Abstract
Glycoprotein hormone receptors [thyrotropin (TSHr), luteinizing hormone/chorionic gonadotropin (LH/CGr), follicle stimulating hormone (FSHr)] are rhodopsin-like G protein-coupled receptors with a large extracellular N-terminal portion responsible for hormone recognition and binding. In structural models, this ectodomain is composed of two cysteine clusters flanking nine leucine-rich repeats (LRRs). The LRRs form a succession of β-strands and α-helices organized into a horseshoe-shaped structure. It has been proposed that glycoprotein hormones interact with residues of the β-strands making the concave surface of the horseshoe. Gain-of-function homology scanning of the β-strands of glycoprotein hormone receptors allowed identification of the critical residues responsible for the specificity towards human chorionic gonadotropin (hCG). Substitution of eight or two residues of the LH/CGr into the TSHr or FSHr, respectively, resulted in constructs displaying almost the same affinity and sensitivity for hCG as wild-type LH/CGr. Molecular dynamics simulations and additional site-directed mutagenesis provided a structural rationale for the evolution of binding specificity in this duplicated gene family.
Keywords: duplicated genes/glycoprotein hormone receptors/G-protein coupled receptors/leucine-rich repeats/molecular dynamics
Introduction
The glycoprotein hormones, thyrotropin (TSH), lutropin (LH), chorionic gonadotropin (hCG), follitropin (FSH) and their receptors (TSHr, LH/CGr, FSHr) constitute an interesting example of coevolution. The hormones are heterodimeric glycoproteins made of an α subunit common to all four proteins and hormone-specific β subunits encoded by paralogous genes. The corresponding receptors are also encoded by paralogous genes: they constitute a subfamily of G protein-coupled receptors characterized by a large N-terminal extension (359–414 residues) and a rhodopsin-like transmembrane domain (see Figure 1A; for reviews see Dias and Van Roey, 2001; Ascoli et al., 2002; Szkudlinski et al., 2002). While the serpentine domains of individual glycoprotein hormone receptors are functionally interchangeable and display high sequence identity (∼70%), the ectodomains are less similar (∼40%) and are responsible for the specificity of hormone recognition and binding (Braun et al., 1991; Cornelis et al., 2001; Remy et al., 2001; Schmidt et al., 2001).
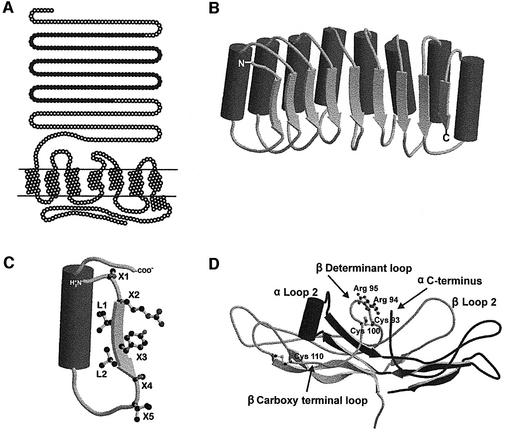
Fig. 1. Schematic representations of TSHr, the LRR portion of TSHr, a single LRR and hCG. (A) Schematic representation of TSHr. The seven transmembrane helices are drawn as helical nets. Closed circles in the N-terminal extension represent the LRR portion of the domain modelled by Kajava et al. (1995), comprising residues 54–254. (B) Schematic representation of LRR portion of TSHr. The α-helices are drawn as solid tubes and the β-strands as flat arrows. (C) Schematic representation of a single LRR. According to proposed structural models (Kajava et al., 1995; Bhowmick et al., 1996), the inner surface of the horseshoe is composed of seven residues: X1X2LX3LX4X5. The motif X2LX3L is considered to be a typical β-strand (represented by a flat arrow) while X1 and X4, X5 are parts of the neighbouring loops (Kajava and Kobe, 2002). (D) Schematic representation of the crystal structure of hCG (Protein Data Bank entry: 1HCN). The ‘seat-belt’ portion of the β-subunit is composed of the ‘Determinant loop’ and the ‘Carboxy terminal loop’ (respectively between Cys93 and Cys100, and between Cys100 and Cys110 ). Figures were created using MolScript v2.1.1 (Kraulis, 1991) and Raster3D v2.5 (Merritt and Bacon, 1997).
The atomic structures of partially deglycosylated hCG and FSH have been determined (Lapthorn et al., 1994; Wu et al., 1994; Fox et al., 2001), showing that the glycoprotein hormones belong to the cystine-knot protein family (Figure 1D). The structures of the ectodomains of the receptors are not available, but their sequences are suggested to contain nine leucine-rich repeats (LRRs) flanked by cysteine-rich domains. This allowed modelling of the LRR portion of the ectodomains of the TSHr and LH/CGr (Jiang et al., 1995; Kajava et al., 1995; Bhowmick et al., 1996) on the template of the crystal structure of the ribonuclease inhibitor (Kobe and Deisenhofer, 1993). According to these models, each LRR is made up of 20–24 amino acids forming a β-strand followed by an α-helix. The LRR units are arranged with their β-strands and α-helices parallel to a common axis and organized spatially to form a horseshoe-shaped molecule, with the β-strands and α-helices making the concave and convex surfaces of the horseshoe, respectively (Figure 1B). By analogy with the atomic structure of the ribonuclease–ribonuclease inhibitor complex (Kobe and Deisenhofer, 1995), it is assumed that hormones make contact, mainly but not exclusively, with the β-sheets of the inner concave portion of the horseshoe.
Guided by the structure of hCG, several groups have shown, by gain-of-function studies with chimeric molecules, that glycoprotein hormone specificity is strongly determined by the ‘seat-belt’ portion of the β subunits (Dias et al., 1994; Moyle et al., 1994; Grossmann et al., 1997). In addition, epitope mapping with monoclonal antibodies suggested that the hormones contact their receptors by their concave side (which corresponds to the β seat belt, β loop 2, α loop 2 and α C-terminus; see Figure 1D) (Moyle et al., 1995; Remy et al., 1996) and, at least for hCG and FSH, seem to dock with their receptors in the same orientation (Wang et al., 2000). Up to now, the structural determinants of the receptors involved in recognition and binding of the hormones have been studied mainly by a strategy based on loss of function (Dias and Van Roey, 2001; Ascoli et al., 2002; Szkudlinski et al., 2002).
In the present study, we relied on the predicted three-dimensional structure of the LRR domains of the glycoprotein hormone receptors to devise a gain-of-function site-directed mutagenesis strategy, with the aim of exchanging the recognition specificity of some of the hormone– receptor couples. We show that substitution of eight residues of the TSHr or two residues of the FSHr into the corresponding amino acids of the LH/CGr resulted in constructs displaying almost the same affinity and sensitivity for hCG as the wild-type (wt) LH/CGr. Molecular dynamics simulations and additional site-directed mutagenesis provided a structural rationale for the evolution of binding specificity between these two families of duplicated genes.
