Abstract
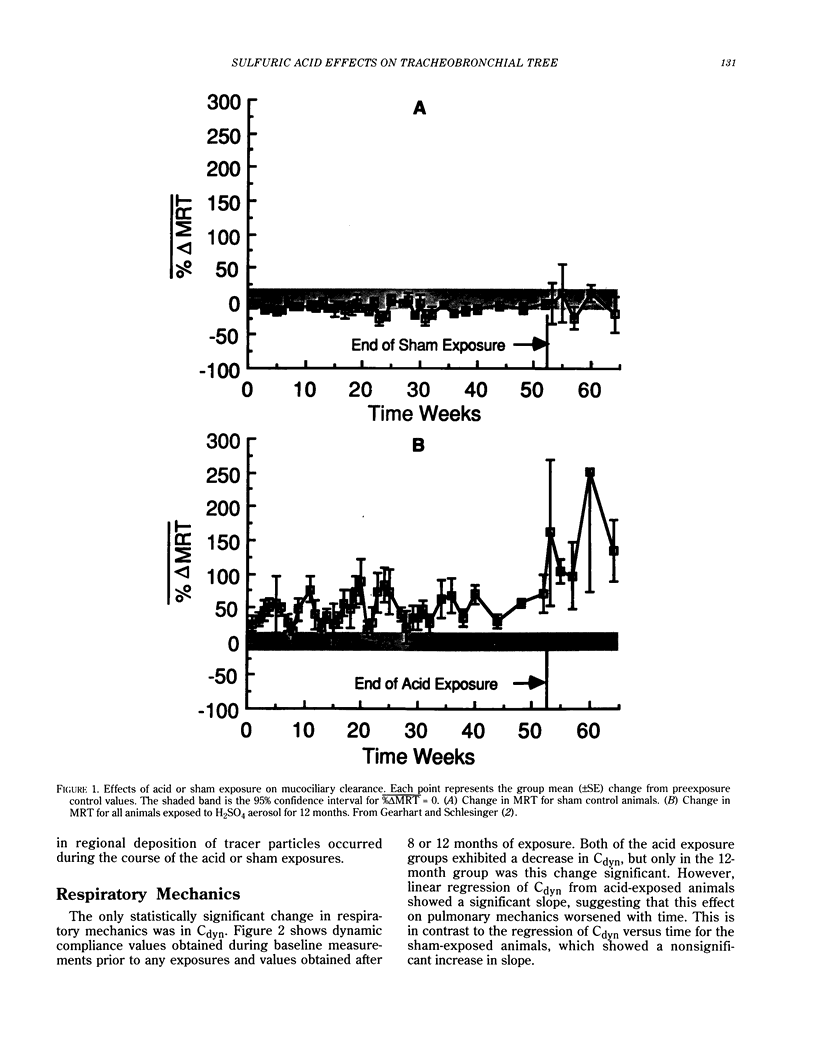
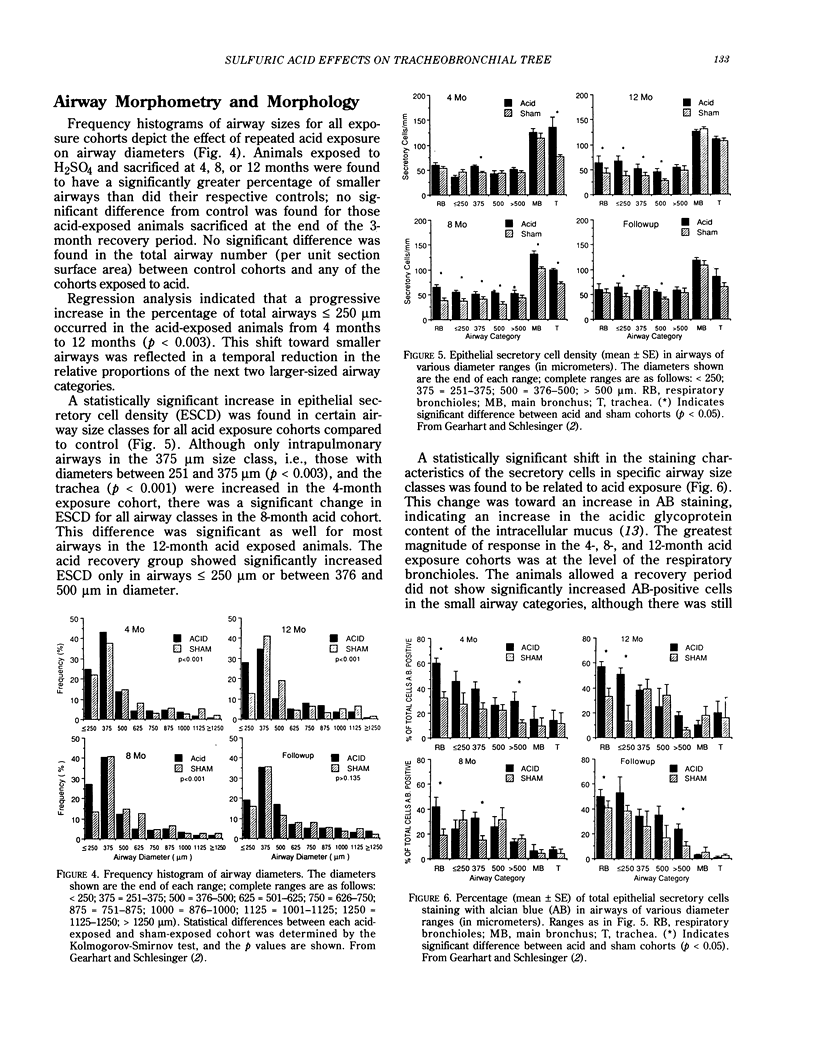
Sulfuric acid aerosols occur in the ambient particulate mode due to atmospheric conversion from sulfur dioxide (SO2). This paper describes the response of the rabbit tracheobronchial tree to daily exposures to sulfuric acid (H2SO4) aerosol, relating physiological and morphological parameters. Rabbits were exposed to filtered air (sham control) or to submicrometer-sized H2SO4 at 250 micrograms/m3 H2SO4, for 1 hr/day, 5 days/week, with sacrifices after 4, 8, and 12 months of acid (or sham) exposure; some rabbits were allowed a 3-month recovery after all exposures ended. H2SO4 produced a slowing of tracheobronchial mucociliary clearance during the first weeks of exposure; this change became significantly greater with continued exposures and did not improve after exposures ended. Airway hyperresponsiveness was evident by 4 months of acid exposure; the condition worsened by 8 months of exposure and appeared to stabilize after this time. Standard pulmonary mechanics parameters showed no significant trends with repeated acid exposure, except for a decline in dynamic lung compliance in animals exposed to acid for 12 months. Lung tissue samples obtained from exposed animals showed a shift toward a greater frequency of smaller airways compared to control, an increase in epithelial secretory cell density in smaller airways, and a shift from neutral to acidic glycoproteins in the secretory cells. The effect on airway diameter resolved after the exposures ceased, but the secretory cell response did not return to normal within the recovery period. No evidence of inflammatory cell infiltration was found due to H2SO4 exposure. Thus, significant alterations in the physiology of the tracheobronchial tree have been demonstrated due to repeated 1-hr exposures to a concentration of H2SO4 that is one-fourth the current 8-hr threshold limit value for exposure in the work environment. The cumulative dose inhaled by the rabbits is similar to current peak daily doses from ambient exposure in North America. The results obtained in the rabbit model provide insight into early changes in the tracheobronchial tree due to repeated irritant exposure and may be involved in the pathogenesis of chronic airway disease.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert R. E., Spiegelman J., Lippmann M., Bennett R. The characteristics of bronchial clearance in the miniature donkey. Arch Environ Health. 1968 Jul;17(1):50–58. doi: 10.1080/00039896.1968.10665188. [DOI] [PubMed] [Google Scholar]
- Camner P., Mossberg B., Philipson K. Tracheobronchial clearance and chronic obstructive lung disease. Scand J Respir Dis. 1973;54(5):272–281. [PubMed] [Google Scholar]
