Abstract
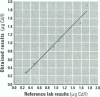
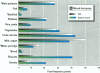
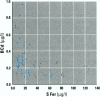
Measurements of intake and uptake of cadmium in relation to diet composition were carried out in 57 nonsmoking women, 20-50 years of age. A vegetarian/high-fiber diet and a mixed-diet group were constructed based on results from a food frequency questionnaire. Duplicate diets and the corresponding feces were collected during 4 consecutive days in parallel with dietary recording of type and amount of food ingested for determination of the dietary intake of cadmium and various nutrients. Blood and 24-hr urine samples were collected for determination of cadmium, hemoglobin, ferritin, and zinc. There were no differences in the intake of nutrients between the mixed-diet and the high-fiber diet groups, except for a significantly higher intake of fiber (p < 0.001) and cadmium (p < 0.002) in the high-fiber group. Fecal cadmium corresponded to 98% in the mixed-diet group and 100% in the high-fiber diet group. No differences in blood cadmium (BCd) or urinary cadmium (UCd) between groups could be detected. There was a tendency toward higher BCd and UCd concentrations with increasing fiber intake; however, the concentrations were not statistically significant at the 5% level, indicating an inhibitory effect of fiber on the gastrointestinal absorption of cadmium. Sixty-seven percent of the women had serum ferritin < 30 micrograms/l, indicating reduced body iron stores, which were highly associated with higher BCd (irrespective of fiber intake). BCd was mainly correlated with UCd, serum ferritin, age, anf fibre intake. UCd and serum ferritin explained almost 60% of the variation in BCd.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buchet J. P., Lauwerys R., Roels H., Bernard A., Bruaux P., Claeys F., Ducoffre G., de Plaen P., Staessen J., Amery A. Renal effects of cadmium body burden of the general population. Lancet. 1990 Sep 22;336(8717):699–702. doi: 10.1016/0140-6736(90)92201-r. [DOI] [PubMed] [Google Scholar]
- Elinder C. G., Friberg L., Lind B., Jawaid M. Lead and cadmium levels in blood samples from the general population of Sweden. Environ Res. 1983 Feb;30(1):233–253. doi: 10.1016/0013-9351(83)90183-4. [DOI] [PubMed] [Google Scholar]
- Elinder C. G., Lind B., Kjellström T., Linnman L., Friberg L. Cadmium in kidney cortex, liver, and pancreas from Swedish autopsies. Estimation of biological half time in kidney cortex, considering calorie intake and smoking habits. Arch Environ Health. 1976 Nov-Dec;31(6):292–302. doi: 10.1080/00039896.1976.10667239. [DOI] [PubMed] [Google Scholar]
- Flanagan P. R., McLellan J. S., Haist J., Cherian G., Chamberlain M. J., Valberg L. S. Increased dietary cadmium absorption in mice and human subjects with iron deficiency. Gastroenterology. 1978 May;74(5 Pt 1):841–846. [PubMed] [Google Scholar]
- Friberg L., Vahter M. Assessment of exposure to lead and cadmium through biological monitoring: results of a UNEP/WHO global study. Environ Res. 1983 Feb;30(1):95–128. doi: 10.1016/0013-9351(83)90171-8. [DOI] [PubMed] [Google Scholar]
- HARE R. S. Endogenous creatinine in serum and urine. Proc Soc Exp Biol Med. 1950 May;74(1):148–151. doi: 10.3181/00379727-74-17837. [DOI] [PubMed] [Google Scholar]
- Hamilton D. L., Valberg L. S. Relationship between cadmium and iron absorption. Am J Physiol. 1974 Nov;227(5):1033–1037. doi: 10.1152/ajplegacy.1974.227.5.1033. [DOI] [PubMed] [Google Scholar]