Results
Overall strategy
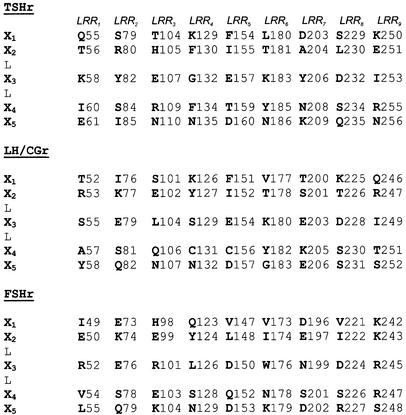
According to proposed structural models (Jiang et al., 1995; Kajava et al., 1995; Bhowmick et al., 1996), the inner surface of the horseshoe is composed of seven residues: X1X2LX3LX4X5 (Figure 1C). The motif X2LX3L is considered to be a typical β-strand while X1 and X4, X5 are parts of the neighbouring loops (Kajava and Kobe, 2002). The side chains of the L residues are pointing inside the hydrophobic core of the protein and are important for its stability. The side chains of the X residues are predicted to be exposed to the solvent, making the surface of the horseshoe available for interaction with the ligands. This view is supported by the observation that X residues are mostly not conserved between the three glycoprotein hormone receptors, including high variability in charged positions, suggesting that they could be important for the binding specificity. Our gain-of-function screening strategy consisted in the replacement of all the X1,2,3,4,5 residues of the β-strands of the LRRs of the TSHr or FSHr by their LH/CGr counterpart (see Figure 2). For simplicity, we will use in the rest of the manuscript the term ‘β-strand’ to describe the X1X2LX3LX4X5 amino acid stretches of the LRRs, even if only X2LX3L are, structurally speaking, typical β-strands. A total of 151 mutants were generated in the course of our study, and tested functionally by transfection in COS-7 cells. Although many contributed in one way or another to the elaboration of the final picture, for convenience, the description and phenotype of the majority of them are described in detail in Supplementary data.
Fig. 2. Alignment of the β-strands of the nine LRRs of the ectodomains of TSHr, LH/CGr and FSHr. In this alignment based on the model illustrated in Figure 1B and C, only the X residues are represented to better visualize the inner cusp of the ectodomain that putatively faces hormones and the possible interactions between side chains of these amino acids. Numbering starts from the first amino acid of the signal peptide of each receptor.
Transformation of TSHr into LH/CGr
First we exchanged each X1 and X5 residue of the TSHr, individually, into their LH/CGr counterparts (Figure 2). Similarly, the X2, X3, X4 residues (X2,3,4) in each β-strand were exchanged three at a time. In a second step, we dissected the X2,3,4 triple mutants displaying interesting phenotypes into individual (X2, X3 or X4) or double (X2,3, X2,4, X3,4) mutants. In a last step, we combined into individual constructs, selected mutations responsible for a gain-of-function phenotype. All these mutants are described in Supplementary table I.
The vast majority of the mutants were well expressed at the cell surface, with only a limited number of exceptions. First, when I60A mutation in the first LRR of the TSHr was combined with other mutations, a moderate to drastic reduction of expression was observed. Also, a drastic decrease in expression was observed when combining triple (X2,3,4) mutants of LRR3 and LRR7. Expression could be rescued, however, by further inclusion of triple X2,3,4 mutations of LRR4. These data illustrate the tolerance (and its limits) of the LRR structure to amino acid substitutions. They are described in detail in Supplementary table I.
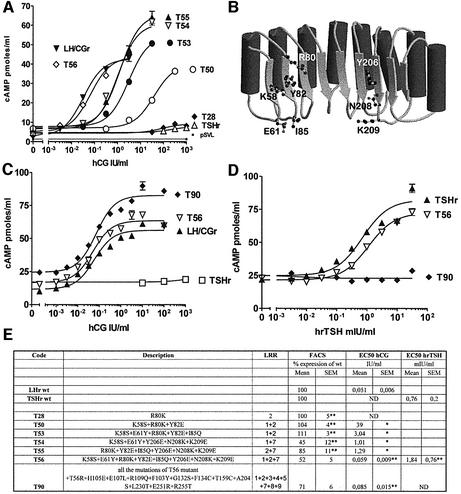
The stepwise exchange strategy described above led to the identification of eight amino acids of the TSHr that, upon replacement by the corresponding residues of the LH/CGr, conferred individually a gain of sensitivity towards hCG (see Table I, Figure 3B and Supplementary figure 1). Two of these residues belong to the first LRR, three to the second and three to the seventh (Figure 3B). No X1 residues are involved. Interestingly, the gain of sensitivity of the individual mutants was additive, as the stepwise inclusion of mutations led to an eight residue TSHr mutant displaying the same sensitivity and affinity towards hCG as the wt LH/CGr (Figures 3A and C, and 5: mutant T56). Unexpectedly, this mutant still exhibits a close to wild-type sensitivity to TSH, making it a dual specificity receptor recognizing equally well TSH and hCG (Figure 3C and D). With the aim to loose recognition of TSH, while keeping the gain of function towards hCG, we exchanged nearly all the X2,3,4,5 residues of the TSHr with their LH/CGr counterparts. The resulting mutant, T90, combining the eight amino acid mutations of T56, plus 12 additional substitutions, has completely lost responsiveness to TSH, while showing sensitivity and affinity for hCG identical to that of the wt LH/CGr (Figures 3C and D, and 5).
Table I. Identification of eight residues of TSHr conferring individually a gain of sensitivity towards hCG.
| Code | Description | LRR | FACS |
hCG screening |
Emax hCG |
EC50 hCG |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| % expression of wt |
100 IU/ml |
1000 IU/ml or plateau |
IU/ml |
|||||||
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | |||
| LH/CGr wt | 100 | 0.051 | 0.006 | |||||||
| TSHr wt | 100 | 1.14 | 0.03 | 1.30 | 0.04 | ND | ||||
| Mutants positive in screening | ||||||||||
| X5 mutants | ||||||||||
| T8 | E61Y | 1 | 75 | 6 | 3.50 | 0.42 | 3.96 | 0.52 | ND | |
| T9 | I85Q | 2 | 107 | 8 | 1.53 | 0.09 | 2.06 | 0.11 | ND | |
| T11 | K209E | 7 | 91 | 10 | 4.09 | 0.27 | 4.60 | 0.29 | 25 | 4 |
| X2-X3-X4 mutants | ||||||||||
| T14 | T56R+K58S+I60A | 1 | 38 | 6a | 2.38 | 0.24 | 2,55 | 0.04a | 88 | 12a |
| T15 | R80K+Y82E | 2 | 110 | 18 | 2.53 | 0.38 | 3.00 | 0.53a | ND | |
| T19 | A204S+Y206E+N208K | 7 | 28 | 7 | 8.04 | 2.09 | 6.12 | 0.66 | 31 | 6 |
| Residues implicated in the gain of sensitivity towards hCG of the X2-X3-X4 mutants | ||||||||||
| T26 | K58S | 1 | 95 | 2 | 2.08 | 0.14 | 2.72 | 0.32 | 43 | 6 |
| T28 | R80K | 2 | 100 | 5a | 1.59 | 0.03a | 1.72 | 0.06 | ND | |
| T29 | Y82E | 2 | 87 | 14a | 1.40 | 0.36a | 2.20 | 0.11 | ND | |
| T34 | Y206E | 7 | 67 | 3 | 2.91 | 0.29 | 4.18 | 0.41 | ND | |
| T35 | N208K | 7 | 46 | 3 | 2.44 | 0.20 | 4.07 | 0.46 | ND | |
List and characteristics of the mutants displaying a gain of sensitivity to hCG. Column 1: code name of the mutants. Column 2: full description of the mutants, with numbering as in Figure 2. Column 3: indication of the LRR β-strands bearing the mutations. Column 4: amount of mutant receptors present at the surface of transfected COS-7 cells (in % of wild type, see Materials and methods). Column 5: fold stimulation of cAMP accumulation over basal, in transfected COS cells after stimulation by 100 IU/ml of hCG. Column 6: fold stimulation of cAMP accumulation over basal, but for stimulation by 1000 IU/ml of hCG, or by a concentration giving a maximal (plateau) response of cAMP accumulation. Column 7: EC50 values in IU/ml for stimulation by hCG of mutant TSHr sensitive enough to obtain a plateau.
aTwo measurements were performed (results expressed as mean ± range of duplicates). ND, EC50 not determined because concentration–action curve does not reach a plateau of maximal stimulation.
