2353–2362. doi: 10.1016/0006-2952(90)90733-2. [DOI] [PubMed] [Google Scholar]
- Bridgewater L. C., Manning F. C., Woo E. S., Patierno S. R. DNA polymerase arrest by adducted trivalent chromium. Mol Carcinog. 1994 Mar;9(3):122–133. doi: 10.1002/mc.2940090304. [DOI] [PubMed] [Google Scholar]
- Chang S. C., Wooden S. K., Nakaki T., Kim Y. K., Lin A. Y., Kung L., Attenello J. W., Lee A. S. Rat gene encoding the 78-kDa glucose-regulated protein GRP78: its regulatory sequences and the effect of protein glycosylation on its expression. Proc Natl Acad Sci U S A. 1987 Feb;84(3):680–684. doi: 10.1073/pnas.84.3.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran G. B., Ray S. D. The role of the nucleus and other compartments in toxic cell death produced by alkylating hepatotoxicants. Toxicol Appl Pharmacol. 1992 Apr;113(2):167–183. doi: 10.1016/0041-008x(92)90112-6. [DOI] [PubMed] [Google Scholar]
- Costa M., Cantoni O., de Mars M., Swartzendruber D. E. Toxic metals produce an S-phase-specific cell cycle block. Res Commun Chem Pathol Pharmacol. 1982 Dec;38(3):405–419. [PubMed] [Google Scholar]
- De Flora S., Bagnasco M., Serra D., Zanacchi P. Genotoxicity of chromium compounds. A review. Mutat Res. 1990 Mar;238(2):99–172. doi: 10.1016/0165-1110(90)90007-x. [DOI] [PubMed] [Google Scholar]
- Dorner A. J., Wasley L. C., Raney P., Haugejorden S., Green M., Kaufman R. J. The stress response in Chinese hamster ovary cells. Regulation of ERp72 and protein disulfide isomerase expression and secretion. J Biol Chem. 1990 Dec 15;265(35):22029–22034. [PubMed] [Google Scholar]
- Eastman A. Activation of programmed cell death by anticancer agents: cisplatin as a model system. Cancer Cells. 1990 Aug-Sep;2(8-9):275–280. [PubMed] [Google Scholar]
- Elias Z., Poirot O., Pezerat H., Suquet H., Schneider O., Danière M. C., Terzetti F., Baruthio F., Fournier M., Cavelier C. Cytotoxic and neoplastic transforming effects of industrial hexavalent chromium pigments in Syrian hamster embryo cells. Carcinogenesis. 1989 Nov;10(11):2043–2052. doi: 10.1093/carcin/10.11.2043. [DOI] [PubMed] [Google Scholar]
- Ewig R. A., Kohn K. W. DNA-protein cross-linking and DNA interstrand cross-linking by haloethylnitrosoureas in L1210 cells. Cancer Res. 1978 Oct;38(10):3197–3203. [PubMed] [Google Scholar]
- Georgiev G. P., Vassetzky Y. S., Jr, Luchnik A. N., Chernokhvostov V. V., Razin S. V. A. E. Braunstein Plenary Lecture. Nuclear skeleton, DNA domains and control of replication and transcription. Eur J Biochem. 1991 Sep 15;200(3):613–624. doi: 10.1111/j.1432-1033.1991.tb16224.x. [DOI] [PubMed] [Google Scholar]
- Hamilton J. W., Wetterhahn K. E. Chromium (VI)-induced DNA damage in chick embryo liver and blood cells in vivo. Carcinogenesis. 1986 Dec;7(12):2085–2088. doi: 10.1093/carcin/7.12.2085. [DOI] [PubMed] [Google Scholar]
- Hamilton J. W., Wetterhahn K. E. Differential effects of chromium(VI) on constitutive and inducible gene expression in chick embryo liver in vivo and correlation with chromium(VI)-induced DNA damage. Mol Carcinog. 1989;2(5):274–286. doi: 10.1002/mc.2940020508. [DOI] [PubMed] [Google Scholar]
- Kasprzak K. S. The role of oxidative damage in metal carcinogenicity. Chem Res Toxicol. 1991 Nov-Dec;4(6):604–615. doi: 10.1021/tx00024a002. [DOI] [PubMed] [Google Scholar]
