Abstract
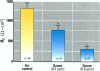
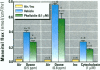
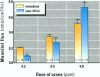
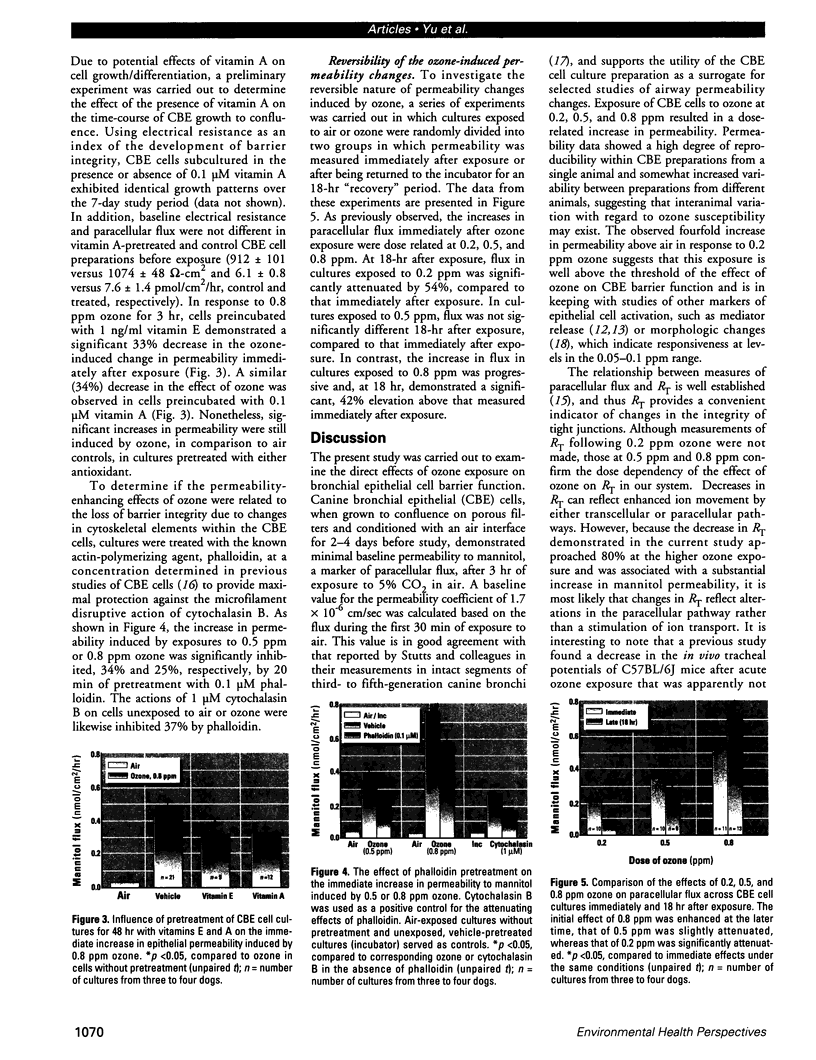
The epithelial cells lining the small, peripheral airways function as important targets for the action of inspired ozone. Loss of epithelial barrier integrity in these regions is a common element in ozone-induced airway inflammation. To investigate the direct effect of ozone on epithelial barrier function, canine bronchial epithelial (CBE) cells grown with an air interface were exposed for 3 hr to 0.2, 0.5, or 0.8 ppm ozone or to air. Mannitol flux, used as an index of paracellular permeability, increased above air controls by 461%, 774%, and 1172% at the three ozone concentrations, respectively. Transcellular electrical resistance exhibited a dose-related decrease. The immediate effect of 0.8 ppm ozone on permeability was significantly inhibited by preincubation for 48 hr in the presence of 1 ng/ml vitamin E (33%) or 1 microM vitamin A (34%). Responses to 0.5 ppm or 0.8 ppm were inhibited by pretreatment of the cells with 0.1 microM of the actin polymerizing agent phalloidin (34% and 25% inhibition, respectively). The increases in permeability induced by 0.2 and 0.5 ppm ozone were attenuated by 54% and 22%, respectively, at 18 hr after exposure, whereas that to 0.8 ppm was further enhanced by 42% at this time. The effects of ozone are modulated by the availability of antioxidants to the cells and appear to be associated with cytoskeletal dysfunction in CBE cells. The data are consistent with a loss of barrier function linked to a direct oxidative effect of ozone on individual CBE cells and indicate that the reversible or progressive nature of this effect is dose dependent.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. S., Hechtman H. B., Shepro D. Phalloidin enhances endothelial barrier function and reduces inflammatory permeability in vitro. Microvasc Res. 1988 May;35(3):308–315. doi: 10.1016/0026-2862(88)90085-4. [DOI] [PubMed] [Google Scholar]
- Alpert S. E., Kramer C. M., Hayes M. M., Dennery P. A. Morphologic injury and lipid peroxidation in monolayer cultures of rabbit tracheal epithelium exposed in vitro to ozone. J Toxicol Environ Health. 1990 Aug;30(4):287–304. doi: 10.1080/15287399009531430. [DOI] [PubMed] [Google Scholar]
- Bassett D. J., Bowen-Kelly E., Brewster E. L., Elbon C. L., Reichenbaugh S. S., Bunton T., Kerr J. S. A reversible model of acute lung injury based on ozone exposure. Lung. 1988;166(6):355–369. doi: 10.1007/BF02714068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla D. K., Crocker T. T. Tracheal permeability in rats exposed to ozone. An electron microscopic and autoradiographic analysis of the transport pathway. Am Rev Respir Dis. 1986 Sep;134(3):572–579. doi: 10.1164/arrd.1986.134.3.572. [DOI] [PubMed] [Google Scholar]
- Bhalla D. K., Rasmussen R. E., Tjen S. Interactive effects of O3, cytochalasin D, and vinblastine on transepithelial transport and cytoskeleton in rat airways. Am J Respir Cell Mol Biol. 1990 Aug;3(2):119–129. doi: 10.1165/ajrcmb/3.2.119. [DOI] [PubMed] [Google Scholar]