Fig. 3. Transformation of TSHr into LH/CGr. Concentration–action curves for TSHr mutants under stimulation by increasing concentrations of hCG or hrTSH. COS-7 cells transiently transfected with the various constructs were stimulated by increasing concentrations of hCG or hrTSH and intracellular cAMP values were determined (see Materials and methods). Each curve is representative of at least two separate experiments. The levels of cAMP observed for the various constructs in the absence of stimulation by a hormone reflect their constitutive activity and their different level of expression. (A) The effects of the eight residues found to be sensitive to hCG (see text and B) are additive: T28 has one residue, T50 three, T53 and T54 five, T55 six and T56 eight residues mutated (see E for details). (B) Schematic representation of the LRR portion of TSHr with the eight residues giving a gain of function towards hCG highlighted. (C and D) T56 is as sensitive to hCG as the wt LH/CGr and nearly as sensitive to hrTSH as the wt TSHr. T90 (with the same eight mutated residues as T56, plus 12 others, see E) is as sensitive to hCG as the wt LH/CGr, but does not respond to hrTSH. (E) Short table summarizing the data shown in the figure. Column 1, code name of the mutants; column 2, full description of the mutants, with numbering as in Figure 2; column 3, indication of the LRR β-strand bearing the mutations; column 4, amount of mutant receptors present at the surface of transfected COS cells (as percentage of wild type, see Materials and methods); column 5, EC50 values in IU/ml for stimulation by hCG of mutant TSHr sensitive enough to obtain a plateau; column 6, EC50 values in mIU/ml for stimulation by hrTSH of mutant TSHr sensitive enough to obtain a plateau. *A single measurement was performed; **two measurements were performed (result expressed as mean ± range of duplicates). ND, EC50 not determined because concentration–action curve does not reach a plateau of maximal stimulation.
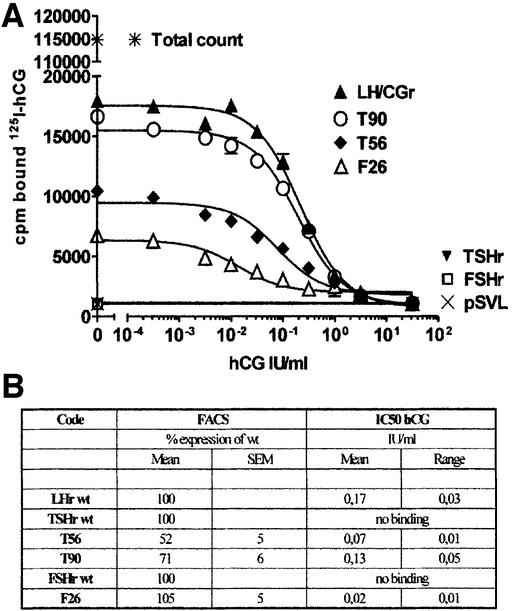
Fig. 5. hCG binding. (A) Displacement curves of [125I]hCG by increasing concentrations of hCG. COS-7 cells transfected with the various constructs were incubated overnight at room temperature in the presence of ± 120 000 c.p.m. of [125I]hCG and graded concentrations of cold hCG (see Materials and methods). Prism v3.03 was used for curve fitting and for IC50 calculation. (B) Short table presenting expression and IC50 values.
Analysis of the eight residues of the TSHr implicated in the gain of sensitivity to hCG
To explore whether the gain of sensitivity of the TSHr mutants towards hCG was due to the establishment of new interactions or loss of repulsion, we mutated individually to alanine the eight residues of TSHr identified above. In the same vein, residue K58 was also mutated to Arg or Asp. The detailed results and interpretation of their effects are presented in Supplementary data (figure 1 and table I). In summary, they stress the importance of our gain-of-function screening strategy for the study of recognition specificity in duplicated gene families. Indeed, depending on the residue involved, Ala mutants presented no (e.g. E61,Y82), lower (e.g. Y206, K209), the same (e.g. K58, I85, N208) or a better (e.g. R80) gain of sensitivity towards hCG, when compared with substitution by the homologous residue of LH/CGr.
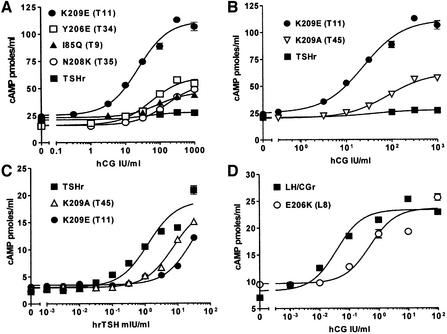
Residue X5 of LRR7 (K209 in TSHr, E206 in LH/CGr) deserves special attention. Its substitution in TSHr for the corresponding LH/CGr residue (K209E, mutant T11) produced the strongest gain in sensitivity to hCG for a single mutant (Figure 4A and Table I). At the same time, the mutant displayed a severe loss of sensitivity to TSH (Figure 4C). These effects are probably due to charge reversal, since mutation K209A (mutant T45) was two to three times less effective than K209E, for both the gain of sensitivity to hCG and the loss of sensitivity to TSH (Figure 4B and C). In addition, the reciprocal mutation in LH/CGr (E206K) causes an important loss of sensitivity and binding to hCG (Figure 4D and Supplementary figure 2). Together, these results suggest that X5 of LRR7 is involved in an electrostatic interaction of opposed charges in the TSH–TSHr and hCG–LH/CGr hormone receptor complexes.
Fig. 4. X5 residue of LRR7 (K209 in TSHr, E206 in LH/CGr) is important for hCG and TSH specificity. Concentration–action curves for TSHr or LH/CGr mutants under stimulation by increasing concentrations of hCG or hrTSH. COS-7 cells were transfected and handled as described in the legend to Figure 3. Each curve is representative of at least two separate experiments. Expression values are (as percentage of wild type): 107 ± 8% for I85Q, 67 ± 3% for Y206E, 46 ± 3% for N208K, 91 ± 10% for K209E, 106 ± 12% for K209A and 62 ± 8% for E206K. (A) The K209E mutation is much more effective than the individual mutations of the other seven residues (for comparison with the other residues, see Supplementary table I). (B) The greater effect due to charge reversal than to charge loss suggests that K209 is involved in an electrostatic interaction with hCG (K209E, EC50 for hCG = 25 ± 4 IU/ml). (C) These results suggest that K209 interacts with TSH by an electrostatic interaction. (D) E206K mutation provokes a large loss-of-function effect (EC50 for hCG = 0.05 ± 0.01 IU/ml for LH/CGr and 0.26 ± 0.12 IU/ml for E206K).