- Kastan M. B., Onyekwere O., Sidransky D., Vogelstein B., Craig R. W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991 Dec 1;51(23 Pt 1):6304–6311. [PubMed] [Google Scholar]
- Klein C. B., Frenkel K., Costa M. The role of oxidative processes in metal carcinogenesis. Chem Res Toxicol. 1991 Nov-Dec;4(6):592–604. doi: 10.1021/tx00024a001. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R., Stevens S. A rapid procedure for measurement of DNA repair in human fibroblasts and for complementation analysis of xeroderma pigmentosum cells. Mutat Res. 1980 Jan;69(1):177–190. doi: 10.1016/0027-5107(80)90187-6. [DOI] [PubMed] [Google Scholar]
- Levis A. G., Buttignol M., Bianchi V., Sponza G. Effects of potassium dichromate on nucleic acid and protein syntheses and on precursor uptake in BHK fibroblasts. Cancer Res. 1978 Jan;38(1):110–116. [PubMed] [Google Scholar]
- Li X. A., Lee A. S. Competitive inhibition of a set of endoplasmic reticulum protein genes (GRP78, GRP94, and ERp72) retards cell growth and lowers viability after ionophore treatment. Mol Cell Biol. 1991 Jul;11(7):3446–3453. doi: 10.1128/mcb.11.7.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu E. S., Lee A. S. Common sets of nuclear factors binding to the conserved promoter sequence motif of two coordinately regulated ER protein genes, GRP78 and GRP94. Nucleic Acids Res. 1991 Oct 11;19(19):5425–5431. doi: 10.1093/nar/19.19.5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léger J. G., Montpetit M. L., Tenniswood M. P. Characterization and cloning of androgen-repressed mRNAs from rat ventral prostate. Biochem Biophys Res Commun. 1987 Aug 31;147(1):196–203. doi: 10.1016/s0006-291x(87)80106-7. [DOI] [PubMed] [Google Scholar]
- Léonard A. Mechanisms in metal genotoxicity: the significance of in vitro approaches. Mutat Res. 1988 Apr;198(2):321–326. doi: 10.1016/0027-5107(88)90009-7. [DOI] [PubMed] [Google Scholar]
- Manning F. C., Xu J., Patierno S. R. Transcriptional inhibition by carcinogenic chromate: relationship to DNA damage. Mol Carcinog. 1992;6(4):270–279. doi: 10.1002/mc.2940060409. [DOI] [PubMed] [Google Scholar]
- Miller C. A., 3rd, Cohen M. D., Costa M. Complexing of actin and other nuclear proteins to DNA by cis-diamminedichloroplatinum(II) and chromium compounds. Carcinogenesis. 1991 Feb;12(2):269–276. doi: 10.1093/carcin/12.2.269. [DOI] [PubMed] [Google Scholar]
- Miller C. A., 3rd, Costa M. Characterization of DNA-protein complexes induced in intact cells by the carcinogen chromate. Mol Carcinog. 1988;1(2):125–133. doi: 10.1002/mc.2940010208. [DOI] [PubMed] [Google Scholar]
- Montaldi A., Zentilin L., Paglialunga S., Levis A. G. Solubilization by nitrilotriacetic acid (NTA) of genetically active Cr(VI) and Pb(II) from insoluble metal compounds. J Toxicol Environ Health. 1987;21(3):387–394. doi: 10.1080/15287398709531027. [DOI] [PubMed] [Google Scholar]
- Muschel R. J., Zhang H. B., Iliakis G., McKenna W. G. Cyclin B expression in HeLa cells during the G2 block induced by ionizing radiation. Cancer Res. 1991 Oct 1;51(19):5113–5117. [PubMed] [Google Scholar]
- O'Connor P. M., Wassermann K., Sarang M., Magrath I., Bohr V. A., Kohn K. W. Relationship between DNA cross-links, cell cycle, and apoptosis in Burkitt's lymphoma cell lines differing in sensitivity to nitrogen mustard. Cancer Res. 1991 Dec 15;51(24):6550–6557. [PubMed] [Google Scholar]