- Casale T. B., Rhodes B. J., Donnelly A. L., Weiler J. M. Airway responses to methacholine in asymptomatic nonatopic cigarette smokers. J Appl Physiol (1985) 1987 May;62(5):1888–1892. doi: 10.1152/jappl.1987.62.5.1888. [DOI] [PubMed] [Google Scholar]
- Cosio M. G., Hale K. A., Niewoehner D. E. Morphologic and morphometric effects of prolonged cigarette smoking on the small airways. Am Rev Respir Dis. 1980 Aug;122(2):265–221. doi: 10.1164/arrd.1980.122.2.265. [DOI] [PubMed] [Google Scholar]
- Fanta C. H. Clinical aspects of mucus and mucous plugging in asthma. J Asthma. 1985;22(6):295–301. doi: 10.3109/02770908509087113. [DOI] [PubMed] [Google Scholar]
- Foster W. M., Langenback E. G., Bergofsky E. H. Disassociation in the mucociliary function of central and peripheral airways of asymptomatic smokers. Am Rev Respir Dis. 1985 Sep;132(3):633–639. doi: 10.1164/arrd.1985.132.3.633. [DOI] [PubMed] [Google Scholar]
- Gearhart J. M., Schlesinger R. B. Sulfuric acid-induced airway hyperresponsiveness. Fundam Appl Toxicol. 1986 Nov;7(4):681–689. doi: 10.1016/0272-0590(86)90118-1. [DOI] [PubMed] [Google Scholar]
- Hogg J. C., Macklem P. T., Thurlbeck W. M. Site and nature of airway obstruction in chronic obstructive lung disease. N Engl J Med. 1968 Jun 20;278(25):1355–1360. doi: 10.1056/NEJM196806202782501. [DOI] [PubMed] [Google Scholar]
- Jones R., Reid L. Secretory cell hyperplasia and modification of intracellular glycoprotein in rat airways induced by short periods of exposure to tobacco smoke, and the effect of the antiinflammatory agent phenylmethyloxadiazole. Lab Invest. 1978 Jul;39(1):41–49. [PubMed] [Google Scholar]
- Lippmann M., Albert R. E. The effect of particle size on the regional deposition of inhaled aerosols in the human respiratory tract. Am Ind Hyg Assoc J. 1969 May-Jun;30(3):257–275. doi: 10.1080/00028896909343120. [DOI] [PubMed] [Google Scholar]
- Lopata M., Barton A. D., Lourenço R. V. Biochemical characteristics of bronchial secretions in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1974 Dec;110(6):730–739. doi: 10.1164/arrd.1974.110.6P1.730. [DOI] [PubMed] [Google Scholar]
- Matsuba K., Thurlbeck W. M. The number and dimensions of small airways in nonemphysematous lungs. Am Rev Respir Dis. 1971 Oct;104(4):516–524. doi: 10.1164/arrd.1971.104.4.516. [DOI] [PubMed] [Google Scholar]
- Maxwell G. M. The problem of mucus plugging in children with asthma. J Asthma. 1985;22(3):131–137. doi: 10.3109/02770908509073131. [DOI] [PubMed] [Google Scholar]
- Miskovits G., Appel J., Szüle P. Ultrastructural changes of ciliated columnar epithelium and goblet cells in chronic bronchitis biopsy material. Acta Morphol Acad Sci Hung. 1974;22(1):91–103. [PubMed] [Google Scholar]
- Mossberg B., Camner P. Impaired mucociliary transport as a pathogenetic factor in obstructive pulmonary diseases. Chest. 1980 Feb;77(2 Suppl):265–266. doi: 10.1378/chest.77.2.265. [DOI] [PubMed] [Google Scholar]