Transformation of FSHr into LH/CGr
The same screening strategy was applied to the FSHr. However, as no X1 residues appeared to be involved in the gain of sensitivity of the TSHr mutants towards hCG, we restricted mutagenesis to X5 and X2,3,4 residues (respectively as single residue and three-at-a-time mutants). It must be noted that the wt FSHr is slightly sensitive to purified and recombinant hCG (for concentrations >30 IU/ml and 100 IU/ml, respectively) (see Figures 6 and 7 and Schubert et al., 2003) and that it is moderately less permissive than TSHr to mutation, for its expression at the cell surface (see Figures 6 and 7 and data not shown).
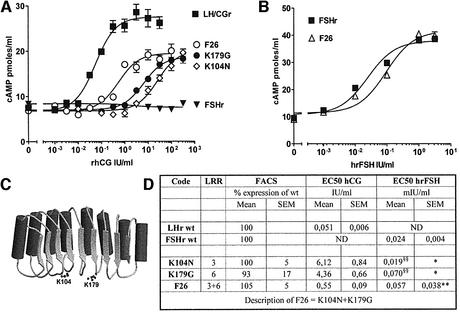
Fig. 6. Transformation of FSHr into LH/CGr. Concentration–action curves for FSHr mutants under stimulation by increasing concentrations of hCG, recombinant hCG (rhCG) or human recombinant FSH (hrFSH). COS-7 cells were transfected and handled as described in the legend to Figure 3. (A) the effects of the two substitutions (K104N and K179G) are additive (F26 is the double mutant). F26 is only 1 log less sensitive to hCG than LH/CGr. (B) F26 shows only a slight decrease in sensitivity to FSH. (C) Schematic representation of the LRR portion of FSHr with the two residues giving a gain of function towards hCG highlighted. (D) Short table (designed as in Figure 3) summarizing the data shown in the figure. §§Not illustrated in the figure.
Fig. 7. Various substitutions for the two residues in FSHr that gave a gain of function towards hCG. Concentration–action curves for FSHr mutantated in position K104 or K179, under stimulation by increasing concentrations of hCG. COS-7 cells transfected with the various constructs were handled as described in the legend to Figure 3. (A) Only the K104N mutant gave a drastic gain of sensitivity towards hCG. Loss of a basic residue (K104A) or charge reversal (K104D) gave only a very slight gain of function towards hCG. (B) The loss of basic character in position 179 is important; but full gain of function is observed only if a small neutral residue (Gly or Ala) is substitued for K179. (C) Short table presenting expression and EC50 values for hCG stimulation (designed as in Figure 3).
In contrast to the observation made for the TSHr, we found only two residues of FSHr that, upon substitution with their LH/CGr counterpart, gave a gain of sensitivity towards hCG: K104N and K179G (see Figure 6A and D). Individually the effect of each mutation was much stronger than in the case of the TSHr. However, here also, the effects of the two mutations were additive and the double mutant, F26, displays dual specificity. It retains near normal sensitivity to FSH (Figure 6B and D), while becoming highly sensitive to hCG (Figure 6A and D). Surprisingly, in competition binding assays, the F26 mutant displays an affinity for hCG eight times higher than the wt LH/CGr (Figure 5). The reason for this discrepancy between sensitivity and affinity is not clear. Current models suggest that activation of the serpentine portions of glycoprotein hormone receptors would be exerted by an activated conformation of the ectodomain (Sangkuhl et al., 2002; Vlaeminck-Guillem et al., 2002). It is thus conceivable that F26 ectodomain would be slightly crippled in its ability to achieve the fully activating conformation, while displaying strong binding capacity.
Analysis of the two residues of the FSHr implicated in the gain of sensitivity to hCG
To better understand the molecular mechanisms implicated in the gain of sensitivity of FSHr mutants to hCG, we mutated individually the two Lys in several residues (see Figure 7 and Supplementary data). These mutations are of interest because they show clearly that specificity could be achieved by different molecular mechanisms. For position 104, it is the absence of an Asn that is of importance to avoid promiscuous stimulation of the FSHr by hCG (Figure 7A), while in position 179, it is the presence of a large, preferably basic residue (Figure 7B).
Molecular dynamics simulations of the extracellular domain of the LH/CG, TSH and FSH receptors
Figure 8A and B shows results representative of unrestrained molecular dynamics simulations of the LRR portions of TSHr, LH/CGr and FSHr ectodomains, performed in water (see Material and methods). A detailed analysis of these simulations is presented in Supplementary data. We will only discuss here the most salient features.
Fig. 8. Representative models of the molecular dynamics simulations. The polar side chain interactions of the X residues of the β-strands of the LRRs of wt TSHr, LH/CGr, FSHr and T56, T90 and F26 mutants were determined by molecular dynamics simulations (see Materials and methods). Row A: X residues of the nine LRRs of wt TSHr, LH/CGr and FSHr are represented as in Figure 2. Acidic and basic residues are highlighted (respectively in red and in blue) and their side chain interactions are represented by arrows. Row B: representative structures obtained during simulations of the LRR portion of the three receptors with polar side chains of interest highlighted. The interactions represented schematically in row A are clearly visible. Row C: electrostatic potential maps constructed with the GRASP software (see methods) using the structures of the LRR portions shown in row B. The positions of the charged side chains are indicated. The three receptors display very different patterns: TSHr is positively charged in its N-terminal and centre portions, LH/CGr has an acidic groove in the lower central portion (created by E154, D157 and E206) and FSHr is acidic in its N-terminal portion and around D150, but is positively charged in the C-terminal region and in the region of all the X5 residues. Row D: amino acid sequence and detailed view of the seat-belt portion of the β-subunit (in red) of the TSH model (the structure was obtained by comparative modelling from the 1HCN structure), the crystal structure of hCG (PDB entry 1HCN) and the crystal structure of FSH (1FL7). The side chains of the residues putatively involved in the interaction with the receptors are drawn in ball-and-stick format. Row E: electrostatic potential maps of the T56, T90 and F26 mutants (representative structures are shown in Supplementary figure 5). In contrast to wt TSHr and FSHr, and in common with the wt LH/CGr, the T56, T90 and F26 mutants display an acidic groove in the lower central portion of the LRRs, generated by E157, D160 and E209 in T56 and T90, and by D150, D153 and D202 in F26 (see text for details).
The numerous charged residues inside the horseshoe form a network of polar interactions (represented as arrows in Figure 8A). The resulting molecular electrostatic potential at the accessible surface of the models is represented in Figure 8C for the wild-type receptors (see Material and methods). In LH/CGr, the most salient feature is the presence of an ‘acidic groove’ (coloured in red) in the centre of the LRRs, created by the free acidic side chains of E154, D157 and E206. For TSHr, in contrast, the centre of the LRRs appears positively charged, due to the presence of R80, R109, K183 and K209, which completely shield the acidic residues E107, E157 and D160 (Figure 8C, left panel). In FSHr, the simulation predicts that the negative area existing in the vicinity of E154 in the LH/CGr is also present around residue D150 (compare middle and right panels in Figure 8C). However, in contrast to LH/CGr, the bottom part of LRRs5–7 of FSHr is positively charged due to the presence of K104, K179 and R227, which hide the acidic residues D153 and D202 (see right panel of Figure 8C).