- Ciaccio M., Valenza M., Tesoriere L., Bongiorno A., Albiero R., Livrea M. A. Vitamin A inhibits doxorubicin-induced membrane lipid peroxidation in rat tissues in vivo. Arch Biochem Biophys. 1993 Apr;302(1):103–108. doi: 10.1006/abbi.1993.1186. [DOI] [PubMed] [Google Scholar]
- Cooper J. A. Effects of cytochalasin and phalloidin on actin. J Cell Biol. 1987 Oct;105(4):1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das N. P. Effects of vitamin A and its analogs on nonenzymatic lipid peroxidation in rat brain mitochondria. J Neurochem. 1989 Feb;52(2):585–588. doi: 10.1111/j.1471-4159.1989.tb09159.x. [DOI] [PubMed] [Google Scholar]
- Devlin R. B., McDonnell W. F., Mann R., Becker S., House D. E., Schreinemachers D., Koren H. S. Exposure of humans to ambient levels of ozone for 6.6 hours causes cellular and biochemical changes in the lung. Am J Respir Cell Mol Biol. 1991 Jan;4(1):72–81. doi: 10.1165/ajrcmb/4.1.72. [DOI] [PubMed] [Google Scholar]
- Fabbri L. M., Aizawa H., Alpert S. E., Walters E. H., O'Byrne P. M., Gold B. D., Nadel J. A., Holtzman M. J. Airway hyperresponsiveness and changes in cell counts in bronchoalveolar lavage after ozone exposure in dogs. Am Rev Respir Dis. 1984 Feb;129(2):288–291. [PubMed] [Google Scholar]
- Gerrity T. R., Weaver R. A., Berntsen J., House D. E., O'Neil J. J. Extrathoracic and intrathoracic removal of O3 in tidal-breathing humans. J Appl Physiol (1985) 1988 Jul;65(1):393–400. doi: 10.1152/jappl.1988.65.1.393. [DOI] [PubMed] [Google Scholar]
- Hiroshima K., Kohno T., Owada H., Hayashi Y. A morphological study of the effects of ozone on rat lung. I. Short-term exposure. Exp Mol Pathol. 1987 Dec;47(3):327–345. doi: 10.1016/0014-4800(87)90017-7. [DOI] [PubMed] [Google Scholar]
- Kessler H., Haupt A., Frimmer M., Ziegler K. Synthesis of a cyclic retro analogue of somatostatin suitable for photoaffinity labelling. Int J Pept Protein Res. 1987 May;29(5):621–628. doi: 10.1111/j.1399-3011.1987.tb02292.x. [DOI] [PubMed] [Google Scholar]
- Kleeberger S. R., Hudak B. B. Acute ozone-induced change in airway permeability: role of infiltrating leukocytes. J Appl Physiol (1985) 1992 Feb;72(2):670–676. doi: 10.1152/jappl.1992.72.2.670. [DOI] [PubMed] [Google Scholar]
- Leung H. W., Vang M. J., Mavis R. D. The cooperative interaction between vitamin E and vitamin C in suppression of peroxidation of membrane phospholipids. Biochim Biophys Acta. 1981 May 22;664(2):266–272. doi: 10.1016/0005-2760(81)90049-7. [DOI] [PubMed] [Google Scholar]
- Madden M. C., Friedman M., Hanley N., Siegler E., Quay J., Becker S., Devlin R., Koren H. S. Chemical nature and immunotoxicological properties of arachidonic acid degradation products formed by exposure to ozone. Environ Health Perspect. 1993 Jun;101(2):154–164. doi: 10.1289/ehp.93101154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell E. M., Keenan K. P., Huang M. Effects of vitamin A-deprivation on hamster tracheal epithelium. A quantitative morphologic study. Virchows Arch B Cell Pathol Incl Mol Pathol. 1984;45(2):197–219. doi: 10.1007/BF02889865. [DOI] [PubMed] [Google Scholar]