- Holbrook J. T., Patterson K. Y., Bodner J. E., Douglas L. W., Veillon C., Kelsay J. L., Mertz W., Smith J. C., Jr Sodium and potassium intake and balance in adults consuming self-selected diets. Am J Clin Nutr. 1984 Oct;40(4):786–793. doi: 10.1093/ajcn/40.4.786. [DOI] [PubMed] [Google Scholar]
- Huebers H. A., Huebers E., Csiba E., Rummel W., Finch C. A. The cadmium effect on iron absorption. Am J Clin Nutr. 1987 May;45(5):1007–1012. doi: 10.1093/ajcn/45.5.1007. [DOI] [PubMed] [Google Scholar]
- Isaksson B. A critical evaluation of the duplicate-portion technique in dietary surveys. Eur J Clin Nutr. 1993 Jul;47(7):457–460. [PubMed] [Google Scholar]
- Johansson G., Callmer E., Gustafsson J. A. Changing from a mixed diet to a Scandinavian vegetarian diet: effects on nutrient intake, food choice, meal pattern and cooking methods. Eur J Clin Nutr. 1992 Oct;46(10):707–716. [PubMed] [Google Scholar]
- Kiyozumi M., Mishima M., Noda S., Miyata K., Takahashi Y., Mizunaga F., Nakagawa M., Kojima S. Studies on poisonous metals. IX. Effects of dietary fibers on absorption of cadmium in rats. Chem Pharm Bull (Tokyo) 1982 Dec;30(12):4494–4499. doi: 10.1248/cpb.30.4494. [DOI] [PubMed] [Google Scholar]
- Kjellström T., Borg K., Lind B. Cadmium in feces as an estimator of daily cadmium intake in Sweden. Environ Res. 1978 Apr;15(2):242–251. doi: 10.1016/0013-9351(78)90101-9. [DOI] [PubMed] [Google Scholar]
- Kjellström T., Linnman L., Elinder CarlGustaf Variation of cadmium concentration in Swedish wheat and barley. An indicator of changes in daily cadmium intake during the 20th century. Arch Environ Health. 1975 Jul;30(7):321–328. doi: 10.1080/00039896.1975.10666714. [DOI] [PubMed] [Google Scholar]
- Milman N., Kirchhoff M. Iron stores in 1359, 30- to 60-year-old Danish women: evaluation by serum ferritin and hemoglobin. Ann Hematol. 1992 Jan;64(1):22–27. doi: 10.1007/BF01811467. [DOI] [PubMed] [Google Scholar]
- Milman N., Pedersen N. S., Visfeldt J. Serum ferritin in healthy Danes: relation to marrow haemosiderin iron stores. Dan Med Bull. 1983 Mar;30(2):115–120. [PubMed] [Google Scholar]
- Moberg A., Hallmans G., Sjöström R., Wing K. R. The effect of wheat bran on the absorption and accumulation of cadmium in rats. Br J Nutr. 1987 Nov;58(3):383–391. doi: 10.1079/bjn19870107. [DOI] [PubMed] [Google Scholar]
- Sartor F. A., Rondia D. J., Claeys F. D., Staessen J. A., Lauwerys R. R., Bernard A. M., Buchet J. P., Roels H. A., Bruaux P. J., Ducoffre G. M. Impact of environmental cadmium pollution on cadmium exposure and body burden. Arch Environ Health. 1992 Sep-Oct;47(5):347–353. doi: 10.1080/00039896.1992.9938373. [DOI] [PubMed] [Google Scholar]
- Vahter M., Berglund M., Lind B., Jorhem L., Slorach S., Friberg L. Personal monitoring of lead and cadmium exposure--a Swedish study with special reference to methodological aspects. Scand J Work Environ Health. 1991 Feb;17(1):65–74. doi: 10.5271/sjweh.1732. [DOI] [PubMed] [Google Scholar]
- Valberg L. S., Sorbie J., Hamilton D. L. Gastrointestinal metabolism of cadmium in experimental iron deficiency;. Am J Physiol. 1976 Aug;231(2):462–467. doi: 10.1152/ajplegacy.1976.231.2.462. [DOI] [PubMed] [Google Scholar]
- Wing A. M. The effects of whole wheat, wheat bran and zinc in the diet on the absorption and accumulation of cadmium in rats. Br J Nutr. 1993 Jan;69(1):199–209. doi: 10.1079/bjn19930022. [DOI] [PubMed] [Google Scholar]