In search of a structural characteristic common to the three constructs displaying promiscuous sensitivity to hCG (T56, T90 and F26 mutants), we noted that, for all of them, the simulations predicted the presence of an ‘acidic groove’ reminiscent of the situation in the wt LH/CGr (Figure 8C and E). For T56 and T90 mutants (Figure 8E, left and middle panels), this results from the presence of the free side chains of E157, D160 and E209, which is secondary to the replacement of residues in the seventh LRR. For the F26 mutant, the K104N and K179G substitutions generate the missing negative area at the bottom part of LRRs5–7, properly mimicking the ‘acidic groove’ observed in the LH/CGr. Together, these simulations predict that an acidic groove located in the middle portion of the LRR domain constitutes an important structural determinant for the normal or promiscuous interaction of hCG with the glycoprotein hormone receptors.
Loss of function mutants
In the last part of this study, we tested some of our mutants for loss of function towards their natural agonists, which allowed identification of several residues potentially implicated in homologous recognition. Thereafter, for TSHr and FSHr, we tested whether these residues, when introduced in the corresponding position of another receptor, would confer a gain of sensitivity to TSH or FSH, respectively. Briefly, these experiments demonstrate that different portions of the concave surface of the horseshoes are important in the three receptors for sensitivity to their natural agonists: (i) for LH/CGr, X3,4,5 residues of LRR7; (ii) for TSHr, X2,3,4 residues of LRR3 and LRR9 and X5 residue of LRR7; (iii) for FSHr, X5 residue of LRR1. The detailed pertinent results of these experiments are presented as Supplementary figures 2–4. In addition, mutations centred on residue X5 of LRR1 suggest that FSHr and TSHr receptors would be functionally closer to each other than FSHr and LH/CGr (Supplementary figure 4). This suggests that FSHr and TSHr could have evolved from a more recent common ancestor and parallels functional data obtained with the hormones (Campbell et al., 1997; Grossmann et al., 1997; Li and Ford, 1998).
Discussion
Combining molecular modelling, sequence conservation information and mutagenesis, we have identified key residues implicated in the specificity of hormone recognition by glycoprotein hormone receptors. Our strategy was built on the premise that both the receptors and the hormones are encoded by paralogous genes, which suggested that the ligand binding process would be similar for all hormone–receptor couples. The available structural models of the ectodomains of the glycoprotein hormone receptors were used to predict which amino acids in the LRRs must be exchanged between receptors to alter hormone specificity. Substitution of eight and two residues of TSH and FSH receptors, respectively, by their LH/CGr counterparts causes a gain of sensitivity towards hCG, without altering significantly sensitivity to TSH or FSH. This is a strong indication that the current molecular models of the LRR portion of glycoprotein hormone receptors must be reasonably accurate (Jiang et al., 1995; Kajava et al., 1995; Bhowmick et al., 1996). The effects of individual mutations are additive, yielding mutant receptors displaying sensitivity to hCG comparable to that of the LH/CGr, but keeping normal sensitivity to their natural agonist (i.e. receptors with dual specificity). In order to completely invert recognition specificity of the TSHr (i.e. making it sensitive to hCG, while losing sensitivity to TSH), it was necessary to introduce 12 additional mutations in the LRRs (mutant T90, Figure 3), which means that evolution targeted different residues of the individual receptors to achieve recognition specificity.
Additional site-directed mutagenesis experiments and molecular dynamics simulations showed the importance of an ‘acidic groove’ in the centre of the LRR domain of the receptor/mutants displaying high sensitivity to hCG (wt LH/CGr, T56, T90 and F26 mutants). This acidic groove is created by three free acidic residues in positions X3 and X5 of LRR5 and X5 of LRR7. Together with previous studies (Bhowmick et al., 1996, 1999; Smits et al., 2002), our results suggest that these residues could be ‘energetic hot spots’ (Clackson and Wells, 1995; Chen and Shapiro, 1997), i.e. those with an important energetic contribution to the formation of the hCG–receptor complex, for which it is tempting to identify the partners in hCG.
Our data suggest that X5 of LRR7 (K209 in TSHr, E206 in LH/CGr) is involved in an electrostatic interaction of opposed charges in TSH–TSHr and in hCG–LH/CGr complexes (Figure 4). Gain-of-function mutagenesis studies of glycoprotein hormones (Dias et al., 1994; Moyle et al., 1994; Campbell et al., 1997; Grossmann et al., 1997) have shown that the ‘determinant loop’ (Figures 1D and 8D) of hCG harbours determinants of luteotropic activity whereas those of TSH, or FSH, both confer thyrotropic activity. Interestingly, the determinant loop of hCG has two Arg (R94 and R95), while TSH and FSH have acidic residues in these positions (D110 and D93, see Figure 8D). This makes the electrostatic surface of this part of the determinant loops positive in hCG and negative in FSH and TSH (Lapthorn et al., 1994; Szkudlinski et al., 1996; Fox et al., 2001). Accordingly, we propose that this portion of the determinant loop of glycoprotein hormones would face residue X5 of the seventh LRR of their receptors.
Next, we considered the residue in position X3 of the fifth LRR (E157 in TSHr, E154 in LH/CGr and D150 in FSHr), as it is conserved in all known glycoprotein hormone receptors and could play a role in the interaction of hCG with LH/CGr (Bhowmick et al., 1996). In Smits et al. (2002), we showed that the gain of sensitivity to hCG of K183 TSHr mutants resulted from the release of the side chain of E157 from an ionic interaction with K183, making it available for interaction with hCG. We observe now that these K183 TSHr mutants display also a 5- to 7-fold increase in sensitivity to human recombinant TSH (hrTSH) when compared with the wt TSHr (see Supplementary figure 6). These results suggest that a free acidic side chain in position X3 of the fifth LRR could face a positive charge common to TSH and hCG (and possibly FSH) in hormone–receptor complexes. The observation that, in the TSHr, the negative charge in position X3 of the fifth LRR (E157) is neutralized, suggests that the receptor may have ‘sacrificed’ part of its affinity for TSH to ‘minimize’ promiscuous interaction with the other glycoprotein hormones. It is tempting to view this as a general evolutionary mechanism contributing to the establishment of recognition specificity in multigenic families of agonists and receptors submitted to homeostatic regulation. Indeed, in the case of the glycoprotein hormones and their receptors, for example, the negative feedback loops characteristic of the hypothalamic– pituitary–end organ axis, would allow for the independent adjustment of the binding affinity of each agonist–receptor couple.
The negative determinant theory (Moyle et al., 1994) contends that specificity of hormone–receptor recognition in duplicated gene families is achieved mainly by repulsive residues. Our results demonstrate that specificity of agonist recognition by glycoprotein hormone receptors is actually achieved by a combination of different molecular mechanisms. This is nicely illustrated by the following examples of single residue mutants: (i) only an asparagine substitution for lysine in position 104 of the FSHr increases sensitivity to hCG, suggesting that a specific interaction is established; (ii) a basic residue in position 179 of the FSHr (the wild-type lysine, or an arginine substitution) is required to avoid promiscuous activation by hCG, suggesting that an electrostatic repulsive mechanism is at work here; (iii) the X5 residue of LRR7 is attractive for hCG and repulsive for TSH when it is acidic, as in the LH/CGr (E206); in contrast, the same residue is attractive for TSH and repulsive for hCG when it is basic, as in the TSHr (K209).