- Obi F. O., Ryan A. J., Billett M. A. Preferential binding of the carcinogen benzo[a]pyrene to DNA in active chromatin and the nuclear matrix. Carcinogenesis. 1986 Jun;7(6):907–913. doi: 10.1093/carcin/7.6.907. [DOI] [PubMed] [Google Scholar]
- Okada S., Taniyama M., Ohba H. Mode of enhancement in ribonucleic acid synthesis directed by chromium (III)-bound deoxyribonucleic acid. J Inorg Biochem. 1982 Aug;17(1):41–49. doi: 10.1016/s0162-0134(00)80228-7. [DOI] [PubMed] [Google Scholar]
- Patierno S. R., Banh D., Landolph J. R. Transformation of C3H/10T1/2 mouse embryo cells to focus formation and anchorage independence by insoluble lead chromate but not soluble calcium chromate: relationship to mutagenesis and internalization of lead chromate particles. Cancer Res. 1988 Sep 15;48(18):5280–5288. [PubMed] [Google Scholar]
- Patierno S. R., Sugiyama M., Basilion J. P., Costa M. Preferential DNA-protein cross-linking by NiCl2 in magnesium-insoluble regions of fractionated Chinese hamster ovary cell chromatin. Cancer Res. 1985 Nov;45(11 Pt 2):5787–5794. [PubMed] [Google Scholar]
- Resendez E., Jr, Wooden S. K., Lee A. S. Identification of highly conserved regulatory domains and protein-binding sites in the promoters of the rat and human genes encoding the stress-inducible 78-kilodalton glucose-regulated protein. Mol Cell Biol. 1988 Oct;8(10):4579–4584. doi: 10.1128/mcb.8.10.4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow E. T., Xu L. S. Chromium(III) bound to DNA templates promotes increased polymerase processivity and decreased fidelity during replication in vitro. Biochemistry. 1991 Nov 26;30(47):11238–11245. doi: 10.1021/bi00111a007. [DOI] [PubMed] [Google Scholar]
- Standeven A. M., Wetterhahn K. E. Is there a role for reactive oxygen species in the mechanism of chromium(VI) carcinogenesis? Chem Res Toxicol. 1991 Nov-Dec;4(6):616–625. doi: 10.1021/tx00024a003. [DOI] [PubMed] [Google Scholar]
- Sugiyama M., Patierno S. R., Cantoni O., Costa M. Characterization of DNA lesions induced by CaCrO4 in synchronous and asynchronous cultured mammalian cells. Mol Pharmacol. 1986 Jun;29(6):606–613. [PubMed] [Google Scholar]
- Tsapakos M. J., Wetterhahn K. E. The interaction of chromium with nucleic acids. Chem Biol Interact. 1983 Sep 1;46(2):265–277. doi: 10.1016/0009-2797(83)90034-0. [DOI] [PubMed] [Google Scholar]
- Warpehoski M. A., Hurley L. H. Sequence selectivity of DNA covalent modification. Chem Res Toxicol. 1988 Nov-Dec;1(6):315–333. doi: 10.1021/tx00006a001. [DOI] [PubMed] [Google Scholar]
- Wedrychowski A., Ward W. S., Schmidt W. N., Hnilica L. S. Chromium-induced cross-linking of nuclear proteins and DNA. J Biol Chem. 1985 Jun 10;260(11):7150–7155. [PubMed] [Google Scholar]
- Wise J. P., Leonard J. C., Patierno S. R. Clastogenicity of lead chromate particles in hamster and human cells. Mutat Res. 1992 Jan;278(1):69–79. doi: 10.1016/0165-1218(92)90287-a. [DOI] [PubMed] [Google Scholar]
- Wise J. P., Orenstein J. M., Patierno S. R. Inhibition of lead chromate clastogenesis by ascorbate: relationship to particle dissolution and uptake. Carcinogenesis. 1993 Mar;14(3):429–434. doi: 10.1093/carcin/14.3.429. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Xu J., Wise J. P., Patierno S. R. DNA damage induced by carcinogenic lead chromate particles in cultured mammalian cells. Mutat Res. 1992 Aug;280(2):129–136. doi: 10.1016/0165-1218(92)90008-n. [DOI] [PubMed] [Google Scholar]