- McKinnon K., Noah T., Madden M., Koren H., Devlin R. Cultured human bronchial epithelial cells release cytokines, fibronectin, and lipids in response to ozone exposure. Chest. 1992 Mar;101(3 Suppl):22S–22S. doi: 10.1378/chest.101.3_supplement.22s. [DOI] [PubMed] [Google Scholar]
- Mustafa M. G. Biochemical basis of ozone toxicity. Free Radic Biol Med. 1990;9(3):245–265. doi: 10.1016/0891-5849(90)90035-h. [DOI] [PubMed] [Google Scholar]
- Mustafa M. G., Tierney D. F. Biochemical and metabolic changes in the lung with oxygen, ozone, and nitrogen dioxide toxicity. Am Rev Respir Dis. 1978 Dec;118(6):1061–1090. doi: 10.1164/arrd.1978.118.6.1061. [DOI] [PubMed] [Google Scholar]
- Pino M. V., Levin J. R., Stovall M. Y., Hyde D. M. Pulmonary inflammation and epithelial injury in response to acute ozone exposure in the rat. Toxicol Appl Pharmacol. 1992 Jan;112(1):64–72. doi: 10.1016/0041-008x(92)90280-6. [DOI] [PubMed] [Google Scholar]
- Pino M. V., Stovall M. Y., Levin J. R., Devlin R. B., Koren H. S., Hyde D. M. Acute ozone-induced lung injury in neutrophil-depleted rats. Toxicol Appl Pharmacol. 1992 Jun;114(2):268–276. doi: 10.1016/0041-008x(92)90077-6. [DOI] [PubMed] [Google Scholar]
- Rasmussen R. E., Bhalla D. K. Transport across rat trachea in vitro after exposure to cytoskeleton-active drugs in vitro or to ozone in vivo. Exp Lung Res. 1989 Mar;15(2):253–268. doi: 10.3109/01902148909087857. [DOI] [PubMed] [Google Scholar]
- Samet J. M., Noah T. L., Devlin R. B., Yankaskas J. R., McKinnon K., Dailey L. A., Friedman M. Effect of ozone on platelet-activating factor production in phorbol-differentiated HL60 cells, a human bronchial epithelial cell line (BEAS S6), and primary human bronchial epithelial cells. Am J Respir Cell Mol Biol. 1992 Nov;7(5):514–522. doi: 10.1165/ajrcmb/7.5.514. [DOI] [PubMed] [Google Scholar]
- Shasby D. M., Shasby S. S., Sullivan J. M., Peach M. J. Role of endothelial cell cytoskeleton in control of endothelial permeability. Circ Res. 1982 Nov;51(5):657–661. doi: 10.1161/01.res.51.5.657. [DOI] [PubMed] [Google Scholar]
- Stutts M. J., Bromberg P. A. Effects of ozone on airway epithelial permeability and ion transport. Toxicol Lett. 1987 Feb;35(2-3):315–319. [PubMed] [Google Scholar]
- Stutts M. J., Schwab J. H., Chen M. G., Knowles M. R., Boucher R. C. Effects of Pseudomonas aeruginosa on bronchial epithelial ion transport. Am Rev Respir Dis. 1986 Jul;134(1):17–21. doi: 10.1164/arrd.1986.134.1.17. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Kleeberger S. R., Croxton T. L. Effects of ozone on transepithelial potential of mouse trachea. Am J Physiol. 1993 Jul;265(1 Pt 1):L33–L37. doi: 10.1152/ajplung.1993.265.1.L33. [DOI] [PubMed] [Google Scholar]
- Young C., Bhalla D. K. Time course of permeability changes and PMN flux in rat trachea following O3 exposure. Fundam Appl Toxicol. 1992 Feb;18(2):175–180. doi: 10.1016/0272-0590(92)90043-h. [DOI] [PubMed] [Google Scholar]
- Yu X. Y., Schofield B. H., Croxton T., Takahashi N., Gabrielson E. W., Spannhake E. W. Physiologic modulation of bronchial epithelial cell barrier function by polycationic exposure. Am J Respir Cell Mol Biol. 1994 Aug;11(2):188–198. doi: 10.1165/ajrcmb.11.2.8049079. [DOI] [PubMed] [Google Scholar]