The observation that limited structural changes in the FSHr can lead to a receptor with dual specificity, recognizing both FSH and hCG, may have medical implications. Natural mutations affecting codon 104 of the FSHr could generate a K104N mutant displaying responsiveness to hCG (see Figure 7), while still responding normally to FSH (Figure 6D). Spontaneous ovarian hyperstimulation syndrome (Schenker, 1999), could well be caused by such a mutation, since its phenotype would be readily explained by the promiscuous hyperstimulation of a mutated FSHr by hCG during the first trimester of pregnancy.
Our ability to switch with relative ease recognition specificity between glycoprotein hormones receptors underscores the notion that evolution has ideally shaped the LRR motifs to achieve versatile molecular recognition in phenomena as diverse as hormone action, development and innate immunity (>2500 entries in Trembl/Swissprot databases) (Kobe and Kajava, 2001). Although the final word on the structure of ligand–receptor complexes will obviously need to wait for crystallographic data, the present study indicates that our screening strategy, associated with molecular modelling, allows identification of residues with functional significance (Chen and Shapiro, 1997; Schreiber, 2002) in the recognition mechanisms involving the large LRR superfamily. Finally, our results illustrate the power of the gain-of-function approach in the study of structure–function relationships in duplicated gene families.
Materials and methods
Reagents
Plasmid pBluescript SK+ was from Stratagene (La Jolla, CA, USA); plasmid pSVL from Amersham Pharmacia Biotech (Roosendaal, The Netherlands); restriction enzymes from Life Technologies (Merelbeke, Belgium) and New England Biolabs (Beverly, MA, USA); Pfu Turbo polymerase from Stratagene. Monoclonal antibody (Mab) BA8 (Costagliola et al., 1998) is directed against a conformational epitope on the TSHr ectodomain. Mab 16B5 and 5B2 were obtained by genetic immunization (Costagliola et al., 1998) with the cDNA encoding the human LH/CGr and FSHr, respectively. Mab 106-105, recognizing human FSHr (Lindau-Shepard et al., 2001), was a generous gift from Dr J.A.Dias. Purified hCG, recombinant hCG and purified bovine TSH were from Sigma Chemical Co. (St Louis, MO, USA); human recombinant FSH from Organon Belge (Brussels, Belgium) and hrTSH from Genzyme Corporation (Cambridge, MA, USA).
Construction of the TSHr, LH/CGr, FSHr mutants
Mutations were introduced in the hTSHr, hLH/CGr or hFSHr by site mutagenesis as described previously (Vlaeminck-Guillem et al., 2002). The primers are available upon request. The appropriate mutated portions of SK+ hTSHr mutants were subcloned in pSVL-hTSHr cDNA (between XhoI and XbaI sites). An EcoRV site was introduced at position 1065 (GACATT→GATATC) of the SK+ hLH/CGr cDNA allowing the subcloning of the PCR-generated extracellular part of LH/CGr mutants upstream of a wild-type serpentine portion in pSVL-hLH/CGr cDNA. The natural XbaI site in position 665 of hFSHr cDNA was eliminated to allow its cloning in pSVL between XhoI and XbaI sites. The appropriate mutated portions of SK+ hFSHr mutants were subcloned in the pSVL-hFSHr cDNA using natural restriction sites. The strategy of construction of each mutant is available upon request. All constructs were amplified in DH5αF′ competent cells and recombinant DNA from selected clones was purified and sequenced for confirmation of the sequences of the PCR-generated areas.
Transfection experiments
COS-7 cells were used for all transient expression experiments, which were performed according two protocols. The first ‘standard protocol’ (Vlaeminck-Guillem et al., 2002) was used for bindings, stimulations by purified hCG in screening steps and for testing single residue TSHr mutants. Briefly, 300 000 cells were seeded in 3.5 cm culture dishes at day 1, and transfected at day 2 by the DEAE–dextran method, as described previously (Vlaeminck-Guillem et al., 2002). Two days after transfection, cells were used for cAMP determinations and flow immunocytofluorometry (see below). The second, ‘24 well’ protocol was used for all other experiments, with the aim of minimizing the amount of hormones. Briefly, 2 million cells were seeded in 10 cm dishes at day 1 and transfected at day 2 as described above. At day 3 cells were trypsinized, detached and centrifuged at 700 g for 2 min. They were suspended in 16 ml of culture medium and seeded (1 ml/well) in 24 well plates (Nunc Brand Products, Sanbio b.v., Uden, The Netherlands). At day 5, cells were used for cAMP determinations and flow immunocytofluorometry. Duplicate dishes were used for each assay. Cells transfected with pSVL alone were always run as controls.
Quantification of cell surface expression of TSHr, LH/CGr, FSHr constructs by FACS
Expression of TSHr, LH/CGr, FSHr and the various mutants was quantified at the surface of COS-7 cells by flow imunocytometry, exactly as described previously (Costagliola et al., 1998), with the panel of monoclonal antibodies described under the ‘Reagents’ section. The fluorescence of 10 000 cells per tube was assayed by a FACScan Flow cytofluorometer (Becton Dickinson, Eerenbodegem, Belgium). Cells transfected by pSVL alone and by pSVL-hTSHr, hLH/CGr or hFSHr wt cDNA were always run as negative and positive controls, respectively.
Determination of cAMP production
For cAMP determinations, culture medium was removed 48 h after transfection and replaced by Krebs–Ringer–HEPES buffer (KRH) for 30 min. Thereafter, cells were incubated for 60 min in fresh KRH supplemented with 25 µM of the phosphodiesterase inhibitor Rolipram (Laboratoire Logeais, Paris, France) and graded concentrations of the various hormones. The medium was discarded and replaced with HCl 0.1 M and the extracts were dried under vacuum, resuspended in water and diluted appropriately for cAMP measurements according to Brooker et al. (1979). Duplicate samples were assayed in all experiments; results are expressed in pmol/ml. Concentration–action curves were fitted with Prism v3.03 (GraphPad Software, Inc., San Diego, CA, USA).
Binding
Ligand binding was measured on COS-7 cells transfected according to the ‘standard protocol’ by wild-type or mutant receptor constructs. Cells were incubated at room temperature in 1 ml of modified Hanks’ buffer without NaCl (isotonicity maintained with 280 mM sucrose), supplemented with 2.5% low fat milk, [125I]glycoprotein hormone (120 000 c.p.m.) and graded concentrations of cold glycoprotein hormones. Incubation was for 4 h for [125I]TSH, and overnight for [125I]hCG or [125I]FSH. Thereafter, the cells were rapidly rinsed with the same ice-cold buffer, solubilized with 1 ml 1 N NaOH, and radioactivity was measured in a γ counter. All experiments were carried out in duplicate and results are expressed as c.p.m. bound.
