Abstract
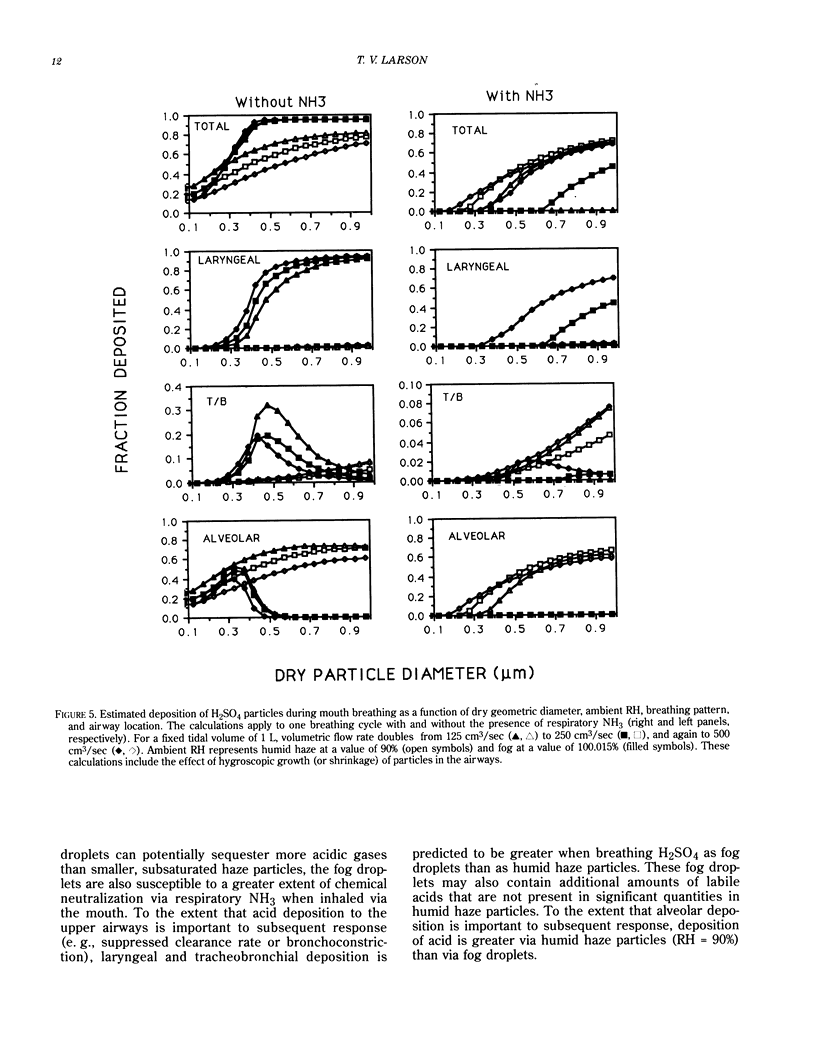
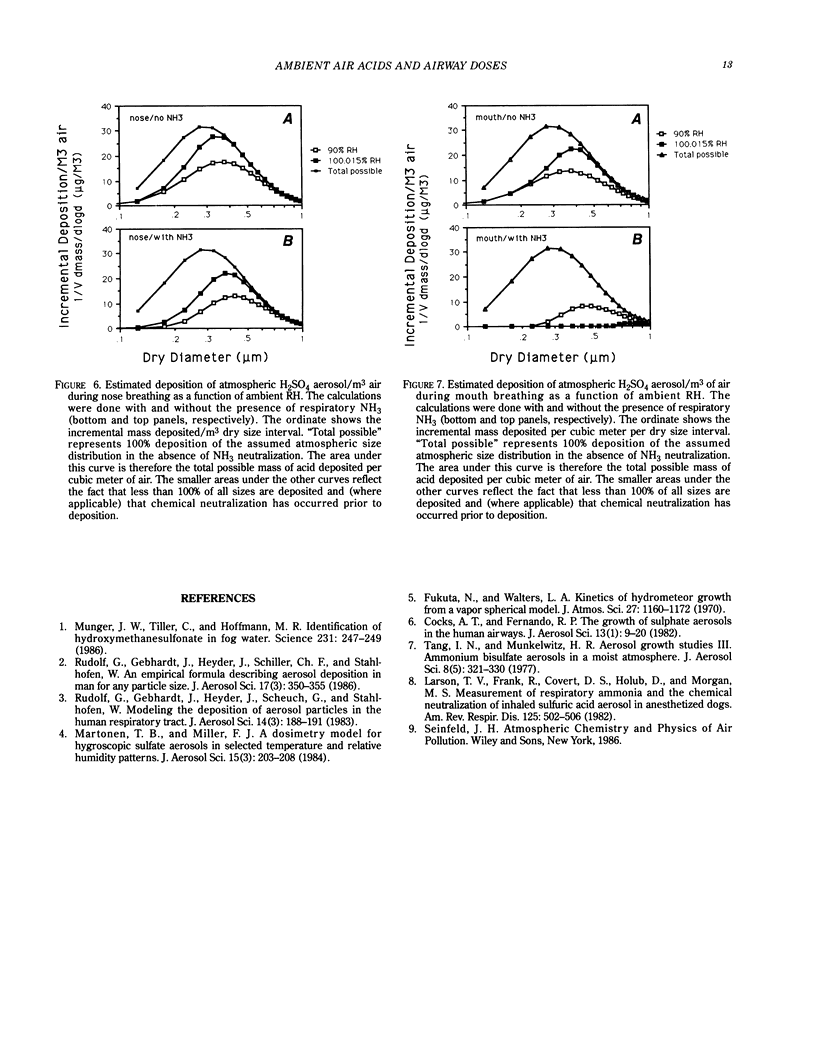
The effects of ambient relative humidity and particle size on acid deposition within the airways have been examined with a computer model. For H2SO4 particles initially at 90% relative humidity in ambient air that are inhaled via the nose or mouth, there is significant deposition of acid in the airways even in the presence of typical values of respiratory NH3. When these same particles are found in a fog at 100.015% relative humidity, there is significant deposition of acid in the nasal region during nose breathing but insignificant deposition to the deep lung for either nose or mouth breathing. The factors governing the partitioning of labile acid gases in the gas and liquid phases prior to inhalation are also discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Larson T. V., Frank R., Covert D. S., Holub D., Morgan M. S. Measurements of respiratory ammonia and the chemical neutralization of inhaled sulfuric acid aerosol in anesthetized dogs. Am Rev Respir Dis. 1982 May;125(5):502–506. doi: 10.1164/arrd.1982.125.5.502. [DOI] [PubMed] [Google Scholar]
- Munger J. W., Tiller C., Hoffmann M. R. Identification of hydroxymethanesulfonate in fog water. Science. 1986 Jan 17;231(4735):247–249. doi: 10.1126/science.231.4735.247. [DOI] [PubMed] [Google Scholar]