- Niewoehner D. E., Kleinerman J., Rice D. B. Pathologic changes in the peripheral airways of young cigarette smokers. N Engl J Med. 1974 Oct 10;291(15):755–758. doi: 10.1056/NEJM197410102911503. [DOI] [PubMed] [Google Scholar]
- Orehek J., Gayrard P., Smith A. P., Grimaud C., Charpin J. Airway response to carbachol in normal and asthmatic subjects: distinction between bronchial sensitivity and reactivity. Am Rev Respir Dis. 1977 Jun;115(6):937–943. doi: 10.1164/arrd.1977.115.6.937. [DOI] [PubMed] [Google Scholar]
- Ramsdale E. H., Roberts R. S., Morris M. M., Hargreave F. E. Differences in responsiveness to hyperventilation and methacholine in asthma and chronic bronchitis. Thorax. 1985 Jun;40(6):422–426. doi: 10.1136/thx.40.6.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsdell J. W., Nachtwey F. J., Moser K. M. Bronchial hyperreactivity in chronic obstructive bronchitis. Am Rev Respir Dis. 1982 Nov;126(5):829–832. doi: 10.1164/arrd.1982.126.5.829. [DOI] [PubMed] [Google Scholar]
- Schlesinger R. B. Factors affecting the response of lung clearance systems to acid aerosols: role of exposure concentration, exposure time, and relative acidity. Environ Health Perspect. 1989 Feb;79:121–126. doi: 10.1289/ehp.8979121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger R. B., Naumann B. D., Chen L. C. Physiological and histological alterations in the bronchial mucociliary clearance system of rabbits following intermittent oral or nasal inhalation of sulfuric acid mist. J Toxicol Environ Health. 1983 Aug-Sep;12(2-3):441–465. doi: 10.1080/15287398309530440. [DOI] [PubMed] [Google Scholar]
- Schlesinger R. B., Vollmuth T. A., Naumann B. D., Driscoll K. E. Measurement of particle clearance from the alveolar region of the rabbit respiratory tract. Fundam Appl Toxicol. 1986 Aug;7(2):256–263. doi: 10.1016/0272-0590(86)90155-7. [DOI] [PubMed] [Google Scholar]
- Shelhamer J. H., Kaliner M. A. Respiratory mucus production in asthma. Bull Eur Physiopathol Respir. 1985 Jul-Aug;21(4):301–307. [PubMed] [Google Scholar]
- Taylor R. G., Joyce H., Gross E., Holland F., Pride N. B. Bronchial reactivity to inhaled histamine and annual rate of decline in FEV1 in male smokers and ex-smokers. Thorax. 1985 Jan;40(1):9–16. doi: 10.1136/thx.40.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utell M. J. Effects of inhaled acid aerosols on lung mechanics: an analysis of human exposure studies. Environ Health Perspect. 1985 Nov;63:39–44. doi: 10.1289/ehp.856339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner A. The role of mucociliary dysfunction in bronchial asthma. Am J Med. 1979 Sep;67(3):477–485. doi: 10.1016/0002-9343(79)90797-6. [DOI] [PubMed] [Google Scholar]