Molecular modelling and molecular dynamics simulations of the extracellular domain of the TSHr, LH/CGr and FSHr
The atomic coordinates of the extracellular domain of the TSHr (Kajava et al., 1995), constructed from the structure of the porcine ribonuclease inhibitor (Kobe and Deisenhofer, 1993), were used in the simulations. The backbone of the LH/CGr was constructed from the TSHr coordinates, replacing the VYSG227 loop of the TSHr by the ATG223 loop of LH/CGr, using the WDN402 coordinates of endoglucanase D (PDB entry 1CLC). The backbone of the FSHr was constructed from the LH/CGr model, replacing the SSESNF151 loop of the LH/CGr by the SLQKV147 loop of FSHr, using the EFVEG67 coordinates of transthyretin (1TTB). SCWRL-2.1 was employed to add the side chains of the non-conserved residues based on a backbone-dependent rotamer library (Dunbrack and Cohen, 1997). Molecular models for the mutant receptors were built from these coordinates by amino acid replacement. The resulting structures were placed in a rectangular box containing Monte Carlo-equilibrated TIP3P water molecules. The periodic boxes were ∼75 × 50 × 50 Å, and contained between 3956 and 4205 water molecules in addition to the extracellular domain. Initially, the atoms of the receptor were kept fixed whereas the water molecules were energy minimized (500 steps). Subsequently, the entire systems were energy minimized (500 steps), heated (from 0 to 300 K in 15 ps), equilibrated (15–200 ps), and the production run (200–500 ps) was carried out at constant pressure using the particle mesh Ewald method to evaluate electrostatic interactions (Darden et al., 1993). Structures were collected every 5 ps during the last 300 ps of simulation (60 structures per simulation). The molecular dynamics simulations were run with the Sander module of AMBER 5 (Pearlman et al., 1995 and http://www.amber.ucsf.edu/amber), the all-atom force field (Cornell, 1995), SHAKE bond constraints in all bonds (Ryckaert et al., 1977), a 2 fs integration time step and constant temperature of 300 K coupled to a heat bath. The molecular electrostatic potentials on the accessible surface of representative structures were calculated and displayed with GRASP (Nicholls et al., 1991 and http://trantor.bioc.columbia.edu/grasp), using the Cornell atomic charges (Cornell, 1995), with the colour scale going from –4 (red) to +4 (blue).
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank C.Gervy for expert assistance in radioimmunoassays. Computer facilities were provided by the Centre de Computació i Comunicacions de Catalunya. S.C. is Research Associate at the FNRS. This study was supported by the Belgian State, Prime Minister’s office, Service for Sciences, Technology and Culture. Also supported by grants from the FRSM, FNRS, ARBD and BRAHMS Diagnostics. Also supported by grants from CICYT (SAF2002–01509), and the Improving Human Potential of the European Community (HPRI-CT-1999-00071).
References
- Ascoli M., Fanelli,F. and Segaloff,D.L. (2002) The lutropin/choriogonadotropin receptor, a 2002 perspective. Endocr. Rev., 23, 141–174. [DOI] [PubMed] [Google Scholar]
- Bhowmick N., Huang,J., Puett,D., Isaacs,N.W. and Lapthorn,A.J. (1996) Determination of residues important in hormone binding to the extracellular domain of the luteinizing hormone/chorionic gonadotropin receptor by site-directed mutagenesis and modeling. Mol. Endocrinol., 10, 1147–1159. [DOI] [PubMed] [Google Scholar]
- Bhowmick N., Narayan,P. and Puett,D. (1999) Identification of ionizable amino acid residues on the extracellular domain of the lutropin receptor involved in ligand binding. Endocrinology, 140, 4558–4563. [DOI] [PubMed] [Google Scholar]
- Braun T., Schofield,P.R. and Sprengel,R. (1991) Amino-terminal leucine-rich repeats in gonadotropin receptors determine hormone selectivity. EMBO J., 10, 1885–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker G., Harper,J.F., Terasaki,W.L. and Moylan,R.D. (1979) Radioimmunoassay of cyclic AMP and cyclic GMP. Adv. Cyclic Nucleotide Res., 10, 1–33. [PubMed] [Google Scholar]
- Campbell R.K., Bergert,E.R., Wang,Y., Morris,J.C. and Moyle,W.R. (1997) Chimeric proteins can exceed the sum of their parts: implications for evolution and protein design. Nat. Biotechnol., 15, 439–443. [DOI] [PubMed] [Google Scholar]
- Chen C.Z. and Shapiro,R. (1997) Site-specific mutagenesis reveals differences in the structural bases for tight binding of RNase inhibitor to angiogenin and RNase A. Proc. Natl Acad. Sci. USA, 94, 1761–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clackson T. and Wells,J.A. (1995) A hot spot of binding energy in a hormone–receptor interface. Science, 267, 383–386. [DOI] [PubMed] [Google Scholar]
- Cornelis S., Uttenweiler-Joseph,S., Panneels,V., Vassart,G. and Costagliola,S. (2001) Purification and characterization of a soluble bioactive amino-terminal extracellular domain of the human thyrotropin receptor. Biochemistry, 40, 9860–9869. [DOI] [PubMed] [Google Scholar]
- Cornell W.D. (1995) A second generation force field for the simulation of proteins, nucleic acids and organic molecules. J. Am. Chem. Soc., 117, 5179–5197. [Google Scholar]
- Costagliola S., Khoo,D. and Vassart,G. (1998) Production of bioactive amino-terminal domain of the thyrotropin receptor via insertion in the plasma membrane by a glycosylphosphatidylinositol anchor. FEBS Lett., 436, 427–433. [DOI] [PubMed] [Google Scholar]
- Darden T., York,D. and Pedersen,L. (1993) Particle mesh Ewald: An N · log(N) method for Ewald sums in large systems. J. Chem. Phys., 98, 10089–10092. [Google Scholar]
- Dias J.A. and Van Roey,P. (2001) Structural biology of human follitropin and its receptor. Arch. Med. Res., 32, 510–519. [DOI] [PubMed] [Google Scholar]
- Dias J.A., Zhang,Y. and Liu,X. (1994) Receptor binding and functional properties of chimeric human follitropin prepared by an exchange between a small hydrophilic intercysteine loop of human follitropin and human lutropin. J. Biol. Chem., 269, 25289–25294. [PubMed] [Google Scholar]
- Dunbrack R.L. Jr and Cohen,F.E. (1997) Bayesian statistical analysis of protein side-chain rotamer preferences. Protein Sci., 6, 1661–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K.M., Dias,J.A. and Van Roey,P. (2001) Three-dimensional structure of human follicle-stimulating hormone. Mol. Endocrinol., 15, 378–389. [DOI] [PubMed] [Google Scholar]
- Grossmann M., Szkudlinski,M.W., Wong,R., Dias,J.A., Ji,T.H. and Weintraub,B.D. (1997) Substitution of the seat-belt region of the thyroid-stimulating hormone (TSH) β-subunit with the corresponding regions of choriogonadotropin or follitropin confers luteotropic but not follitropic activity to chimeric TSH. J. Biol. Chem., 272, 15532–15540. [DOI] [PubMed] [Google Scholar]
- Jiang X., Dreano,M., Buckler,D.R., Cheng,S., Ythier,A., Wu,H., Hendrickson,W.A. and el Tayar,N. (1995) Structural predictions for the ligand-binding region of glycoprotein hormone receptors and the nature of hormone–receptor interactions. Structure, 3, 1341–1353. [DOI] [PubMed] [Google Scholar]
- Kajava A.V. and Kobe,B. (2002) Assessment of the ability to model proteins with leucine-rich repeats in light of the latest structural information. Protein Sci., 11, 1082–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajava A.V., Vassart,G. and Wodak,S.J. (1995) Modeling of the three-dimensional structure of proteins with the typical leucine-rich repeats. Structure, 3, 867–877. [DOI] [PubMed] [Google Scholar]
- Kobe B. and Deisenhofer,J. (1993) Crystal structure of porcine ribonuclease inhibitor, a protein with leucine-rich repeats. Nature, 366, 751–756. [DOI] [PubMed] [Google Scholar]
- Kobe B. and Deisenhofer,J. (1995) A structural basis of the interactions between leucine-rich repeats and protein ligands. Nature, 374, 183–186. [DOI] [PubMed] [Google Scholar]
- Kobe B. and Kajava,A.V. (2001) The leucine-rich repeat as a protein recognition motif. Curr. Opin. Struct. Biol., 11, 725–732. [DOI] [PubMed] [Google Scholar]
- Kraulis P.J. (1991) MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallogr., 24, 945–950. [Google Scholar]
- Lapthorn A.J., Harris,D.C., Littlejohn,A., Lustbader,J.W., Canfield,R.E., Machin,K.J., Morgan,F.J. and Isaacs,N.W. (1994) Crystal structure of human chorionic gonadotropin. Nature, 369, 455–461. [DOI] [PubMed] [Google Scholar]
- Li M.D. and Ford,J.J. (1998) A comprehensive evolutionary analysis based on nucleotide and amino acid sequences of the α- and β-subunits of glycoprotein hormone gene family. J. Endocrinol., 156, 529–542. [DOI] [PubMed] [Google Scholar]
- Lindau-Shepard B., Brumberg,H.A., Peterson,A.J. and Dias,J.A. (2001) Reversible immunoneutralization of human follitropin receptor. J. Reprod. Immunol., 49, 1–19. [DOI] [PubMed] [Google Scholar]
- Merritt E.A. and Bacon,D.J. (1997) Raster3D: photorealistic molecular graphics. Methods Enzymol., 277, 505–524. [DOI] [PubMed] [Google Scholar]
- Moyle W.R., Campbell,R.K., Myers,R.V., Bernard,M.P., Han,Y. and Wang,X. (1994) Co-evolution of ligand–receptor pairs. Nature, 368, 251–255. [DOI] [PubMed] [Google Scholar]
- Moyle W.R., Campbell,R.K., Rao,S.N., Ayad,N.G., Bernard,M.P., Han,Y. and Wang,Y. (1995) Model of human chorionic gonadotropin and lutropin receptor interaction that explains signal transduction of the glycoprotein hormones. J. Biol. Chem., 270, 20020–20031. [DOI] [PubMed] [Google Scholar]
- Nicholls A., Sharp,K.A. and Honig,B. (1991) Protein folding and association: insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins, 11, 281–296. [DOI] [PubMed] [Google Scholar]
- Pearlman D.A., Case,D.A., Caldwell,J.W., Ross,W.S., Cheatam,T.E., DeBolt,S., Ferguson,D.M., Seibel,G. and Kollman,P.A. (1995) AMBER, a package of computer programs for applying molecular mechanics, normal mode analysis, molecular dynamics and free energy calculations to simulate the structural and energetic properties of molecules. Comput. Phys. Commun., 91, 1–41. [Google Scholar]
- Remy J.J., Couture,L., Pantel,J., Haertle,T., Rabesona,H., Bozon,V., Pajot-Augy,E., Robert,P., Troalen,F., Salesse,R. and Bidart,J.M. (1996) Mapping of HCG–receptor complexes. Mol. Cell. Endocrinol., 125, 79–91. [DOI] [PubMed] [Google Scholar]
- Remy J.J., Nespoulous,C., Grosclaude,J., Grebert,D., Couture,L., Pajot,E. and Salesse,R. (2001) Purification and structural analysis of a soluble human chorionogonadotropin hormone–receptor complex. J. Biol. Chem., 276, 1681–1687. [DOI] [PubMed] [Google Scholar]
- Ryckaert J.P., Ciccotti,G. and Berendsen,H. (1977) Numerical integration of the cartesian equation of motion of a system with constraints: molecular dynamics of n-alkanes. J. Comput. Phys., 00, 327–341. [Google Scholar]
- Sangkuhl K., Schulz,A., Schultz,G. and Schoneberg,T. (2002) Structural requirements for mutational lutropin/choriogonadotropin receptor activation. J. Biol. Chem., 277, 47748–47755. [DOI] [PubMed] [Google Scholar]
- Schenker J.G. (1999) Clinical aspects of ovarian hyperstimulation syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol., 85, 13–20. [DOI] [PubMed] [Google Scholar]
- Schmidt A., MacColl,R., Lindau-Shepard,B., Buckler,D.R. and Dias,J.A. (2001) Hormone-induced conformational change of the purified soluble hormone binding domain of follitropin receptor complexed with single chain follitropin. J. Biol. Chem., 276, 23373–23381. [DOI] [PubMed] [Google Scholar]
- Schreiber G. (2002) Kinetic studies of protein–protein interactions. Curr. Opin. Struct. Biol., 12, 41–47. [DOI] [PubMed] [Google Scholar]
- Schubert R.L., Narayan,P. and Puett,D. (2003) Specificity of cognate ligand–receptor interactions: fusion proteins of human chorionic gonadotropin and the heptahelical receptors for human luteinizing hormone, thyroid-stimulating hormone and follicle-stimulating hormone. Endocrinology, 144, 129–137. [DOI] [PubMed] [Google Scholar]
- Smits G., Govaerts,C., Nubourgh,I., Pardo,L., Vassart,G. and Costagliola,S. (2002) Lysine 183 and glutamic acid 157 of the TSH receptor: two interacting residues with a key role in determining specificity toward TSH and human CG. Mol. Endocrinol., 16, 722–735. [DOI] [PubMed] [Google Scholar]
- Szkudlinski M.W., Teh,N.G., Grossmann,M., Tropea,J.E. and Weintraub,B.D. (1996) Engineering human glycoprotein hormone superactive analogues. Nat. Biotechnol., 14, 1257–1263. [DOI] [PubMed] [Google Scholar]
- Szkudlinski M.W., Fremont,V., Ronin,C. and Weintraub,B.D. (2002) Thyroid-stimulating hormone and thyroid-stimulating hormone receptor structure–function relationships. Physiol. Rev., 82, 473–502. [DOI] [PubMed] [Google Scholar]
- Vlaeminck-Guillem V., Ho,S.C., Rodien,P., Vassart,G. and Costagliola,S. (2002) Activation of the cAMP pathway by the TSH receptor involves switching of the ectodomain from a tethered inverse agonist to an agonist. Mol. Endocrinol., 16, 736–746. [DOI] [PubMed] [Google Scholar]
- Wang Y., Bernard,M.P. and Moyle,W.R. (2000) Bifunctional hCG analogs adopt different conformations in LH and FSH receptor complexes. Mol. Cell. Endocrinol., 170, 67–77. [DOI] [PubMed] [Google Scholar]
- Wu H., Lustbader,J.W., Liu,Y., Canfield,R.E. and Hendrickson,W.A. (1994) Structure of human chorionic gonadotropin at 2.6 Å resolution from MAD analysis of the selenomethionyl protein. Structure, 2, 545–558. [DOI] [PubMed] [Google Scholar]